Back to Journals » Patient Preference and Adherence » Volume 14
Comparison of Real-World Treatment Patterns Among Psoriasis Patients Treated with Ixekizumab or Adalimumab
Authors Blauvelt A , Shi N, Burge R , Malatestinic WN , Lin CY, Lew CR, Zimmerman NM , Goldblum OM, Zhu B, Murage MJ
Received 9 October 2019
Accepted for publication 11 February 2020
Published 9 March 2020 Volume 2020:14 Pages 517—527
DOI https://doi.org/10.2147/PPA.S233993
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Andrew Blauvelt,1 Nianwen Shi,2 Russel Burge,3,4 William N Malatestinic,3 Chen-Yen Lin,3 Carolyn R Lew,2 Nicole M Zimmerman,2 Orin M Goldblum,3 Baojin Zhu,3 Mwangi J Murage3
1Oregon Medical Research Center, Portland, OR, USA; 2IBM Watson Health, Cambridge, MA, USA; 3Eli Lilly and Company, Indianapolis, IN, USA; 4University of Cincinnati, Cincinnati, OH, USA
Correspondence: Mwangi J Murage
Global Patient Outcomes and Real World Evidence (GPORWE), Eli Lilly and Company, LCT – South Building 171-2, Drop Code 5221, 1555 Harding St, Indianapolis, IN 46221, USA
Tel +1-317-460-3619
Email [email protected]
Background: There is lack of real-world treatment pattern comparison data between ixekizumab and adalimumab which are approved for the treatment of moderate-to-severe plaque psoriasis.
Objective: To compare real-world treatment patterns among psoriasis patients initiating ixekizumab or adalimumab in the United States.
Methods: Psoriasis patients with ≥ 1 claim for ixekizumab or adalimumab between March 1, 2016, and May 31, 2018, were identified (index date = date of first ixekizumab or adalimumab claim) from the IBM Watson Health MarketScan® databases. Patients were required to be continuously enrolled for ≥ 12 months before the index date and followed for a minimum of 6 months until inpatient death, enrollment end, or study end, whichever occurred first. Treatment persistence, adherence, discontinuation, restart, and switching were analyzed. Inverse probability of treatment weighting and multivariable regression modeling were employed to address cohort imbalances and estimate the adjusted risk of non-persistence, discontinuation, and switching, and the odds of adherence.
Results: A total of 646 ixekizumab and 3668 adalimumab users were included and followed for a mean of 14.0 and 16.5 months, respectively. Compared to adalimumab, ixekizumab was associated with 19% lower risk of non-persistence (hazard ratio [HR]=0.81, 95% confidence interval [CI]: 0.69– 0.95), 26% lower risk of discontinuation (HR=0.74, 95% CI: 0.62– 0.88), and 28% lower risk of switching (HR=0.72, 95% CI: 0.57– 0.91). Ixekizumab users had higher odds of medication possession ratio ≥ 80% (odds ratio [OR]=1.36, 95% CI: 1.10– 1.69) but similar odds by proportion of days covered ≥ 80% (OR=1.22, 95% CI: 0.98– 1.53).
Conclusion: Psoriasis patients treated with ixekizumab demonstrated longer persistency, higher adherence and were less likely to discontinue or switch treatment compared to adalimumab users. However, while patients achieving highly adherent threshold significantly differed by MPR ≥ 80%, it did not by PDC ≥ 80%; hence, further analysis using fixed-length follow-up is required.
Keywords: ixekizumab, adalimumab, psoriasis, treatment switching, discontinuation, persistence
Plain Language Summary
Ixekizumab was approved by the United States Food and Drug Administration (FDA) to treat moderate-to-severe psoriasis in March 2016. Although IXE has demonstrated superior efficacy over etanercept and ustekinumab in clinical trials, there is a paucity of real-world studies comparing the effectiveness and drug survival of ixekizumab to other biologics indicated for psoriasis. This retrospective cohort study is the first direct comparison of treatment patterns between ixekizumab and adalimumab among psoriasis patients using real-world data in the United States. A total of 646 ixekizumab and 3668 adalimumab users were identified from administrative claims data between 2016 and 2018 and followed for an average of 14 and 17 months, respectively. Advanced robust analyses (inverse probability of treatment weights [IPTW] and multivariable regression modeling) were used to address baseline cohort imbalances. Ixekizumab recipients were associated with lower risks of non-persistence, treatment discontinuation, and treatment switching than adalimumab users. Findings from this study provide direct evidence for cost-effectiveness decisions in psoriasis management.
Introduction
Adalimumab (ADA), a tumor necrosis factor (TNF) inhibitor, and more recently approved ixekizumab (IXE), an interleukin-17A (IL-17) inhibitor, are among commonly used biologics for the treatment of moderate-to-severe psoriasis and their efficacy in clinical trials has raised patient expectations for achievable disease control.1,2 In large clinical trials, 63–80% of ADA patients and 87–90% of IXE patients achieved a 75% or more reduction in Psoriasis Area and Severity Index (PASI), while 40–56% of ADA patients and 68–73% of IXE patients achieved a 90% or more reduction in PASI.3–8 Furthermore, 13% and 38% of ADA and IXE patients achieved complete resolution of psoriatic plaques (PASI 100) at week 12, respectively.4,5 In a recent review of 15 years of real-world data, ADA was highlighted for its superior short-term effectiveness and drug survival compared with etanercept.9 Although IXE has demonstrated superior efficacy over etanercept and ustekinumab in clinical trials,5,10,11 there is a paucity of real-world studies comparing the effectiveness and drug survival of IXE to those of other biologics, and there has been no direct comparison between IXE and ADA in psoriasis patients.12 To fill this gap, this retrospective analysis aimed to evaluate and compare real-world treatment patterns, including treatment persistence (drug survival), adherence, discontinuation, switching, and restart, among psoriasis patients treated with either IXE or ADA in the United States (U.S.).
Materials and Methods
Study Design and Data Source
A retrospective, inverse probability-weighted cohort design was used to compare treatment patterns between IXE and ADA among psoriasis patients in the US. The analysis was performed using patient-level administrative claims data extracted from IBM Watson Health MarketScan® Commercial Claims and Encounters (Commercial), Medicare Supplemental and Coordination of Benefits (Medicare), and Early View databases from July 1, 2015 through May 31, 2018.
The Commercial database contains inpatient, outpatient, and outpatient prescription drug experience of approximately 147.9 million employees and their dependents covered under both fee-for-service and managed care health plans between 1995 and 2017. The Medicare database contains medical and prescription data on approximately 10.6 million retirees with Medicare supplemental insurance paid for by employers between 1995 and 2017, including the Medicare-covered portion of the payment (Coordination of Benefits Amount, or COB) and the employer-paid portion. The Early View database (January–May 2018) includes the same components in the standard commercial and Medicare databases, but captures healthcare services incurred as late as 45 days before the data release. Data are fully compliant with the Health Insurance Portability and Accountability Act of 1996. As this study did not involve the collection, use, or transmittal of individually identifiable data, it was exempted from Institutional Review Board approval.
Patient Selection and Study Cohorts
Patients with ≥1 inpatient or ≥2 outpatient claims ≥30 days apart for psoriasis (ICD-9-CM diagnoses: 696.1x or ICD-10-CM diagnoses: L40.0-L40.4, L40.8) between July 1, 2015, and May 31, 2018, were identified. Claims for diagnostic procedures were excluded. Patients were required to have a claim for IXE or ADA between March 1, 2016, and May 31, 2018, and a psoriasis diagnosis on or before the first drug claim. The date of the first IXE or ADA claim was set as the index date, and patients were classified into the IXE or ADA cohorts accordingly. All study patients were required to be 18 years or older at index and have continuous enrollment with medical and pharmacy benefits for 12 months prior to (pre-index period) and at least 6 months after the index date (follow-up period). To ensure the index drug was prescribed for psoriasis, patients with pre-period diagnoses for non-psoriasis indications for their index drug such as psoriatic arthritis for IXE and ADA, and ankylosing spondylitis, juvenile idiopathic arthritis, rheumatoid arthritis, Crohn’s disease, ulcerative colitis, hidradenitis suppurativa, and uveitis for ADA, were excluded. Additionally, patients were excluded if they had the index drug within 90 days before the index date.
All patients were followed for a minimum 6-month from the index date until inpatient death, the end of database enrollment, or study end (May 31, 2018), whichever occurred first.
Study Measures
Study outcomes included treatment persistence, adherence, discontinuation, restart, and switching. Pharmacy claims billed by National Drug Codes (NDC) and medical claims for injectable medications were assembled to analyze medication utilization. The length of treatment was determined by days’ supply recorded on the outpatient pharmacy claims billed by NDC. When ADA is billed as a medical claim, a 14-day supply was assigned to each claim based on the recommended dosing schedule.13 Allowable gaps of 45-day, 60-day, and 90-day have been used in biologic treatment persistence analyses.14–16 In this study, 60-day gap served as the primary measure and patients reached non-persistence when having a gap of 60 days or longer. Sensitivity analyses were performed using 45-day and 90-day gaps. The persistence end date was set by the day before the first allowable gap. If the last day supply occurred less than the allowable gap to the end of follow-up, the persistence end date was determined by the last day supply. For example, if a patient had a 300-day follow-up period and was on medication until day 270, the patient was flagged as persistent from day 1 to day 270.
Treatment adherence was assessed by medication possession ratio (MPR) as the primary definition and proportion of days covered (PDC) as the secondary definition. MPR was calculated as the total days’ supply during the follow-up period divided by the length of the follow-up period. PDC was calculated as the number of days covered during the follow-up period divided by the length of the follow-up period. When two consecutive scripts overlapped, the overlapping days were appended for MPR and truncated for PDC. Patients were flagged as highly adherent based on MPR and PDC ≥80%, which have been used in prior studies.17,18
Treatment discontinuation occurred when having a gap of 90 days or longer without index medication on hand.14 The last days’ supply before the first 90-day gap set the discontinuation date. Restart was flagged if the patients had a new claim for the index drug after discontinuation.
Treatment switching was defined as having a new psoriasis agent alone for 30 days or longer after discontinuing the index drug. Psoriasis agents included biologics (brodalumab, certolizumab, etanercept, guselkumab, infliximab, secukinumab, IXE [for ADA users], ADA [for IXE users], ustekinumab), apremilast, acitretin, cyclosporine, or methotrexate. If the new agent overlapped with the index drug for less than 30 days, the switching date was set as the date of the first claim of the new therapy. If it overlapped 30 days or more, the switching date was set as the first day on the new medication after the overlapping period. Treatment gaps less than 90 days were included in the determination of the length of overlapping period and monotherapy with the new drug.
Other Variables
Demographic characteristics, measured on the index date, included age, gender, geographic region, payer, and health plan type. Baseline clinical characteristics included Deyo Charlson co-morbidity index (DCCI) and co-morbid conditions (anxiety, coronary heart disease, depression, diabetes, hyperlipidemia, hypertension, multiple sclerosis, obesity, osteoarthritis, other autoimmune disorders, peripheral vascular disease, and sleep apnea). Baseline biologic use, the number of unique biologics, the use of systemic agents/targeted oral therapies for psoriasis, topical agents, and phototherapy were captured. Baseline total all-cause and psoriasis-related healthcare costs were assessed. The length of the follow-up period was reported.
Statistical Analysis
To address baseline cohort differences, inverse probability of treatment weights (IPTW) were obtained from a logistic regression model with IXE versus ADA as the dependent variable. Covariates included the demographic, clinical, and medication variables listed in the “Other Variables” section (except for multiple sclerosis, which occurred to <1% of patients), as well as baseline psoriasis-related healthcare costs. Descriptive results were adjusted by IPTW and standardized difference (StdDiff) was used to evaluate cohort balances. Cohorts were considered balanced if StdDiff was 0.1 or lower.
Descriptive analyses were performed for all study variables. Categorical measures were summarized as counts and percentages. Continuous measures were presented as means and standard deviations. Statistical tests of significance were conducted for outcome variables using chi-squared tests for categorical variables and two-sample t-tests for continuous variables.
Kaplan-Meier estimation with Log rank test was employed to estimate the probabilities of persistence, discontinuation, and switching using the weighted data. Cox proportional hazards regression analyses were performed to estimate the risk of treatment non-persistence, discontinuation, and switching. Logistic regression was used to estimate the odds of being highly adherent to treatment. The same list of covariates and multiple sclerosis was included in all regression models. All models were weighted by IPTW and used a robust “sandwich” covariance approach to estimate the standard error.19 P values <0.05 were considered, a priori, to be statistically significant.
Descriptive analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC). Weighted descriptive analyses, Kaplan–Meier curves, and multivariable analyses were generated with R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 646 IXE users and 3668 ADA users were included in the study (Figure 1). Before weighting, IXE users were, on average, 2.5 years older than ADA users (49.7±2.0 years vs 47.2±12.9 years, Table 1). IXE users had worse baseline health status as demonstrated by higher mean DCCI (0.6±1.2 vs 0.4±1.0, StdDiff =0.128) and higher pre-period rates for co-morbid conditions, including hypertension (40.6% vs 33.6%, StdDiff =0.145), hyperlipidemia (36.4% vs 29.4%, StdDiff =0.148), obesity (26.0% vs 20.3%, StdDiff =0.135), diabetes (20.3% vs 14.0%, StdDiff =0.166), and sleep apnea (14.2% vs 9.1%, StdDiff =0.193). In addition, IXE users had a higher rate of pre-period biologic use (65.8% vs 35.8%, StdDiff =0.628), higher mean number of unique biologics (0.7±0.6 vs 0.4±0.5, StdDiff =0.679), and higher rate of prior systemic treatment/targeted oral therapies (60.5% vs 53.1%, StdDiff =0.151) than ADA users. Total baseline all-cause and psoriasis-related healthcare costs were also higher for IXE than for ADA.
 |
Table 1 Baseline Patient Characteristics, Before and After Inverse Probability of Treatment Weighting (IXE versus ADA) |
 |
Figure 1 Patient selection. Abbreviations: ADA, adalimumab; ICD-9/ICD-10-CM, international classification of diseases, ninth/tenth revision clinical modification; IXE, ixekizumab. |
All baseline demographic and clinical characteristics were well balanced after weighting (Table 1). The mean length of the follow-up period was 14.0±4.9 months for IXE users and 16.5±6.4 months for ADA users, respectively.
Treatment Persistence
IXE users had a significantly higher persistence rate than ADA users during the entire variable follow-up period (54.5% vs 42.2%, P <0.001; Table 2) based on 60-day allowable gap. IXE users also spent a larger proportion of follow-up time on persistent treatment than ADA users (70.2%±35.2% vs 62.5%±35.4%, P <0.001; Table 2). Kaplan-Meier estimation showed the probability of persistence was significantly higher for IXE users (P = 0.004; Figure 2). The difference in the persistence rates between IXE and ADA increased over time, from 3% (70%/IXE vs 67%/ADA) at day 180 to 8% (55%/IXE vs 47%/ADA) at day 360. The sensitivity analyses using 45-day and 90-day gaps depicted a similar pattern (P = 0.051 and P < 0.001, respectively; eFigures 1–2).
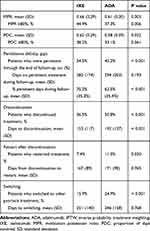 |
Table 2 Weighted Treatment Pattern Outcomes During the Follow-Up Period (IXE versus ADA) |
 |
Figure 2 Kaplan–Meier curve (weighted) for probability of persistence (60-day gap) (IXE versus ADA). Abbreviations: ADA, adalimumab; IXE, ixekizumab. |
Treatment Adherence
Compared to ADA users, IXE users had significantly higher mean MPR (0.66±0.29 vs 0.61±0.30, P = 0.003) and higher percentage of patients with MPR ≥80% (44.9% vs 37.3%, P = 0.006; Table 2) over an average of 14.0- and 16.5-month follow-up for IXE and ADA users, respectively. PDC-based adherence analysis indicated IXE users had significantly higher mean PDC than ADA users (0.62±0.29 vs 0.58±0.29, P = 0.022) but no statistically significant differences were detected in the percentage of patients with PDC ≥80% (38.2% vs 33.1%, P = 0.061).
Treatment Discontinuation and Restart
The discontinuation rate was significantly lower among IXE users than ADA users (36.5% vs 50.8%, P < 0.001; Table 2). Based on Kaplan–Meier estimation, 1-year probability of survival from discontinuation was 0.63 for IXE and 0.52 for ADA (P < 0.001), respectively. The proportion of patients who restarted index treatment after discontinuation was significantly lower among IXE users than ADA users (7.4% vs 11.3%, P = 0.020). The mean time between treatment discontinuation and restart was comparable between IXE and ADA users (5.6±3.0 months and 5.7±3.0 months, P = 0.765) based on a mean length of follow-up of 14.0 and 16.5 months, respectively.
Switching
Compared to ADA users, IXE users were less likely to switch to another psoriasis agent (15.9% vs 24.9%, P < 0.001; Table 2). Kaplan–Meier estimation showed that 1-year probability of staying on index drug was 0.87 for IXE and 0.78 for ADA (P = 0.003), respectively. The top four agents IXE users switched to were ustekinumab (3.0%), secukinumab (2.9%), guselkumab (2.5%), and apremilast (2.5%), whereas the top new agents ADA users switched to were ustekinumab (7.5%), secukinumab (5.4%), IXE (4.3%), and apremilast (3.0%).
Multivariable Analyses
After adjusting for baseline patient characteristics, IXE was associated with a 19% lower risk of non-persistence (hazard ratio [HR] = 0.81, 95% confidence interval [CI]: 0.69–0.95) than ADA (Figure 3). Sensitivity analyses using 45-day and 90-day gaps confirmed similar patterns, although the result with the 45-day gap was not statistically significant. In addition, IXE users had a 26% lower risk of discontinuation (HR = 0.74, 95% CI: 0.62–0.88) and a 28% lower risk of switching (HR = 0.72, 95% CI: 0.57–0.91) compared with ADA users. IXE users had 36% higher odds of being highly adherent to treatment than ADA based on MPR≥80% (OR = 1.36, 95% CI: 1.10–1.69). Findings based on PDC were similar, but the differences were statistically insignificant (OR = 1.22, 95% CI: 0.98–1.53).
Discussion
Drug treatment patterns have been used as a surrogate of real-world therapeutic effectiveness and tolerability in psoriasis patients.20,21 This retrospective, claims-based analysis is the first to examine real-world treatment patterns of IXE and ADA among psoriasis patients in the US. After adjusting for baseline patient demographic and clinical characteristics, psoriasis patients treated with IXE demonstrated better treatment pattern outcomes across all measures examined compared to ADA users, including 19% lower risk of non-persistence, 26% lower risk of discontinuation, and 28% lower risk of switching.
Real-world treatment patterns among ADA users have been analyzed previously and our findings are consistent with previous reports.12,15,17,20,22–33 Using a single payer database and a 60-day allowable gap, Chastek et al found that 42% of ADA users were persistent at 1 year and the mean length of persistence was 291 days over a maximum 2-year follow-up.25 This is comparable to the 47% persistent rate and the mean length of the persistence of 294 days over an average of 16.5 months of follow-up observed among ADA users in this study. In a study of Medicare patients, 40.7% of ADA users were adherent (PDC ≥80%) and 43.4% discontinued treatment (90-day gap) in the first year of treatment.15 In our study, fewer ADA users were adherent (33.1%) and more discontinued treatment (50.8%), but this was reasonable given the roughly 38% longer mean follow-up period.
In this study, approximately 25% of ADA users switched to another psoriasis treatment and 20% switched to another biologic during a mean length of 16.5-month follow-up, which is higher than previous estimates (6–10%) based on 1-year follow-up.15,23,26,29 While the definition of switching may vary among different studies, the higher switching rate observed in our study, in part, may reflect the introduction of new treatment options for psoriasis, such as IXE, with stronger efficacy and fewer required injections. Three of the top four drugs that ADA users switched to were approved after September 2014. In addition, 64% of ADA users in the current study were biologic-naïve before starting ADA, whereas only 34% of IXE users were biologic-naïve.
IXE is a newer agent approved in 2016,34 and there are limited real-world data available. Based on 801 IXE users, Murage et al reported an 80% persistence rate over an average of 7.5 months. In addition, 12.5% and 7.1% IXE users discontinued IXE and switched treatment, respectively.12 The discontinuation and switching rates were higher and the persistence rate was lower in our study, likely due to the length of follow-up being twice as long as reported in the Murage study.12
Data used in cost-effectiveness models for new biologics like IXE or secukinumab are mostly based on clinical trials, which can differ substantially from routine clinical practice.22,35 In the Institute for Clinical and Economic Review evidence rating for a head-to-head comparison between IXE and ADA, IXE was rated as “C+” (comparable or better net health benefit with moderate certainty in the evidence) and ADA as “C-” (moderate certainty that comparative net health benefit is comparable or inferior) based on indirect evidence.36 Our study provides direct evidence based on real-world data comparing treatment patterns between IXE and ADA among psoriasis patients, which may inform clinicians and payers making treatment decisions for their patients and formulary decisions for their organizations.
Interpretation of findings from this study should consider several limitations. Clinical variables were captured based on diagnosis codes, procedure codes, and pharmacy prescriptions from claims, which are subject to data coding limitations and data entry error. The Early View database may have missed 1–3% of pharmacy claims due to shorter adjudication period. Treatment pattern analyses were built on the assumption that patients took medications as prescribed. These information biases, however, affected both groups equally. Psoriasis severity was not measurable from claims. As a remedy, pre-period biologic use and number of unique biologics were captured as a proxy for severity. IPTW and multivariable modeling were employed to address observable imbalances between patient cohorts, but unobservable differences from claims remained. Treatment adherence findings from this study should be considered as inconclusive since IXE users had a shorter mean length of follow-up than ADA users did. The adherence outcomes were assessed via a logistic regression model, which did not account for variable length follow-up. Future analysis using fixed-length follow-up is required to evaluate these findings. Finally, the chosen databases were limited to only those individuals with commercial health coverage or private Medicare supplemental coverage. Consequently, the results of this analysis may not be generalizable to psoriasis patients with other types of insurance or the uninsured.
Conclusions
This study provided direct evidence of real-world treatment pattern comparison between psoriasis patients treated with either IXE or ADA. Compared to ADA recipients, psoriasis patients initiating IXE treatment had typically worse general health and were more likely to have failed prior biologics. Nonetheless, IXE users depicted better treatment pattern outcomes overall with the longer persistency and lower likelihood of discontinuing treatment or switching to another medication compared to patients treated with ADA.
Abbreviations
ADA, Adalimumab; CI, Confidence Interval; COB, Coordination of Benefits; DCCI, Deyo Charlson Co-Morbidity Index; IBM, International Business Machines; HR, Hazard Ratio; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IL-17, Interleukin-17A; IPTW, inverse probability of treatment weighting; IXE, Ixekizumab; MPR, Medication Possession Ratio; NDC, National Drug Codes; OR, Odds Ratio; PASI, Psoriasis Area and Severity Index; PDC, Proportion of Days Covered; SAS, Statistical Analytic System; StdDiff, Standardized Difference; TNF, Tumor Necrosis Factor; U.S., United States.
Ethics Approval and Informed Consent
This study used deidentified data; therefore, it was exempted from Institutional Review Board approval.
Acknowledgments
Natalie N Boytsov, Meghan E Jones, and Alan J M Brnabic of Eli Lilly and Company provided a critical review for the analyses. Aswin Kalyanaraman and Arun Eswaran of IBM Watson Health provided programming for the study. Santosh Tiwari of IBM Watson Health provided medical writing support in accordance with Good Publication Practice guidelines (www.ismpp.org/gpp3).
Author Contributions
Nianwen Shi had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Andrew Blauvelt, Nianwen Shi, Russel Burge, William N Malatestinic, Orin M Goldblum, Baojin Zhu, Mwangi J. Murage. Acquisition, analysis, or interpretation of data: Andrew Blauvelt, Nianwen Shi, Russel Burge, William N Malatestinic, Chen-Yen Lin, Carolyn R. Lew, Nicole M. Zimmerman, Orin M Goldblum, Baojin Zhu, Mwangi J. Murage. Critical revision of the manuscript for important intellectual content: Andrew Blauvelt, Nianwen Shi, Russel Burge, William N Malatestinic, Chen-Yen Lin, Carolyn R. Lew, Nicole M. Zimmerman, Orin M Goldblum, Baojin Zhu, Mwangi J. Murage. Statistical analysis: Nicole M. Zimmerman. Supervision: Mwangi J. Murage, Nianwen Shi. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was funded by Eli Lilly and Company, USA. Employees from Eli Lilly participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure
Andrew Blauvelt has served as a scientific adviser and/or clinical study investigator for AbbVie Aclaris, Akros, Allergan, Almirall, Amgen, Arena, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Inc., Eli Lilly and Company, Galderma, Genentech/Roche, GlaxoSmithKline, Janssen, Leo, Meiji, Merck Sharp & Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Revance, Sandoz, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB Pharma, Valeant, and Vidac, and as a paid speaker for Janssen, Regeneron, and Sanofi Genzyme. He also reports grants, personal fees from Eli Lilly and Novartis, during the conduct of the study. Russel Burge, William N Malatestinic, Chen-Yen Lin, Orin M Goldblum, Baojin Zhu, and Mwangi J. Murage are employees and shareholders of Eli Lilly and Company, who provided funding for this project. Nianwen Shi, Carolyn R. Lew, and Nicole M. Zimmerman are employees of IBM Watson Health and served as a consultant for this project. The authors report no other conflicts of interest in this work.
References
1. Belinchón I, Rivera R, Blanch C, Comellas M, Lizán L. Adherence, satisfaction and preferences for treatment in patients with psoriasis in the European Union: a systematic review of the literature. Patient Prefer Adherence. 2016;10:2357–2367. doi:10.2147/PPA
2. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18(11):2297. doi:10.3390/ijms18112297
3. Asahina A, Nakagawa H, Etoh T, Ohtsuki M. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol. 2010;37(4):299–310. doi:10.1111/jde.2010.37.issue-4
4. Cai L, Gu J, Zheng J, et al. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a Phase 3, randomized, placebo-controlled, double-blind study. J Eur Acad Dermatol Venereol. 2017;31(1):89–95. doi:10.1111/jdv.13746
5. Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551. doi:10.1016/S0140-6736(15)60125-8
6. Leonardi C, Maari C, Philipp S, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: three-year results from the UNCOVER-3 study. J Am Acad Dermatol. 2018;79(5):824–830.e822. doi:10.1016/j.jaad.2018.05.032
7. Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a Phase III study. Br J Dermatol. 2017;177(4):1014–1023. doi:10.1111/bjd.15666
8. Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158(3):558–566. doi:10.1111/j.1365-2133.2007.08315.x
9. Sator P. Safety and tolerability of adalimumab for the treatment of psoriasis: a review summarizing 15 years of real-life experience. Ther Adv Chronic Dis. 2018;9(8):147–158. doi:10.1177/2040622318772705
10. Menter A, Warren RB, Langley RG, et al. Efficacy of ixekizumab compared to etanercept and placebo in patients with moderate-to-severe plaque psoriasis and non-pustular palmoplantar involvement: results from three phase 3 trials (UNCOVER-1, UNCOVER-2 and UNCOVER-3). J Eur Acad Dermatol Venereol. 2017;31(10):1686–1692. doi:10.1111/jdv.14237
11. Revicki D, Willian MK, Saurat JH, et al. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2008;158(3):549–557. doi:10.1111/j.1365-2133.2007.08236.x
12. Murage MJ, Gilligan A, Tran O, Brinson J, Goldbum O, Burge R Treatment patterns and healthcare utilization and costs for ixekizumab-treated psoriasis patients.
13. Humira® (adalimumab) injection prescribing information; 2018. Available from: https://www.rxabbvie.com/pdf/humira.pdf.
14. Bonafede M, Johnson BH, Tan DH, Harrison DJ, Stolshek BS. Compliance and cost of biologic therapies for rheumatoid arthritis. Am J Pharm Benefits. 2017;9(5):84–90.
15. Doshi JA, Takeshita J, Pinto L, et al. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US Medicare population. J Am Acad Dermatol. 2016;74(6):1057–1065.e1054. doi:10.1016/j.jaad.2016.01.048
16. Harnett J, Gerber R, Gruben D, Koenig AS, Chen C. Evaluation of real-world experience with tofacitinib compared with adalimumab, etanercept, and abatacept in RA patients with 1 previous biologic DMARD: data from a U.S. administrative claims database. J Manag Care Spec Pharm. 2016;22(12):1457–1471. doi:10.18553/jmcp.2016.22.12.1457
17. Murage MJ, Tongbram V, Feldman SR, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence. 2018;12:1483–1503. doi:10.2147/PPA
18. Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303–2310. doi:10.1185/03007990903126833
19. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–5655. doi:10.1002/sim.v35.30
20. No DJ, Inkeles MS, Amin M, Wu JJ. Drug survival of biologic treatments in psoriasis: a systematic review. J Dermatolog Treat. 2018;29(5):460–466. doi:10.1080/09546634.2017.1398393
21. van den Reek J, Kievit W, Gniadecki R, et al. Drug survival studies in dermatology: principles, purposes, and pitfalls. J Invest Dermatol. 2015;135(7):1–5. doi:10.1038/jid.2015.171
22. Ahn CS, Gustafson CJ, Sandoval LF, Davis SA, Feldman SR. Cost effectiveness of biologic therapies for plaque psoriasis. Am J Clin Dermatol. 2013;14(4):315–326. doi:10.1007/s40257-013-0030-z
23. Bonafede M, Fox KM, Watson C, Princic N, Gandra SR. Treatment patterns in the first year after initiating tumor necrosis factor blockers in real-world settings. Adv Ther. 2012;29(8):664–674. doi:10.1007/s12325-012-0037-5
24. Bonafede M, Johnson BH, Fox KM, Watson C, Gandra SR. Treatment patterns with etanercept and adalimumab for psoriatic diseases in a real-world setting. J Dermatolog Treat. 2013;24(5):369–373. doi:10.3109/09546634.2012.755255
25. Chastek B, Fox KM, Watson C, Kricorian G, Gandra SR. Psoriasis treatment patterns with etanercept and adalimumab in a United States health plan population. J Dermatolog Treat. 2013;24(1):25–33. doi:10.3109/09546634.2012.661038
26. Feldman SR, Zhao Y, Navaratnam P, Friedman HS, Lu J, Tran MH. Patterns of medication utilization and costs associated with the use of etanercept, adalimumab, and ustekinumab in the management of moderate-to-severe psoriasis. J Manag Care Spec Pharm. 2015;21(3):201–209. doi:10.18553/jmcp.2015.21.3.201
27. Gniadecki R, Bang B, Bryld LE, Iversen L, Lasthein S, Skov L. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol. 2015;172(1):244–252. doi:10.1111/bjd.2015.172.issue-1
28. Lunder T, Marko P, Koser Kolar N, Kralj B, Kecelj Leskovec N. Drug survival of biologic therapies for the treatment of psoriasis: results of Slovenian national registry. Biologicals. 2018;54:44–49. doi:10.1016/j.biologicals.2018.04.003
29. Malatestinic W, Nordstrom B, Wu JJ, et al. Characteristics and medication use of psoriasis patients who may or may not qualify for randomized controlled trials. J Manag Care Spec Pharm. 2017;23(3):370–381. doi:10.18553/jmcp.2017.16367
30. Murage MJ, Anderson A, Casso D, et al. Treatment patterns, adherence, and persistence among psoriasis patients treated with biologics in a real-world setting, overall and by disease severity. J Dermatolog Treat. 2019;30:141–149.
31. Seale L, Cardwell LA, Feldman SR. Adherence to biologics in patients with psoriasis. Expert Rev Clin Immunol. 2018;14(2):155–161. doi:10.1080/1744666X.2018.1427065
32. Vilarrasa E, Notario J, Bordas X, Lopez-Ferrer A, Gich IJ, Puig L. ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis): a retrospective observational study on biologic drug survival in daily practice. J Am Acad Dermatol. 2016;74(6):1066–1072. doi:10.1016/j.jaad.2016.01.037
33. Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632–2640. doi:10.1038/jid.2015.208
34. Taltz® (ixekizumab) injection prescribing information. Available from: http://uspl.lilly.com/taltz/taltz.html#pi.
35. Wu JJ, Feldman SR, Rastogi S, Menges B, Lingohr-Smith M, Lin J. Comparison of the cost-effectiveness of biologic drugs used for moderate-to-severe psoriasis treatment in the United States. J Dermatolog Treat. 2018;29:769–774.
36. Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value; August 2018; Available from: https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Final_Evidence_Report_080118.pdf.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

