Back to Journals » Clinical Ophthalmology » Volume 17
Comparison of Primary Duet Lens Procedures: In-The-Bag Monofocal with Sulcus Multifocal, and Standard Single Multifocal Lens for Cataract Surgery
Authors Harrisberg BP, Chua AW , Chua MJ , Taher A
Received 13 November 2022
Accepted for publication 9 January 2023
Published 19 January 2023 Volume 2023:17 Pages 273—282
DOI https://doi.org/10.2147/OPTH.S396472
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Brian P Harrisberg,1,* Alfred W Chua,2,* Matthew J Chua,3 Amir Taher1
1Department of Ophthalmology, Royal Prince Alfred Hospital, Camperdown, New South Wales, Australia; 2Department of Anesthetics, Royal Prince Alfred Hospital, Camperdown, New South Wales, Australia; 3Department of Anaesthesia and Pain Medicine, Nepean Hospital, Kingswood, New South Wales, Australia
*These authors contributed equally to this work
Correspondence: Brian P Harrisberg, Centre Sydney Eye Surgeons, RPAH Medical Centre, G8/100 Carillon Avenue, Newtown, New South Wales, 2042, Australia, Tel +61 418 201 647, Fax +61 2 9519 3882, Email [email protected]
Purpose: To assess the safety and efficacy of primary duet intraocular lens (IOL) procedure using an in-The-bag monofocal IOL and a sulcus-based multifocal reversible platform for cataract surgery. The visual outcomes were compared with a single in-The-bag multifocal IOL.
Patients and Methods: Retrospective cohort study. Consecutive patients who underwent primary duet IOL procedures were compared with consecutive patients who underwent single multifocal IOL surgery. Primary outcomes were uncorrected distance and near visual acuities (UDVA and UNVA), refraction and spherical equivalent data. Secondary outcomes included surgical complications.
Results: The study group consisted of 32 eyes (22 toric IOLs) whilst the control group had 57 eyes (29 toric IOLs). There were no statistically significant differences between the two groups on post-operative 1-month and 1-year UDVA (p=0.1522 and 0.4926, respectively) and UNVA (p=0.1248 and 0.2738, respectively). There were no statistically significant differences in the postoperative 1-month spherical equivalent within ± 0.5 diopter (p=0.1891). Postoperative intraocular pressure spikes were observed on day-1 in both groups, with most returned to their baseline at 1-month and all were normal at 1-year post surgery. There were no statistically significant differences in intraocular pressure between the two groups on day-1, 1-month and 1-year after surgery (p=0.6421). There were no statistically significant differences in the IOL axis deviation from the intended axis in the toric subgroup analysis (p=0.5843).
Conclusion: Primary duet IOL procedure is equally effective and safe in correcting distance and near vision when compared with single multifocal IOL in the capsular bag.
Keywords: cataract surgery, duet procedure, spectacle independence, 1stQ AddOn
Introduction
Modern cataract surgery facilitates accurate refractive correction and achieves emmetropia (residual refractive error within ± 0.5 diopter) in up to 86% of patients.1–3 In the majority of cases, a capsular-bag-based monofocal intraocular lens (IOL) is implanted to improve distance vision,4 whilst spectacles are required to adjust for near vision. In contrast, multifocal IOLs can generate unaided excellent distant visual acuity as well as enhanced intermediate and/or near vision, enabling spectacle independence.5,6 Globally, approximately 5% of all cataract patients receive multifocal IOLs.4 There is a growing desire for spectacle independence following cataract surgery. Multifocal IOLs play a key role as surgeons shift to optimize spectacle independence and provide better vision.
A major concern with multifocal IOLs is the presence of unwanted light aberrations where external artificial light generates haloes, glare and contrast sensitivity loss.6,7 In rare circumstances, these side effects may become intolerable, leading to an IOL exchange. This is problematic as there are complications associated with IOL exchange procedures, including capsular rupture, vitreous loss and zonular dehiscence.8–10 The primary duet procedure, a capsular-bag-based refractive correcting monofocal IOL with an additional sulcus-based multifocal IOL, offers easy reversibility without interfering with the target distance correction in such circumstances.11,12 The risk of surgical complications from a sulcus explant is significantly reduced when compared with an IOL exchange procedure.4
The concept of having two IOLs in an eye is not new.11 The primary duet procedure (implantation of two IOLs in a single operation) was first described for provision of adequate high IOL power optical correction when there was no single IOL available commercially.13 It has since expanded to include a secondary duet procedure (implantation of a supplementary IOL in a second operation) for indications including secondary correction of pseudophakic refractive errors,14,15 persistent intolerable negative dysphotopsia,16 and improving spectacle independence for previous uncomplicated pseudophakic patients with monofocal lenses.4
This study aims to assess the safety and efficacy of a planned primary duet cataract surgery – a capsular-bag-based monofocal IOL and a sulcus-based trifocal IOL in a reversible platform. Outcomes were compared to traditional cataract surgery with single capsular-bag-based multifocal IOL, of a similar design and optics.
Materials and Methods
This study received ethics approval from the Sydney Local Health District Ethics Review Committee (X21-0126 and 2020/ETH00749). Data were collected in compliance with the National Health and Medical Research Council 2014 recommendations for de-identified Quality Assurance and Evaluation Activities data.17 Consent was not explicitly required as this was a retrospective study using de-identified data.
Patient Selection
We conducted a single-centre retrospective review of consecutive patients undergoing cataract surgery between July 2019 and November 2020. Patients were divided into two groups based on their operation. The first group was made up of patients undergoing primary duet procedure using a capsular-bag-based monofocal IOL (non-toric: Bi-Flex 677PA or toric: Bi-Flex 677TA; Medicontur Medical Engineering Ltd, Zsámbék, Hungary) and a sulcus-based trifocal IOL (1stQ AddOn A4DW0M; Medicontur Medical Engineering Ltd), contrasted to patients who underwent single capsular-bag-based multifocal IOL (Liberty 677MY or Liberty Toric 677MTY; Medicontur Medical Engineering Ltd) insertion. All operations were performed by a single surgeon (BH) eliminating inter-operator variability. A total of 89 eyes from 53 patients were included in this study. The average time interval between surgery of the first and the fellow eye was one week.
Exclusion criteria included pre-existing ocular diseases (eg, congenital eye disease, glaucoma, pigment dispersion syndrome, uveitis, retinal disorders, amblyopia, loose zonules), previous ocular surgery (eg, laser in situ keratomileusis), or significant anatomical abnormalities (eg, shallow anterior chamber depth defined as <2.8mm in phakic eye and pupillary/iris abnormalities).
Primary outcomes measured included uncorrected visual acuity (distance and near) and spherical equivalent (SEQ) data in addition to perioperative and postoperative complications, in particular, intraocular pressure (IOP).
Pre- and Postoperative Examination
Detailed preoperative evaluation included corneal topography, aberrometry (Nidek OPD-Scan III, Nidek Co. Ltd., Aichi, Japan), anterior segment examination (Oculus Pentacam HY Type 70900, Oculus, Wetzlar, Germany), posterior segment examination with a macular optical coherence tomography (Zeiss Cirrus HD-OCT model 5000, Carl Zeiss Meditec, Jena, Germany), and intraocular pressure (IOP) (Goldmann applanation tonometry, Haag-Streit, Essex, United Kingdom). Uncorrected and corrected distance (6m) and near (37cm) visual acuities were tested with a Snellen chart. The IOL power calculation was performed with the IOLMaster 700 machine (Carl Zeiss Meditec, Jena, Germany) using the Barrett TK formula and total keratometry.
Follow-up visits were carried out on day-1, 1-month and 1-year post surgery. Tests performed included general examination under slit lamp, IOP, uncorrected and corrected distance and near visual acuities (UDVA, CDVA, UNVA, CNVA), IOL axis alignment (if applicable), and complications. Additional reviews were scheduled in accordance with necessity.
The 1stQ AddOn IOL Design and Specifications
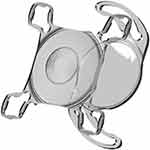
The 1stQ AddOn IOL is a one-piece, hydrophilic acrylic, foldable IOL specifically designed for fixation in the sulcus (Figure 1). It has a square design (to reduce edge light scatter and avoid pupillary capture), 4 soft flexible haptics with zero posterior angulation (to adapt to various sulcus shapes and sizes and to maintain rotational stability), 6mm optic diameter, and an overall diameter of 13mm (to reduce iris capture especially during mydriasis). It has a convex-concave configuration to minimize the risk of interlenticular opacification (ILO) and pigment dispersion.11,18 The AddOn IOL is also available in monofocal and toric designs. Only the trifocal model was considered in this study. The clinical outcomes and safety have been confirmed in other previous studies.4,18,19
According to the manufacturer, the optic design of 1stQ AddOn trifocal IOL is similar to the Liberty trifocal capsular bag IOL, except for fewer diffractive rings (6 rings and 7 rings, respectively).
Surgical Technique for Duet Procedure
All single and duet IOL procedures were performed using sutureless cataract technique and same postoperative management. Pupils were dilated with phenylephrine 10% and cyclopentolate 1% before surgery.
A 2.2mm temporal limbal step incision was made, intracameral lignocaine 2% was administered and the lens was removed using phacoemulsification. The capsular-bag IOL was inserted with an injector (Loadinject 2.2 injector, MDJ SAS, La Monnerie Le Montel, France). The toric IOL was rotated to the intended axis with the assistance of digital alignment system (Callisto eye, Carl Zeiss Meditec, Jena, Germany). The viscoelastic agent was then removed and the corneal wound was enlarged to 2.4mm. Additional viscoelastic agent was administered prior to supplementary IOL insertion into the ciliary sulcus using another injector (Accuject 2.2–1P injector, Medicel, Altenrhein, Switzerland). The correct positioning of all 4 haptics was confirmed before thorough removal of remaining viscoelastic agent. Intracameral acetylcholine and cefuroxime were then administered.
The patients were observed in the recovery room for 60 minutes. All patients that received primary duet procedures underwent slit lamp examination to exclude pupillary capture prior to discharge. Postoperative management included topical chloramphenicol and ketorolac for two weeks, and phenylephrine and prednisolone mixture (Prednefrin Forte, Allergan Australia Pty Ltd, Gordon, New South Wales, Australia) for 3 weeks, commenced immediately following the surgery.
Duration of Procedure
The initial five duet procedures (5/32, 16%) required up to an additional 10 minutes surgical duration when compared with a single IOL procedure. However, the subsequent cases (27/32, 84%) only required an additional 5 minutes of surgical time.
Anaesthetic Technique
The initial three duet procedures (3/32, 9%) were performed under peribulbar block as we anticipated a much longer surgical duration. Subsequent cases of duet procedures (29/32, 91%) and all single IOL procedures (57/57, 100%) were performed under topical anaesthetic with intravenous sedation. Additional intraoperative sedation was not required during all duet procedures.
Statistical Analysis
GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA) was used in the statistical analysis. Descriptive statistics (mean; standard deviation, SD; minimum and maximum values; median; 95% confidence intervals) were calculated for all variables. Comparisons between matching variables on IOP were performed using the two-tailed t-test, Wilcoxon matched-pairs signed rank test and 2-way Analysis of Variance. The Mann–Whitney test and Kolmogorov–Smirnov test were performed on the visual acuity outcomes comparison. Results were presented as mean ± SD. P values of 0.05 or less were considered to be statistically significant in all cases.
Results
Demographic Data
There were 32 eyes of 18 patients (8 males and 10 females) who underwent duet procedure cataract surgery during the study period. Mean age was 63.8 ± 10.2 years. Twenty-two eyes had capsular-bag-based toric IOLs (Bi-Flex 677TA) whilst 10 eyes had non-toric IOLs (Bi-Flex 677PA). All had an additional plano trifocal IOL placed in the sulcus.
There were 57 eyes of 35 patients (14 males and 21 females; mean age was 62.2 ± 10.6 years) who underwent cataract surgery using a single multifocal IOL (non-toric: Liberty 677MY or toric: 677MTY).
In the toric IOL subgroup analysis, there were 22 eyes in the dual IOL group and 29 eyes in the single IOL group. The results of the toric subgroups were not statistically different from that of the non-toric subgroups (data not shown); therefore, we have pooled the toric and non-toric cases together in two study groups (dual and single IOL). The patient demographic data are summarized in Supplemental Table 1.
Visual Outcomes
There were no statistically significant differences at postoperative 1-month and 1-year between the dual IOL and single IOL groups in UDVA (Mann–Whitney test p=0.1522 and 0.4926; Kolmogorov–Smirnov test p=0.6447 and 0.3648, respectively) (Figures 2A and 3A) and UNVA (Mann–Whitney test 0.1248 and 0.2738; Kolmogorov–Smirnov test >0.9999 and 0.9654, respectively) (Figures 2B and 3B). A similar percentage of patients achieved post-operative 1-month spherical equivalent within ± 0.5 diopter [dual IOL 95% (18/19) vs Single IOL 98% (55/56)] (Figure 4) in the two groups and it was not statistically significant (Mann–Whitney test p=0.1891; Kolmogorov–Smirnov test p=0.9981).
The toric IOL axis deviation from the intended axis on post-operation day-1 was similar between dual IOL and single IOL toric subgroups analysis [11/22 (65%) and 13/29 (65%), respectively, had deviation <5 degree from intended axis] and it was not statistically significant (Mann–Whitney test p=0.5843; Kolmogorov–Smirnov test p≤0.9999) (Supplemental Table 2). In some patients, the toric IOL axis was not clearly visualised due to residual pupillary miosis.
Complications
There were no differences in complications associated with primary duet procedures when compared with that of single IOL procedures. There was one case of pupillary capture in the dual IOL group, diagnosed with slit lamp examination prior to discharge, that was corrected under topical anaesthesia with uneventful subsequent recovery.
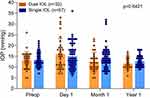
Statistically significant postoperative IOP spikes above their preoperative baseline values were observed in dual IOL (baseline: 13.06±4.08mmHg, day-1: 16.06±7.23mmHg, p=0.0067) and single IOL (baseline: 13.40±3.27mmHg, day-1: 15.08±6.00mmHg, p=0.0067) groups on day-1. One patient had IOP 36mmHg in the single IOL group requiring anterior chamber decompression by paracentesis wound burping at the slit lamp whilst three patients had elevated IOP (30–33mmHg) in the dual IOL group that required no intervention. Their elevated IOPs were monitored conservatively otherwise and they returned to their baseline within the first postoperative month. At 1-month after surgery, three patients in the single IOL groups who had baseline IOP of 15–18mmHg and day-1 post-operation IOP at 16–23 mmHg had an elevated IOP of 30–32 mmHg. The elevated IOPs were thought to be steroid-related, as they promptly normalised after steroid-containing eyedrops were ceased and no further treatment was required. At subsequent additional and 1-year follow-ups, their IOPs were normal. There were no statistically significant differences (p=0.6421) in IOP changes between dual IOL and single IOL groups on postoperative day-1 (16.06±7.23mmHg vs 15.08±6.00mmHg), 1-month (12.37±4.51mmHg vs 14.30±5.42mmHg) and 1-year (11.61± 2.68mmHg vs 12.83± 2.67mmHg) (Figure 5, Supplemental Table 3).
There were no other complications in both groups noted intraoperatively or during the follow-up period of 12 months, including vitreoretinal complications, pupillary block, hyphema, intraocular haemorrhage, glaucoma, corneal decompression, IOL opacification, pigment dispersion syndrome, cystoid macular oedema, endophthalmitis and ILO.
The IOL centration was confirmed by identifying the diffractive rings centred on the pupil. All IOLs were well centred, with no IOL tilt or optic capture observed. There were no differences observed in IOL centration pattern between capsular-bag-based multifocal IOL and sulcus-based multifocal IOL.
At the time of manuscript submission, no patient from the dual IOL group has requested the removal of the sulcus-based IOL.
Discussion
Primary duet procedures, with both IOLs in the capsular bag, were first performed in 1993 for extreme hyperopia resulting in best corrected visual acuity (BCVA) 20/50 in both eyes.13 A later study on primary duet procedures, performed in 15 hypermetropic eyes, reported BCVA 6/12 or better in 100% of eyes that had no pre-existing ocular comorbidities.14 Another study on 20 patients having bilateral surgery at the same time also reported that all patients achieving spectacle independence after 6 months with no adverse events.20 The insertion of a sulcus-based supplementary IOL as a secondary procedure for correcting residual ametropia after cataract surgery and improving near vision for spectacle independence has been reported as effective and safe.5,15,21 In a laboratory study, the optical quality of two IOLs was found comparable to that of a single IOL.22
A prospective randomised study has reported minimal differences in visual outcomes between dual IOL and single IOL groups (all IOLs were Dr Schmidt Intraocularlinsen GmbH, St Augustin, Germany) at one year follow-up: all patients achieved UDVA LogMAR 0.10 or better; 91% of dual IOL and 93% of single IOL achieved near visual acuity LogMAR 0.10 or better.23 An intra-individual study has reported a single IOL providing slightly but statistically better UDVA, CDVA and UNVA when compared with dual IOL (all IOLs were Carl Zeiss Meditec, Jena, Germany).24 In our study, there were no statistically significant differences in the visual outcomes (UDVA and UNVA) between the dual IOL and single IOL groups. Our current study is the first to report these data on Liberty and 1stQ AddOn lenses.
Rotational stability of toric IOL is paramount in visual outcomes. Gundersen et al reported 89% had a toric IOL axis deviation of ≤10 degrees from the intended axis when a supplementary sulcus-based toric IOL was implanted as a secondary procedure in 18 eyes.21 In contrast, all our toric IOLs were implanted in the capsular bag. We examined all toric IOLs for correct axis alignment after AddOn IOL insertion. On some occasions, the toric IOL was accidentally rotated off the intended axis during implantation of the second IOL and it was easily corrected prior to completion of surgery. We found no differences in toric axis deviation from the intended axis between dual IOL and single IOL groups in our study.
A study of secondary duet procedure in pseudophakic patients who wished to become spectacle independent, the supplementary sulcus implants consisted of 27.8% (5/18) plano and 72.2% (13/18) had refractive power.4 In contrast, all the sulcus IOLs were plano in a study of primary duet procedures (100%, 42/42).20 All our sulcus IOLs were plano (100%, 32/32). In patients who are uncertain of their multifocal IOL suitability, the primary duet procedure technique provides an opportunity for the patients to experience multifocal vision. In those who are subsequently intolerant to unwanted side effects, after at least 3 months trial of neuroadaptation, it allows safer and easier removal of the sulcus-based multifocal IOL.11 The risks and difficulties in removing a capsular-bag placed IOL not only impact the ocular structures but also risk not achieving emmetropia with a replacement IOL. It is our belief that leaving the entire correcting power in the capsular bag will be more reliable than removing an in-The-bag multifocal IOL. A concern with a multifocal platform in the sulcus may be the decentration of the diffractive rings. One could expect greater predictability of diffractive ring centration in the bag but there was no evidence of this occurring in our study and we have not been able to identify any differences between the 2 groups.
Persistent postoperative IOP elevation despite topical and systemic therapy, with evidence of pigment dispersion syndrome, was reported in two patients after secondary implantation of a sulcus-based IOL (AcrySof MA60MA), necessitating IOL exchange procedure.25 The authors concluded that the truncated optic edge in that IOL was a poor choice for sulcus-based secondary implantation. No persistent IOP changes have been reported with other sulcus-based secondary IOLs.4,19,21,26 There were 7 patients with an elevated IOP during the early postoperative period in our study (3 patients from the dual IOL group and 4 patients from the single IOL group); however, there were no long-term cases of persistent elevated IOP.
Specific designed sulcus-based IOL has been able to reduce many potential complications associated with placing both IOLs in the capsular bag and later studies have demonstrated the safety of sulcus-based IOL implantation.4,15,21,26–30 Our selection of the 1stQ AddOn IOL as the sulcus-based IOL was determined by its specific designs for sulcus fixation and fewer diffractive rings. The mean distance between the capsular-bag-based IOL (a wide variety of sizes, materials and designs) and the sulcus-based AddOn IOL was 0.68 mm (range: 0.34–1.24 mm) in a study of post mortem pseudophakic human eyes,18 well within the acceptable range. There was no IOL opacification or ILO found in our dual IOL patients during the follow-up period.
Additional peripheral iridotomy for duet procedure has been recommended, especially in hyperopic eyes, by some authors.11 This has been considered but not performed in any of our patients and to date we have not experienced any adverse events such as pupil block glaucoma.
This study has a few limitations. It is a retrospective, single centre study with a relatively small number of subjects. In addition, we have not measured endothelial cell count loss, quality of vision (eg, pupil size, defocus curve and contrast sensitivity), or intermediate visual acuity. However, we followed up the patients for one year, and at the time of manuscript submission, we have not encountered corneal decompensation in any patients. Our study was limited to one experienced surgeon performing all the operations and this might reflect in our lower complications.
Conclusion
Primary duet IOL procedure, although more complex, appeared equally effective and safe in correcting unaided distance and near vision when compared with an in-The bag multifocal lens. The AddOn sulcus-based platform, as a multifocal design, achieved equal functionality to the placement of a single multifocal IOL in the capsular bag.
Data Sharing Statement
Data available from the corresponding author on request due to privacy/ethical reasons.
Ethics Approval Statement
This study received ethics approval from the Sydney Local Health District Ethics Review Committee (X21-0126 and 2020/ETH00749). Data were collected in compliance with the National Health and Medical Research Council 2014 recommendations for de-identified Quality Assurance and Evaluation Activities data. Consent was not explicitly required as this was a retrospective study using de-identified data.
Acknowledgments
The authors wish to thank Ms Thu Nguyen of Central Sydney Eye Surgeons, Sydney, for her assistance in data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; they took part in drafting, revising or critical reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Brian Harrisberg is a consultant for Medicontur Medical Engineering Ltd. The authors declared that there is no other conflict of interest with respect to the research, authorship, and/or publication of this article.
References
1. Rementería-Capelo LA, García-Pérez JL, Gros-Otero J, et al. Visual and refractive outcomes of cataract surgeries performed in one year in a private practice setting: review of 2714 procedures. J Ophthalmol. 2020;2020:2421816. doi:10.1155/2020/2421816
2. Aristodemou P, Knox Cartwright NE, Sparrow JM, et al. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63–71. doi:10.1016/j.jcrs.2010.07.032
3. Behndig A, Montan P, Stenevi U, et al. Aiming for emmetropia after cataract surgery: Swedish National Cataract Register study. J Cataract Refract Surg. 2012;38(7):1181–1186. doi:10.1016/j.jcrs.2012.02.035
4. Palomino-Bautista C, Sánchez-Jean R, Carmona Gonzalez D, et al. Spectacle independence for pseudophakic patients - experience with a trifocal supplementary add-on intraocular lens. Clin Ophthalmol. 2020;14:1043–1054. doi:10.2147/opth.S238553
5. Javitt JC, Wang F, Trentacost DJ, et al. Outcomes of cataract extraction with multifocal intraocular lens implantation: functional status and quality of life. Ophthalmology. 1997;104(4):589–599. doi:10.1016/s0161-6420(97)30265-6
6. Sachdev GS, Sachdev M. Optimizing outcomes with multifocal intraocular lenses. Indian J Ophthalmol. 2017;65(12):1294–1300. doi:10.4103/ijo.IJO_1072_17
7. Alio JL, Plaza-Puche AB, Javaloy J, et al. Comparison of a new refractive multifocal intraocular lens with an inferior segmental near add and a diffractive multifocal intraocular lens. Ophthalmology. 2012;119(3):555–563. doi:10.1016/j.ophtha.2011.08.036
8. Dagres E, Khan MA, Kyle GM, et al. Perioperative complications of intraocular lens exchange in patients with opacified Aqua-Sense lenses. J Cataract Refract Surg. 2004;30(12):2569–2573. doi:10.1016/j.jcrs.2004.04.055
9. Fernández-Buenaga R, Alió JL, Pinilla-Cortés L, et al. Perioperative complications and clinical outcomes of intraocular lens exchange in patients with opacified lenses. Graefe’s Arch Clin Exp Ophthalmol. 2013;251(9):2141–2146. doi:10.1007/s00417-013-2411-7
10. Altaie R, Loane E, O’Sullivan K, et al. Surgical and visual outcomes following exchange of opacified Hydroview intraocular lenses. Br J Ophthalmol. 2007;91:299–302. doi:10.1136/bjo.2006.095414
11. Manzouri B, Dari M-L, Claoué C. Supplementary IOLs: monofocal and multifocal, their applications and limitations. Asia Pac J Ophthalmol. 2017;6:358–363. doi:10.22608/apo.2017110
12. Baur ID, Auffarth GU, Yildirim TM, et al. Reversibility of the duet procedure: bilateral exchange of a supplementary trifocal sulcus-fixated intraocular lens for correction of a postoperative refractive error. Am J Ophthalmol Case Rep. 2020;20:100957. doi:10.1016/j.ajoc.2020.100957
13. Gayton JL, Sanders VN. Implanting two posterior chamber intraocular lenses in a case of microphthalmos. J Cataract Refract Surg. 1993;19(6):776–777. doi:10.1016/s0886-3350(13)80349-5
14. Habot-Wilner Z, Sachs D, Cahane M, et al. Refractive results with secondary piggyback implantation to correct pseudophakic refractive errors. J Cataract Refract Surg. 2005;31(11):2101–2103. doi:10.1016/j.jcrs.2005.05.023
15. Gundersen KG, Potvin R. A review of results after implantation of a secondary intraocular lens to correct residual refractive error after cataract surgery. Clin Ophthalmol. 2017;11:1791–1796. doi:10.2147/opth.S144675
16. Masket S, Fram NR, Cho A, et al. Surgical management of negative dysphotopsia. J Cataract Refract Surg. 2018;44(1):6–16. doi:10.1016/j.jcrs.2017.10.038
17. National Health and Medical Research Council. Statement ethical considerations in quality assurance and evaluation activities (NHMRC); 2014. Available from: https://www.nhmrc.gov.au/about-us/resources/ethical-considerations-quality-assurance-and-evaluation-activities.
18. Reiter N, Werner L, Guan J, et al. Assessment of a new hydrophilic acrylic supplementary IOL for sulcus fixation in pseudophakic cadaver eyes. Eye. 2017;31(5):802–809. doi:10.1038/eye.2016.310
19. Albayrak S, Comba ÖB, Karakaya M. Visual performance and patient satisfaction following the implantation of a novel trifocal supplementary intraocular lens. Eur J Ophthalmol. 2021;31(5):2346–2352. doi:10.1177/1120672120969042
20. Kahraman G, Dragostinoff N, Brezna W, et al. Visual outcomes and patient satisfaction after bilateral sequential implantation of a capsular bag IOL and a Supplementary Sulcus-Fixated Trifocal IOL. J Refract Surg. 2021;37(2):105–111. doi:10.3928/1081597x-20201215-01
21. Gundersen KG, Potvin R. Refractive and Visual Outcomes After Implantation of a Secondary Toric Sulcus Intraocular Lenses. Clin Ophthalmol. 2020;14:1337–1342. doi:10.2147/opth.S255725
22. Łabuz G, Auffarth GU, Knorz MC, et al. Trifocality achieved through polypseudophakia: optical quality and light loss compared with a single trifocal intraocular lens. J Refract Surg. 2020;36(9):570–577. doi:10.3928/1081597x-20200715-01
23. Schrecker J, Feith A, Langenbucher A. Comparison of additional pseudophakic multifocal lenses and multifocal intraocular lens in the capsular bag. Br J Ophthalmol. 2014;98(7):915–919. doi:10.1136/bjophthalmol-2013-304591
24. Muñoz G, Albarrán-Diego C, Belda L, et al. Add-on sulcus-based versus primary in-The-bag multifocal intraocular lens: intraindividual study. J Refract Surg. 2014;30(5):320–325. doi:10.3928/1081597x-20140422-02
25. Iwase T, Tanaka N. Elevated intraocular pressure in secondary piggyback intraocular lens implantation. J Cataract Refract Surg. 2005;31(9):1821–1823. doi:10.1016/j.jcrs.2005.06.034
26. Karjou Z, Jafarinasab MR, Seifi MH, et al. Secondary piggyback intraocular lens for management of residual ametropia after cataract surgery. J Ophthalmic Vis Res. 2021;16:12–20. doi:10.18502/jovr.v16i1.8244
27. Srinivasan S, Scharioth G, Riehl A. Implantation of Scharioth macula lens in patients with age-related macular degeneration: results of a prospective European multicentre clinical trial. BMJ Open Ophthalmol. 2019;4:e000322. doi:10.1136/bmjophth-2019-000322
28. Scharioth GB. New add-on intraocular lens for patients with age-related macular degeneration. J Cataract Refract Surg. 2015;41:1559–1563. doi:10.1016/j.jcrs.2015.07.018
29. Basarir B, Kaya V, Altan C, et al. The use of a supplemental sulcus fixated IOL (HumanOptics Add-On IOL) to correct pseudophakic refractive errors. Eur J Ophthalmol. 2012;22(6):898–903. doi:10.5301/ejo.5000156
30. Gerten G, Kermani O, Schmiedt K, et al. Dual intraocular lens implantation: monofocal lens in the bag and additional diffractive multifocal lens in the sulcus. J Cataract Refract Surg. 2009;35(12):2136–2143. doi:10.1016/j.jcrs.2009.07.014
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.