Back to Journals » International Journal of General Medicine » Volume 15
Comparison of Prognosis and Lymph Node Metastasis in T1-Stage Colonic and Rectal Carcinoma: A Retrospective Study
Authors Deng J, Zhou S, Wang Z, Huang G, Zeng J, Li X
Received 5 January 2022
Accepted for publication 22 March 2022
Published 5 April 2022 Volume 2022:15 Pages 3651—3662
DOI https://doi.org/10.2147/IJGM.S354120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jun Deng, Shifa Zhou, Zhiwen Wang, Genbo Huang, Jingjun Zeng, Xiujiang Li
Department of Emergency Surgery, The First Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China
Correspondence: Xiujiang Li, Department of Emergency Surgery, The First Affiliated Hospital of Nanchang University, Nanchang, 17 Yongwaizheng Street, Nanchang, 330006, Jiangxi, People’s Republic of China, Tel/Fax +86-791-8869-2540, Email [email protected]
Background: Limited evidence and contradictory results have been reported regarding the impact of tumor site on lymph node metastasis (LNM) and prognosis in T1 stage adenocarcinoma (AC). We aimed to compare two anatomic locations in terms of LNM and prognosis using a comprehensive statistical analysis of a large population.
Methods: The Surveillance, Epidemiology, and End Results (SEER) database and our center (First Affiliated Hospital of Nanchang University) were used to extract patient information. Univariate and multivariate logistic or Cox regression and propensity score matching were used to explore the association between LNM/survival and tumor site.
Results: Information for 12,404 patients, including 9655 colonic AC and 2749 rectal AC patients, was extracted from the SEER database. The 516 AC patients included 184 colonic and 332 rectal AC patients from our center. Multivariate logistic regression analysis revealed a correlation between LNM and tumor site (colon vs rectum, odds ratio [OR] =1.52, 95% CI, 1.349– 1.714, P< 0.001). Additionally, we found that younger age, T1b stage, poor differentiation, and lymphatic invasion were risk factors for LNM. After adjusting for confounding factors by PSM, we found that the location of the rectum remained a higher risk factor for LNM. However, we found that patients diagnosed with rectal AC had a prognosis similar to that of patients diagnosed with colonic AC, which was demonstrated by the analysis of SEER data and data from our center.
Conclusion: T1-stage rectal AC may have a higher risk of LNM than colonic AC, while rectal AC has a prognosis similar to that of colonic AC.
Keywords: colorectal adenocarcinoma, anatomic location, SEER, lymph node metastasis, survival
Introduction
As shown by 2020 cancer statistics, globally, colorectal cancer (CRC) ranks second in mortality and third in incidence.1 Fortunately, the rate of mortality and incidence have declined in recent decades.1 More than 90% of CRC is adenocarcinoma, which is derived from epithelial cells of the colorectal mucosa. Based on the World Health Organization (WHO) classification, CRC can be divided into adenocarcinoma (AC) and other rare types of CRC, such as squamous cell carcinoma and spindle cell carcinoma.2 According to the 8th AJCC TNM staging system, CRC is separated into four stages, namely, T1-T4 stages, based on the depth of tumor invasion, and T1 stage tumors are called superficial tumors. With the development and promotion of colonoscopy, the incidence of T1 stage CRC has increased over the past several decades.3 Currently, guidelines recommend that endoscopic resection alone is only suitable for T1 stage CRC without high risks, and additional surgery is necessary for T1 CRC with high risks of lymph node metastasis (LNM).4,5
Based on anatomical location, CRC is divided into right side and left side tumors or colonic and rectal tumors.6,7 The anatomical and physiological structures of the colon and rectum are significantly different. To date, the different clinical features of CRC according to different anatomical locations have received increasing attention from clinicians.6,8 Regarding epidemiological data, rectal cancer accounts for almost one-third of CRC cases, which is due to the rectum being shorter than the colon. However, previous studies have reported that the rectal mucosa has a higher carcinogenic risk than the colonic mucosa.9 In addition, the distribution of intestinal flora, molecular mutations, clinical manifestations, and therapeutic methods are distinct. For instance, metastatic dissemination in rectal cancer involves the lungs and bones more frequently than in colon cancer, whereas colon cancer favors the liver.10 Regarding T1 stage CRC, the difference between colonic and rectal tumors is unclear. Several studies have found that T1 stage rectal cancer is less favorable than colon cancer, which may be related to LNM.11,12 However, many confounding factors in the aforementioned studies make it difficult to conclude that the rectum is a risk factor for LNM. Regarding the difference in survival of patients with colonic or rectal tumors, although recent reports have found a substantial rise in overall survival in recent decades, the 5-year disease-specific survival rate is approximately 59% for colon cancer and 61% for rectal cancer, which accounts for genetic differences, different sensitivities of chemotherapy and different clinical characteristics.13–15 Some studies have suggested that rectal cancer has a better survival than colonic cancer, while other studies have reported that colon cancer has a better 5-year survival rate.13,16,17 Of course, some studies also found that the prognosis of rectal cancer was similar to that of colon cancer.18 However, as for the difference in early-stage CRC survival based on anatomical location, due to no excess mortality and limited samples for early-stage CRC in previous studies, there are few studies to report, and more exploration is needed.
The aim of our study was to assess the difference in LNM and survival between colonic and rectal AC. We performed a comprehensive analysis to determine factors predicting LNM using the SEER database and data from our center. The large population resulting from the two centers provided reliable findings. Moreover, our study allowed for better prognostication and may ultimately influence surgical management for T1 stage CRAC in different locations.
Methods
Patient Selection
All patients with T1 CRAC were retrieved from the SEER database with the National Cancer Institute’s SEER*Stat software (version 8.3.6) and from our center (First Affiliated Hospital of Nanchang University, FAHNU). The patients did not give informed consent because the SEER database is free for public use. All patients underwent surgery and were diagnosed by pathology. According to the International Classification of Diseases in Oncology (ICD-O-3), tumors with codes of 8140, 8144, 8210, 8211, 8213, 8220, 8221, 8262, 8263, 8480, 8481, and 8490 are identified as adenocarcinoma.19 The following criteria were used for patients from the SEER database: (1) patients older than 20 who were diagnosed with T1 stage CRAC by positive histology from 2010 through 2015; (2) patients with concrete location information (C18.0 and C18.1); and (3) patients with detailed information, including race, grade, examined node count, tumor size, N stage, and M stage. In addition, the exclusion criteria were as follows: patients with unknown information about our included clinical features, such as tumor site, T stage and N stage. To extract patients from our center, we selected patients who were diagnosed from January 2010 through December 2018 to collect the clinical characteristics. The inclusion criteria for the included patients were as follows: (1) patients who were diagnosed with T1 stage CRC by pathology and who were over 20 years of age and (2) patients who did not receive preoperative adjuvant therapy. The exclusion criteria included (1) patients without records of T stage, N stage and lymphatic invasion and (2) patients with severe diseases, such as cirrhosis, renal failure and cardiac failure. All our patients were followed up by telephone, and those who were lost to follow-up were excluded when we analyzed the difference in prognosis. All included cases were recorded in the Human Genetic Resources Center of the First Affiliated Hospital of Nanchang University. The research protocol of the Chinese cohort was approved by the Ethics Committee of NCU1h. All the patients provided informed consent.
Clinicopathological Factors
The clinicopathological variables extracted from the SEER database included age, race, sex, pathology grade, LNM, M stage, tumor size, N stage, examined node count, and primary site. Based on the definition of early-onset CRC, we divided patients into the following two age groups: <50 years and ≥50 years. Race was recorded as the following three types: white, black, and other. Sex included male and female. Pathology grade was categorized as well/moderately differentiated type and poorly differentiated/undifferentiated type. LNM was described as N1 (Yes) or N0 (No). M1 (Yes) indicated positive M stage. Tumor size was categorized into two groups as follows: ≤3 cm and >3 cm. With respect to the examined node count, previous studies have indicated that it is better for patients when the number of examined lymph nodes is at least 12.20,21 Therefore, the examined node count was divided into two groups as follows: ≤12 and >12. The primary site was separated into the colon and rectum. In addition, smoking, drinking, lymphatic invasion, and chemotherapy were described as no or yes. Tumor type was recorded as uplift type, ulcerative type, and invasive type. The therapeutic methods included laparoscopy, robot surgery, and normal surgery. In our study, the main observation indicators were LNM status, overall survival (OS), and cancer-specific survival (CSS). CSS was defined as death attributable to AC, while OS included CSS and death attributable to other causes. Detailed information is shown in Tables 1 and 2.
 |
Table 1 Basic Information of Patients with Colorectal Adenocarcinoma in T1 Stage from 2010 Through 2015 in SEER Database |
 |
Table 2 Basic Information of Patients with Colorectal Cancer in T1 Stage from Our Hospital Diagnosed from 2010 Through 2018 |
Study Design
For the basic statistics, patients were divided into two groups, namely, colonic AC and rectal AC, and Pearson’s chi-squared test was utilized to investigate the association among the categorical variables. To explore the potential risk factors for LNM, we performed univariate and multivariate logical regression, and we presented the results as the odds ratio (OR) with the 95% confidence interval (CI). With respect to the OS and CSS of patients with SCC and AC, we generated survival curves using the survminer package in R software. Furthermore, to analyze the related risk factors for survival, we performed multivariate Cox regression.
Regarding the imbalance between the colonic AC and rectal AC groups, we performed propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) to obtain new data for analysis with the MatchIt package in R software. The value of the calliper was set as 0.05, and the effect was evaluated based on the standardized mean difference (SMD) and P value. The effect was balanced when the SMD was less than 0.1 or the P value was more than 0.05. The detailed process is described as follows: 1) we calculated the propensity scores of each patient according to the location (colonic AC and rectal AC) with the multivariate logistic regression model; 2) we matched patients between the two groups at a ratio of 1:1, and the detailed information for all clinical factors is listed in Supplementary Tables 1–3) we analyzed the differences in all variables between the colonic AC and rectal AC groups with the chi-squared test; 4) we explored the correlation between LNM and the location using the univariate logistic regression model and analyzed the relationship between survival and the location using the Cox regression model; and 5) we generated a survival curve plot. The total flow chart is shown in Supplementary Figure 1.
Statistical Analysis
All statistical analyses were performed with R software (version 3.6.1, StataCorp LLC, College Station, Texas). The main packages used in our study included the ggplot2, MatchIt, survival, rms, and survminer packages (https://cran.r-project.org/web/packages). The chi-squared test was performed with SPSS (version 24.0). The results were statistically significant when P < 0.05.
Results
Basic Clinical Information of Patients with Colonic AC and Rectal AC
As shown by the flowchart in Figure 1, we enrolled 12,404 CRAC patients, including 9655 colonic AC and 2749 rectal AC patients. The basic information is shown in Table 1. We found that colonic AC patients were more likely to be female than rectal AC patients (49.38% vs 43.8%). Additionally, the distribution of race was significantly different, and the percentage of white colonic AC patients was lower than that of white rectal AC patients (78.94% vs 80.76%). Significantly, the percentage of younger patients (≤50 years) with rectal AC was more than twice that of younger patients with colonic AC (13.67% vs 6.41%). In addition, colonic AC tended to be more poorly differentiated and was inclined to be smaller tumor size. The number of examined LNs was higher in colonic AC patients than in rectal AC patients (≥12, 69.93% vs 64.31%). The rate of liver metastasis for rectal AC was higher than that for colonic AC (P=0.005); however, there was no significant difference in lung metastasis between the two groups (P=0.479). Regarding the patients in our center, we enrolled 516 patients, including 184 colonic AC and 332 rectal AC patients. The patients from our center are described in Figure 2, and their basic information is listed in Table 2. In our data, the distribution of sex, age, and tumor type in the colonic AC group was similar to that in the rectal AC group (P>0.05). Compared to rectal AC, colonic AC was inclined to be an advanced tumor (T1b, 76.63% vs 64.16%) and poorly differentiated (10.87% vs 6.93%). However, the rate of LNM in rectal cancer was higher than that in colonic AC (12.05% vs 5.95%, P=0.026). Other clinical features, such as lymphatic invasion, metastasis, and tumor size, were not significantly distinct.
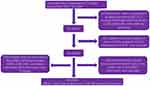 |
Figure 1 Flow chart of patient information extraction from the SEER database. |
 |
Figure 2 Flow chart of patient information extraction from the First Affiliated Hospital of Nanchang University. |
Rectal AC Predicts a Higher Risk of LNM Than Colonic AC
Considering that rectal AC had a larger proportion of cases with LNM than colonic AC, we next investigated whether anatomic location is a relative factor for LNM. Univariate logistic regression analysis showed that rectal location was a risk factor for LNM (OR, 1.635; 95% CI, 1.456–1.836, P≤0.001) (Supplementary Table 1). Additionally, multivariate logistic regression analysis showed that the rectum was an independent risk factor for LNM (Supplementary Table 1). In addition, we found that younger age, poor differentiation, >12 examined lymph nodes and larger tumor size were risk factors for LNM, while white race was a protective factor for LNM (Supplementary Table 1). Regarding the data from our center (Supplementary Table 2), we also found that rectal AC had a higher risk of LNM (OR, 1.56; 95% CI, 0.781–2.569). Moreover, younger age (≤50), poor differentiation and lymphatic invasion were significant risk factors for LNM (Supplementary Table 2). Considering that there were too many confounding factors associated with our observational features in the SEER database, we performed PSM to adjust the imbalance. As shown in Supplementary Table 3, we adjusted the imbalance by matching 2713 colonic AC and 2713 rectal AC patients and found that rectal AC remained a higher risk factor (P<0.001), which was consistent with the data from our center (Supplementary Table 2).
Comparative Survival Rate Between Colonic AC and Rectal AC in T1 Stage
To explore the difference in survival rate between colonic AC and rectal AC, we first performed a K-M survival curve and found that patients with colonic AC in the T1 stage had poorer OS (P=0.0035); however, when we analyzed the difference in CSS, we found that patients with colonic AC had better CSS than those with rectal AC (Figure 3A and B). Due to the confounding factors associated with survival, we matched 2703 colonic AC patients with 2703 rectal AC patients. As shown in Figure 4, the variables after matching were balanced because the value of SMD was less than 0.1, and detailed information on the variables is shown in Supplementary Table 4. The K-M survival curve after PSM demonstrated that anatomic location was not related to survival (Figure 5). In the data from our center, we excluded 218 patients without survival information, including 173 rectal AC and 49 colonic AC patients. In line with that, we also found that colonic AC patients had a comparable survival rate to rectal AC patients from our center (Figure 6). In addition, we found that older age, poor differentiation, large tumor size, positive LNM, and metastasis were independent hazard factors, while female patients with more examined LNs had a better survival rate (P<0.05) (Table 3).
 |
Table 3 Univariate and Multivariate Cox Regression Model for Exploring the Potential Risk Factors for OS in Patients from SEER Database |
 |
Figure 3 Comparison of OS (A) and CSS (B) between T1 stage colonic and rectal AC. |
 |
Figure 4 Standardized mean difference (SMD) across covariates before and after PSM as well as the association between tumor site and survival. |
 |
Figure 5 Comparison of OS (A) and CSS (B) between T1 stage colonic and rectal AC after PSM using the SEER data. |
 |
Figure 6 K-M survival curve analysis comparing T1 stage colonic and rectal AC using the data from our hospital. |
Discussion
With the increase in colorectal endoscopy, the incidence of early colorectal cancer has increased, thereby enhancing the need to understand the rate and risk factors for LNM and survival.22 To date, few studies have focused on this issue. In our study, we focused on patients with pathological T1 stage AC and compared the difference in LNM and survival between colonic AC and rectal AC, demonstrating that rectal AC has a higher rate of LNM and a higher comparative rate of survival than colonic AC. To our knowledge, this is the largest study to date that analyzed the association of anatomic site with LNM and survival, and this study was the first to perform PSM to adjust for confounding factors in populations from two centers, making the results more credible.
Lymph node involvement is considered an important factor for clinical management because lymph node status substantially affects therapeutic methods and prognosis.23 Generally, the rate of LNM for T1 stage CRC is approximately 8%-15%, among which colonic cancers account for approximately 4–12% and rectal tumors account for approximately 12%-18%.22,24–26 For adenocarcinomas, such as mucinous carcinoma, the rate of LNM is higher than that of other types of cancer.27 Regarding the SEER data in our study, we found that the rate of LNM in colonic AC was 11.66%, while the rate of LNM in rectal AC was 17.75%. By comparison, our data from FAHNU showed that the rate was lower for CRAC, with LNM rates of 5.98% for colonic AC and 12.05% for rectal AC. These different results may be attributed to the smaller sample size and heterogeneity of the data from our center. Importantly, both sets of data from different sources demonstrated that rectal AC was a predictive factor for the risk of LNM. The differing LNM risks between the two locations might be due to intrinsic genetic differences and the unique structure. For example, the probability of dMMR/MSI-H is progressively decreased from the right colon (22.3%) and left colon (4.6%) to the rectum (0.7%), and colonic AC has different immune microenvironments.28 Previous studies have also found that left-side CRC or rectal carcinoma has a higher rate of LNM than colonic carcinoma.11,29 Previous studies have also reported that some clinical features, such as tumor size, histological grade, and age, are also different between colonic AC and rectal AC.11,12,26,30 Hence, we performed PSM to reduce the effect of confounding factors. Combining the present data with our previous data indicated that rectal location was an independent risk factor for LNM, which may explain why rectal AC had a higher recurrence rate than colonic AC.11 Additionally, in line with our results, many characteristics may influence LNM, including age, higher T stage, lymphatic invasion, histologic differentiation, tumor size, submucosal depth, and increased number of lymph nodes examined.26,30,31
In general, patients with T1 stage CRC have a good prognosis, and the 5-year OS rate can be up to 85%-90%, which is consistent with our results.32,33 Regardless of colonic AC or rectal AC, the 5-year OS percentages of the patients in the present study were 84.78% (SEER database) and 93% (data from our center), respectively. For the survival analysis according to anatomic location, patients with T1 stage colonic AC have a 5-year survival of 85%-95%, while the 5-year survival rate of rectal AC patients is 80%-90%.34,35 To date, studies have compared the difference in survival between the two types of AC. By collecting patient information from two different centers, our study demonstrated that T1 stage colonic AC patients had a prognosis comparable to that of T1 stage rectal AC patients. Moreover, statistical methods, such as PSM and IPTW, increased the reliability of our results by eliminating the influence of confounding factors.36
In conclusion, according to data from two centers, we found that T1 stage rectal AC patients had a higher risk of LNM than T1 stage colonic AC patients, especially for tumors with poor differentiation and larger tumor sizes (>5 cm). Therefore, patients with T1 stage rectal AC should be more careful when endoscopic treatments, such as ESD and EMR, are performed. More accurate assessment of LNM and R0 resection is imperative for reducing recurrence in rectal T1 stage AC. Furthermore, the comparative prognosis between colonic and rectal AC patients suggests that both patient types require preventive CRC screening and immediate follow-up.
Data Sharing Statement
Our data were extracted from the publicly available SEER database.
Ethics Approval and Consent to Participate
Ethics approval and consent were obtained from the SEER database. Our study was permitted by the Ethics Committee of the First Affiliated Hospital of Nanchang University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors disclose no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi:10.3322/caac.21590
2. Li C, Zheng H, Jia H, Huang D, Gu W. Prognosis of three histological subtypes of colorectal adenocarcinoma: a retrospective analysis of 8005 Chinese patients. Cancer Med. 2019;8:3411–3419. doi:10.1002/cam4.2234
3. Quirke P, Williams GT, Ectors N, Ensari A. The future of the TNM staging system in colorectal cancer: time for a debate? Lancet Oncol. 2007;8:651–657. doi:10.1016/S1470-2045(07)70205-X
4. Watanabe T. Current therapeutic strategies for rectal cancer. Int J Clin Oncol. 2015;20:631–632. doi:10.1007/s10147-015-0878-4
5. Benson AB
6. Salem ME, Weinberg BA, Xiu J, et al. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget. 2017;8:86356–86368. doi:10.18632/oncotarget.21169
7. Meguid RA, Slidell MB, Wolfgang CL. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15:2388–2394. doi:10.1245/s10434-008-0015-y
8. Kamran SC, Clark JW, Zheng H, et al. Primary tumor sidedness is an independent prognostic marker for survival in metastatic colorectal cancer: results from a large retrospective cohort with mutational analysis. Cancer Med. 2018;7:2934–2942. doi:10.1002/cam4.1558
9. Paschke S, Jafarov S, Staib L, et al. Are colon and rectal cancer two different tumor entities? A proposal to abandon the term colorectal cancer. Int J Mol Sci. 2018;19. doi:10.3390/ijms19092577
10. Qiu M, Hu J, Yang D, Cosgrove DP, Xu R. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget. 2015;6:38658–38666. doi:10.18632/oncotarget.6130
11. Chang LC, Shun CT, Lin BR, et al. Recurrence outcomes less favorable in T1 rectal cancer than in T1 colon cancer. Oncologist. 2021;26:e1548–e1554. doi:10.1002/onco.13815
12. Kobayashi H, Mochizuki H, Morita T, et al. Characteristics of recurrence after curative resection for T1 colorectal cancer: Japanese multicenter study. J Gastroenterol. 2011;46:203–211. doi:10.1007/s00535-010-0341-2
13. Buchwald P, Hall C, Davidson C, Dixon L, Dobbs B. Improved survival for rectal cancer compared to colon cancer: the four cohort study. ANZ J Surg. 2018;88:E114–e117. doi:10.1111/ans.13730
14. Hemminki K, Försti A, Hemminki A. Survival in colon and rectal cancers in Finland and Sweden through 50 years. BMJ Open Gastroenterol. 2021;8:e000644. doi:10.1136/bmjgast-2021-000644
15. Phipps AI, Lindor NM, Jenkins MA, Baron JA, Win AK, Gallinger S. Colon and rectal cancer survival by tumor location and microsatellite instability: the Colon Cancer Family Registry. Dis Colon Rectum. 2013;56:937–944. doi:10.1097/DCR.0b013e31828f9a57
16. Aguiar JS, Oliveira MM, Silva D, Mello CAL, Calsavara VF, Curado MP. Survival of patients with colorectal cancer in a cancer center. Arq Gastroenterol. 2020;57:172–177. doi:10.1590/s0004-2803.202000000-32
17. Innos K, Soplepmann J, Suuroja T. Survival for colon and rectal cancer in Estonia: role of staging and treatment. Acta Oncol. 2012;51:521–527. doi:10.3109/0284186X.2011.633928
18. Lee YC, Lee YL, Chuang JP, Lee JC. Differences in survival between colon and rectal cancer from SEER data. PLoS One. 2013;8:e78709. doi:10.1371/journal.pone.0078709
19. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi:10.1111/his.13975
20. Jiang K, Zhu Y, Liu Y, Ye Y, Xie Q. Lymph node ratio as an independent prognostic indicator in stage III colorectal cancer: especially for fewer than 12 lymph nodes examined. Tumour Biol. 2014;35:11685–11690. doi:10.1007/s13277-014-2484-x
21. Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–441. doi:10.1093/jnci/djk092
22. Fields AC, Lu P, Hu F, Hirji S, Irani J, Bleday R. Lymph node positivity in T1/T2 rectal cancer: a word of caution in an era of increased incidence and changing biology for rectal cancer. J Gastrointest Surg. 2021;25:1029–1035. doi:10.1007/s11605-020-04580-z
23. Nakadoi K, Tanaka S, Kanao H, et al. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J Gastroenterol Hepatol. 2012;27:1057–1062. doi:10.1111/j.1440-1746.2011.07041.x
24. Takamaru H, Saito Y, Sekiguchi M, et al. Endoscopic resection before surgery does not affect the recurrence rate in patients with high-risk T1 colorectal cancer. Clin Transl Gastroenterol. 2021;12:e00336.
25. Xu X, Zhang C, Ni X, Wu J, Pan C. Population-based analysis on predictors for lymph node metastasis in T1 colon cancer. Surg Endosc. 2020;34:4030–4040. doi:10.1007/s00464-019-07192-0
26. Okabe S, Shia J, Nash G, et al. Lymph node metastasis in T1 adenocarcinoma of the colon and rectum. J Gastrointest Surg. 2004;8:
27. Hugen N, Brown G, Glynne-Jones R. Advances in the care of patients with mucinous colorectal cancer. Nat Rev Clin Oncol. 2016;13:361–369. doi:10.1038/nrclinonc.2015.140
28. Corrò C, Dutoit V, Koessler T. Emerging trends for radio-immunotherapy in rectal cancer. Cancers. 2021;13:1374. doi:10.3390/cancers13061374
29. Mochizuki K, Kudo SE, Ichimasa K, et al. Left-sided location is a risk factor for lymph node metastasis of T1 colorectal cancer: a single-center retrospective study. Int J Colorectal Dis. 2020;35:1911–1919. doi:10.1007/s00384-020-03668-x
30. Aytac E, Gorgun E, Costedio MM, Stocchi L. Impact of tumor location on lymph node metastasis in T1 colorectal cancer. Langenbecks Arch Surg. 2016;401:627–632. doi:10.1007/s00423-016-1452-x
31. Nascimbeni R, Burgart LJ. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45:200–206. doi:10.1007/s10350-004-6147-7
32. Yamashita K, Oka S, Tanaka S, et al. Preceding endoscopic submucosal dissection for T1 colorectal carcinoma does not affect the prognosis of patients who underwent additional surgery: a large multicenter propensity score-matched analysis. J Gastroenterol. 2019;54:897–906. doi:10.1007/s00535-019-01590-w
33. Tamaru Y, Oka S, Tanaka S, et al. Long-term outcomes after treatment for T1 colorectal carcinoma: a multicenter retrospective cohort study of Hiroshima GI Endoscopy Research Group. J Gastroenterol. 2017;52:1169–1179. doi:10.1007/s00535-017-1318-1
34. Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi64–72. doi:10.1093/annonc/mdt354
35. Morino M, Risio M, Bach S, et al. Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg Endosc. 2015;29:755–773. doi:10.1007/s00464-015-4067-3
36. Tang CT, Chen Y, Zeng C. Prognostic analysis of gastric signet ring cell carcinoma and mucinous carcinoma: a propensity score-matched study and competing risk analysis. Aging. 2020;12:22059–22077. doi:10.18632/aging.104048
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
