Back to Journals » Drug Design, Development and Therapy » Volume 14
Comparison of Pharmacokinetics of a Fixed-Dose Combination of Amlodipine/Losartan/Rosuvastatin with Concomitant Administration of Amlodipine/Losartan and Rosuvastatin in Healthy Volunteers
Authors Yoon DY , Park SI , Jung JA , Kim YI, Jang IJ , Chung JY
Received 30 September 2019
Accepted for publication 29 January 2020
Published 19 February 2020 Volume 2020:14 Pages 661—668
DOI https://doi.org/10.2147/DDDT.S233014
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Deok Yong Yoon,1 Sang-In Park,2 Jin-A Jung,3 Yong-Il Kim,3 In-Jin Jang,1 Jae-Yong Chung4
1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Republic of Korea; 2Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; 3Hanmi Pharm. Co., Ltd., Seoul, Republic of Korea; 4Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Bundang Hospital, Seongnam, Republic of Korea
Correspondence: Jae-Yong Chung
Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Bundang Hospital, 82, Gumi-ro, 173 Beon-gil, Bundang-gu, Seongnam-si, Gyeonggi-do 13620, Republic of Korea
Tel +82-31-787-3955
Fax +82-31-787-4045
Email [email protected]
Background: A fixed-dose combination (FDC) tablet formulation of amlodipine/losartan/rosuvastatin 5/100/20 mg was developed to improve medication compliance in patients with both hypertension and dyslipidemia. The comparative pharmacokinetic study was performed to compare the profile of an FDC tablet formulation of amlodipine/losartan/rosuvastatin with that of concomitant administration of a currently marketed FDC tablet of amlodipine/losartan with a rosuvastatin tablet.
Subjects and Methods: A randomized, open-label, single oral dose, two-way crossover study was conducted in 60 healthy subjects. Subjects were orally administered the FDC tablet of amlodipine/losartan/rosuvastatin and a loose combination (LC) of two tablets comprising an FDC of amlodipine/losartan and rosuvastatin. Blood samples were collected for up to 144 h post dose for pharmacokinetic evaluations. Plasma concentrations of amlodipine, losartan, EXP3174 (an active metabolite of losartan), and rosuvastatin were measured by using liquid chromatography-tandem mass spectrometry. The geometric mean ratio (GMR) and its 90% confidence interval (90% CI) in the FDC treatment to LC treatment for the area under the concentration-time curve from zero to the last quantifiable time point (AUClast) and the maximum plasma concentration (Cmax) were calculated. Safety was monitored throughout the study.
Results: The GMR (90% CI) values of AUClast and Cmax were 0.9946 (0.9663– 1.0238) and 0.9690 (0.9379– 1.0011) for amlodipine, 0.9855 (0.9422– 1.0308) and 0.9178 (0.8349– 1.0089) for losartan, 0.9814 (0.9501– 1.0136) and 0.9756 (0.9313– 1.0219) for EXP3174, and 0.9448 (0.8995– 0.9923) and 0.9609 (0.8799– 1.0494) for rosuvastatin, respectively. No clinically significant changes were observed in any of the safety parameters, including clinical laboratory tests, vital signs, electrocardiograms, and physical examinations, between the FDC treatment and the LC treatment.
Conclusion: We confirmed the pharmacokinetic equivalence of the FDC and LC treatments. This triple combination FDC formulation could be a clinically useful replacement for LC therapy.
Keywords: fixed-dose combination, loose combination, bioequivalence, pharmacokinetics, hypertension, dyslipidemia
Introduction
Cardiovascular diseases (CVDs) are the primary cause of mortality worldwide. In 2015, one-third of the global deaths were caused by CVD, and approximately 422 million prevalent cases were reported.1 The two major contributing risk factors of CVD are dyslipidemia and hypertension. According to the European guideline on CVD, cardiovascular mortality could be reduced by managing cholesterol levels and blood pressure.2 The co-existence of dyslipidemia and hypertension has been reported in 15–30% cases.3,4 Moreover, the number of patients with multiple risk factors of CVD has reportedly increased.5
Statin, a β-hydroxy β-methylglutaryl-CoA reductase inhibitor, is the first line of treatment for controlling blood cholesterol. Rosuvastatin is an effective and safe statin, which acts by decreasing low-density lipoprotein (LDL) cholesterol. In a meta-analysis, 5–40 mg of rosuvastatin regimens reduced the percentage of LDL cholesterol from 41.0% to 56.0%.6 For hypertension, calcium channel blockers (CCBs) and angiotensin II receptor blockers (ARBs) are the first-line therapies. The guideline recommends that patients with high blood pressure take two or more different classes of medications to stabilize blood pressure.7 A fixed-dose combination (FDC) formulation of amlodipine and losartan, classified as a CCB and ARB, respectively, showed superior efficacy compared with monotherapy in a Phase II clinical study. This implied that both systolic blood pressure (SBP) and diastolic blood pressure (DBP) were significantly reduced upon FDC administration.8
To manage both blood pressure and cholesterol levels in patients with multiple risk factors of CVD, concomitant administration of statins and antihypertensive drugs is recommended.9 A meta-analysis in patients with CVD reported that use of an FDC contributed to improved medication compliance.10,11 In order to develop a new FDC formulation, a regimen of co-medication of the same active drugs in the same doses should be clinically established. Furthermore, the pharmacokinetic (PK) profiles between the FDC and loose combination (LC) treatments should be compared.12 The triple combination regimen of amlodipine 5 mg, losartan 100 mg, and rosuvastatin 20 mg was clinically effective in patients with both dyslipidemia and hypertension.13 The pharmacokinetic profiles of the FDC and LC treatments of amlodipine and losartan were compared and found to be similar.14 Consequently, to develop a triple FDC for clinical application, the equivalence of PK parameters must be confirmed between the FDC of the three drugs and the case of concomitant LC administration.
The objective of the study was to compare the PK and safety profiles of an FDC tablet of amlodipine/losartan/rosuvastatin 5/100/20 mg with those of the concomitant administration of an FDC tablet of amlodipine/losartan 5/100 mg with a rosuvastatin 20 mg tablet.
Materials and Methods
Subjects and Study Design
The study protocol and informed consent forms were reviewed and approved by the Korean Ministry of Food and Drug Safety (MFDS) and the Institutional Review Board of Seoul National University Bundang Hospital (No. B-1411-275-006). The study was performed in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice. The study was registered with ClinicalTrials.gov (NCT04039724).
Healthy male subjects aged 19 to 45 years with a body mass index between 18 and 27 kg/m2 were eligible to participate in this study. All participants voluntarily signed the informed consent form. Their health was confirmed based on medical history, physical examination, vital signs, 12-lead-electrocardiogram (ECG), and routine clinical laboratory tests. The key exclusion criteria were as follows: glomerular filtration rate (calculated by MDRD equation) < 80 mL/min/1.73m2; aspartate transaminase and alanine transferase > 2 × upper limit of normal; SBP > 150 or < 100 mmHg, DBP > 95 or < 60 mmHg; QTc interval > 450 ms; administered any ethical-the-counter drug within 14 days and any over-the-counter drug within 7 days before the first administration; blood transfusion or donation within 60 days before the first administration; continuous consumption of caffeine (> 1000 mg/day), cigarettes (> 10 cigarettes/day), or alcohol (> 210 g/week); and participation in any other clinical trials within 60 days before the first administration.
This study was designed as a randomized, open-label, single oral dose, two-way crossover trial. A total of 60 subjects were randomly assigned to two sequence groups. The 30 participants assigned to sequence 1 were administered LC treatment, which comprised a concomitant administration of an FDC tablet of amlodipine/losartan 5/100 mg with rosuvastatin 20 mg tablet at the first period, followed by an FDC tablet of amlodipine/losartan/rosuvastatin 5/100/20 mg after 14 days of the wash-out period. The treatment was reversed in the remaining 30 subjects assigned to sequence 2. All treatments were administered with 200 mL of water after an overnight fast for at least 12 h. In each treatment period, blood samples for PK analysis were collected pre-dose and at 0.5, 0.75, 1, 1.25, 1.5, 2, 2.5, 3, 4, 4.5, 5, 6, 7, 8, 10, 12, 22, 48, 72, 96, and 144 h after administration.
Bioanalytical Methods
Blood samples were centrifuged for 10 min at 3000 rpm at 4°C after which the plasma was collected. The plasma was stored at −70°C until required for analysis. For the sampling of rosuvastatin, 0.75 mL of the plasma was aliquoted into a tube containing 0.2 M of sodium acetate buffer (pH 4.0) and mixed before being stored at −70°C.
The plasma concentrations of amlodipine, losartan, EXP3174, and rosuvastatin were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS). For amlodipine, plasma samples (200 μL) along with an internal standard (10 μL of 200 ng/mL amlodipine-d4) were added into a tube with 1.5 mL of methyl tert-butyl ether. After mixing for 2 min and centrifuging for 5 min at 13,000 rpm, the organic layer was harvested and evaporated under a stream of nitrogen. The residue was reconstituted in 150 μL of mobile phase and 10 μL of the sample was injected into the LC-MS/MS system after filtration. LC-MS/MS was carried out using a 4000 QTRAP (AB SCIEX, USA) on a Shiseido Nanospace SI-2 LC system (Shiseido, Japan). The separation was performed with a C18 column (2.1 mm, i.d. ×100 mm, particle size 3 μm) using an acetonitrile/deionized water/formic acid (50/50/0.01, V/V/V) mobile phase at a flow rate of 0.2 mL/min with 2.5 min of analysis time. Electrospray ionization (ESI) was performed in the positive ion mode. Ion detection was performed by monitoring the m/z transitions of 409.2 → 238.2 for amlodipine and 413.2→ 238.2 for amlodipine-d4. For losartan and EXP3174, plasma samples (200 μL) and internal standards (10 μL of 5000 ng/mL losartan-d4 and 2500 ng/mL EXP3174-d4) were added to a tube with 600 μL of acetonitrile. After mixing for 3 min and centrifuging for 5 min at 13,000 rpm, 50 μL of the supernatant was mixed with 150 μL of the mobile phase and 3 μL of the samples were injected into the LC-MS/MS system. LC-MS/MS was carried out using API 4000 (AB SCIEX, USA) in an ACQUITYTM UPLC system (Waters, USA). The separation was performed with a C18 column (2.0 mm, i.d. ×150 mm, particle size 5 μm) using deionized water/acetonitrile/formic acid (40/60/0.1, V/V/V) as a mobile phase at a flow rate of 0.25 mL/min with 3 min of analysis time. ESI was performed in the positive ion mode. The ion was detected by monitoring the m/z transitions of 423.3 → 207.3 for losartan, 427.3 → 211.3 for losartan-d4, 437.2 → 235.2 for EXP3174, and 441.2 → 239.2 for EXP3174-d4. For rosuvastatin, plasma samples (300 μL) were mixed with 0.2 M sodium acetate trihydrate (pH 4.0; 100 μL). The mixed plasma sample (200 μL) and internal standard (20 μL of 35 ng/mL rosuvastatin-d6) were added to a tube with 1.2 mL of methyl tert-butyl ether. After mixing and centrifuging at 3000 rpm for 5 min each, the organic layer was harvested and evaporated under a stream of nitrogen. The residue was reconstituted in 200 μL of mobile phase and centrifuged for 5 min at 13,000 rpm. After centrifugation, 5 μL of the supernatant was harvested and used for LC-MS/MS analysis. LC-MS/MS was performed using an API 5000 (AB SCIEX, USA) on a Shimadzu UFLC system (Shimadzu, Japan). The separation was performed using a C18 column (2.0 mm, i.d. ×75 mm, particle size 3 μm) with acetonitrile/deionized water/formic acid (45/55/0.01, V/V/V) as the mobile phase at a flow rate of 0.2 mL/min with 4 min of analysis time. ESI was performed in the positive ion mode. The ion was detected by monitoring the m/z transitions of 482.1 → 258.1 for rosuvastatin and 488.1 → 264.1 for rosuvastatin-d6.
The plasma drug concentration was quantified by substitution of the peak area ratio into the prepared calibration curve. The calibration curve was prepared using the peak area ratio of the analyte to that of the internal standard. Incurred sample reanalysis was performed to verify the reliability of the developed method.
Pharmacokinetic and Statistical Assessments
PK analysis was performed using data collected from subjects who completed the study with actual sampling times. PK parameters were calculated by means of a non-compartmental method using WinNonlin® version 8.0 (Certara, St. Louis, MO, USA). The primary PK endpoints were maximum plasma concentration (Cmax) and area under the concentration-time curve from time zero to the last quantifiable time point (AUClast). AUClast was calculated using the linear up log down trapezoidal method. Statistical analysis was conducted using the SAS® software version 9.4 (SAS Institute Inc., Cary, NC, USA). Bioequivalence between both treatment groups was assessed using the geometric mean ratio (GMR) and 90% confidence interval (90% CI) of AUClast and Cmax. The conventional bioequivalence criterion of 0.80–1.25 was used.
Safety Assessment
Safety was monitored through adverse events (AEs), physical examinations, vital signs, 12-lead-ECGs, and routine clinical laboratory tests. Information on the AEs, including the number of different AEs, the number of subjects with AEs, severity, seriousness, and causality was collected. Due to the simultaneous administration of two antihypertensive drugs, blood pressure was measured pre-dose and at 4, 8, 22, 48, and 144 h after administration, including the screening and post-study visits. The number, frequency, severity, and seriousness of AEs, along with relation to treatment, were summarized using descriptive statistics.
Results
Subject Characteristics
A total of 61 male subjects were randomly assigned to two sequence groups. The mean ± standard deviation values of demographic characteristics were as follows: age, 26.0 ± 4.1 years; BMI, 23.2 ± 2.0 kg/m2; height, 174.6 ± 5.2 cm; weight, 70.9 ± 7.8 kg. Both sequence groups had similar demographics (Table 1). Of the 61 participants, 60 were administered the treatment and 56 completed the study. The reasons for withdrawal from the study were as follows; one participant withdrew consent before any treatment was administered, two withdrew consent after administration, and two other subjects opted out of the study because of AEs experienced which were oropharyngeal pain with pyrexia and enteritis during the wash-out period.
 |
Table 1 Demographic Characteristics of Study Subjects |
Pharmacokinetics
PK analysis was performed with the 56 subjects who completed the study without major violation. The PK profiles after the drugs were administered as an FDC and LC were comparable. The mean plasma concentration-time profiles of amlodipine, losartan, EXP3174, and rosuvastatin after administration of the FDC and LC are presented in Figure 1. A summary of the PK parameters is shown in Table 2. The GMR (90% CI) of AUClast and Cmax was 0.9946 (0.9663–1.0238) and 0.9690 (0.9379–1.0011) for amlodipine, 0.9855 (0.9422–1.0308) and 0.9178 (0.8349–1.0089) for losartan, 0.9814 (0.9501–1.0136) and 0.9756 (0.9313–1.0219) for EXP3174, and 0.9448 (0.8995–0.9923) and 0.9609 (0.8799–1.0494) for rosuvastatin, respectively. The highest value of intra-individual coefficient variance (CV) was 30.80%, which was observed in the Cmax of losartan (Table 3).
 |
Table 2 Summary of the PK Parameters of Amlodipine, Losartan, an Active Metabolite of Losartan (EXP3174) and Rosuvastatin When Administered as a Fixed-Dose Combination and as a Loose Combination |
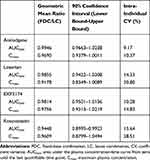 |
Table 3 Comparison of Pharmacokinetic Parameters When Administered as Fixed-Dose Combination or as Loose Combination |
Safety
Safety analysis was conducted on all 60 subjects who were administered the drug at least once. The pattern of change in blood pressure after FDC and LC administration was similar in that both SBP and DBP decreased for 8 h after administration and slowly recovered to the baseline level (Figure 2). A total of 49 AEs were reported in 26 of the 60 subjects, and the number of cases assessed as adverse drug reactions among the AEs was 33 from 21 subjects. All AEs and adverse drug reactions were reported to be mild in their maximum severity. Nervous system disorders such as dizziness and headaches were the most common AEs. There were no clinically significant differences in the safety profile between the two treatments.
Discussion
The aim of the study was to compare the PK and safety profiles of an FDC treatment (administration of an FDC tablet of amlodipine/losartan/rosuvastatin 5/100/20 mg) and an LC treatment (concomitant administration of an FDC tablet of amlodipine/losartan 5/100 mg with a rosuvastatin 20 mg tablet). According to the results obtained, 90% CIs for AUClast and Cmax of amlodipine, losartan, EXP3174, and rosuvastatin were in the range of 0.80–1.25 which was the criteria of bioequivalence between the two treatments. Similar to other comparative studies of FDC formulations of CCBs, ARBs, and statins, GMRs of all primary pharmacokinetic parameters were close to 1.15,16
Approximately 14% of EXP3174, an active metabolite of losartan with a half-life of around 6 to 9 h, is formed by CYP2C9 and CYP3A4, and it is 10- to 40-fold more potent than losartan.17 In addition to the active drugs, the PK profile of EXP3174 was analyzed in this study. Because, it could be assumed that EXP3174 significantly affected the safety and efficacy of the losartan based on pharmacological and PK characteristics of EXP3174. The results of this study indicated that the PK profiles of EXP3174 in both treatments were comparable in common with three active drugs.
This study was well designed and performed because sufficient numbers of participants were enrolled and completed the study. The number of study population which was able to meet the bioequivalence criteria with a significance level of 0.05 and 90% power was statistically calculated based on the previous study in which the highest value of intra-individual CV of PK parameter among the three active drugs and one metabolite was for the Cmax of losartan, which was about 36.5%.16 Of the 56 subjects who completed this study, the highest value of the intra-individual CV was 30.80% (Cmax of losartan). The number of participants enrolled in the study validates the bioequivalence between the two treatments.18 Additionally, the intra-individual CV for Cmax for rosuvastatin was reported as 28.36% in a previous study, which was comparable to the value of 28.51% attained in this study.15
In the Phase III clinical trial of triple combination drug therapy, the efficacy endpoints were the mean changes in SBP and DBP compared to the baseline.13 In this study, intensive observation of blood pressure was conducted and the decrease of the mean SBP and DBP were measured as 10.3 to 12.4 and 12.1 to 14.6 mmHg, respectively. The changes in blood pressure were not as significant in that the changes in the mean SBP and DBP in the phase III study were approximately 16.80 and 9.91 mmHg, respectively.13 The reason for the difference in these results could be potentially because healthy subjects participated in this study, their movement was restricted during hospitalization, and a single oral dose of FDC or LC was administered. Furthermore, the tendency of FDC and LC treatments to lower blood pressure was comparable. In both treatments, SBP and DBP decreased for 8 h after administration and reached the baseline again within 7 days. The changing pattern of blood pressure in this study was similar to that in a previous comparative pharmacokinetic study of an FDC of amlodipine and losartan.14
Amlodipine 5 mg, losartan 100 mg, and rosuvastatin 20 mg along with a combination dosage regimen exhibited clinically significant effectiveness in patients with both dyslipidemia and hypertension.13 In addition to the FDCs studied in this investigation, additional different combinations of these three drugs might be required to adjust and titrate dosages for individual therapy. The pharmacokinetics of amlodipine, losartan, and rosuvastatin are known to be linear and dose-proportional in the range of 5~10 mg, 25~200 mg, and 10~80 mg respectively, and no significant drug-drug interactions were reported (ClinicalTrials.gov: NCT02140489, unpublished data).17,19–21 Therefore, systemic exposure to the drugs in different dosage strengths could be estimated through the results of this study.
Conclusion
In conclusion, this study confirms that there is no significant difference in the PK and safety profiles between the clinical therapeutic doses of an FDC tablet of amlodipine/losartan/rosuvastatin and an LC of amlodipine/losartan and rosuvastatin. Therefore, the triple combination FDC formulation could be a clinically useful replacement for the LC therapy.
Data Sharing Statement
The authors do not intend to share substantial data of this study, but they are ready to share the file of substantial data in excel format and all other study-related documents, at any specific time for any period, if the editorial board requires.
Acknowledgments
Sang-In Park was previously employed at the Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Republic of Korea. Her contribution to this manuscript is based on her prior employment, and the current manuscript does not reflect any position of Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, University of Ulsan College of Medicine. This study was funded by Hanmi Pharm. Co., Ltd., Seoul, Republic of Korea.
Disclosure
Jin-A Jung and Yong-Il Kim are both employees at Hanmi Pharm. Co., Ltd., Seoul, Republic of Korea. All authors declare no other conflicts of interest regarding the publication of this manuscript.
References
1. Roth GA, Johnson C, Abajobir A, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. doi:10.1016/j.jacc.2017.04.052
2. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–2381. doi:10.1093/eurheartj/ehw106
3. Eaton CB, Feldman HA, Assaf AR, et al. Prevalence of hypertension, dyslipidemia, and dyslipidemic hypertension. J Fam Pract. 1994;38(1):17–23.
4. Johnson ML, Pietz K, Battleman DS, Beyth RJ. Prevalence of comorbid hypertension and dyslipidemia and associated cardiovascular disease. Am J Manag Care. 2004;10(12):926–932.
5. Dahlof B. Cardiovascular disease risk factors: epidemiology and risk assessment. Am J Cardiol. 2010;105(1 Suppl):3A–9A. doi:10.1016/j.amjcard.2009.10.007
6. Wlodarczyk J, Sullivan D, Smith M. Comparison of benefits and risks of rosuvastatin versus atorvastatin from a meta-analysis of head-to-head randomized controlled trials. Am J Cardiol. 2008;102(12):1654–1662. doi:10.1016/j.amjcard.2008.08.014
7. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. doi:10.1001/jama.2013.284427
8. Park CG, Youn HJ, Chae SC, et al. Evaluation of the dose-response relationship of amlodipine and losartan combination in patients with essential hypertension: an 8-week, randomized, double-blind, factorial, Phase II, multicenter study. Am J Cardiovasc Drugs. 2012;12(1):35–47. doi:10.2165/11597170-000000000-00000
9. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104.
10. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120(8):713–719. doi:10.1016/j.amjmed.2006.08.033
11. Devabhaktuni M, Bangalore S. Fixed combination of amlodipine and atorvastatin in cardiovascular risk management: patient perspectives. Vasc Health Risk Manag. 2009;5(1):377–387. doi:10.2147/vhrm.s3339
12. World Health Organization Guidelines for registration of fixed-dose combination medicinal products. Available from: http://www.gmp-compliance.org/guidemgr/files/WHO_TRS_929_ANNEX5.PDF.
13. Lee HY, Kim SY, Choi KJ, et al. A randomized, multicenter, double-blind, placebo-controlled study to evaluate the efficacy and the tolerability of a triple combination of amlodipine/losartan/rosuvastatin in patients with comorbid essential hypertension and hyperlipidemia. Clin Ther. 2017;39(12):2366–2379. doi:10.1016/j.clinthera.2017.10.013
14. Choi Y, Lee S, Cho SM, et al. Comparisons of the pharmacokinetics and tolerability of fixed-dose combinations of amlodipine besylate/losartan and amlodipine camsylate/losartan in healthy subjects: a randomized, open-label, single-dose, two-period, two-sequence crossover study. Drug Des Devel Ther. 2016;10:3021–3028. doi:10.2147/DDDT.S113891
15. Oh M, Ghim JL, Park SE, Kim EY, Shin JG. Pharmacokinetic comparison of a fixed-dose combination versus concomitant administration of fimasartan, amlodipine, and rosuvastatin using partial replicated design in healthy adult subjects. Drug Des Devel Ther. 2018;12:1157–1164. doi:10.2147/DDDT.S164215
16. Oh M, Shin JG, Ahn S, et al. Pharmacokinetic comparison of a fixed-dose combination versus concomitant administration of amlodipine, olmesartan, and rosuvastatin in healthy adult subjects. Drug Des Devel Ther. 2019;13:991–997. doi:10.2147/DDDT.S202730
17. Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44(8):797–814. doi:10.2165/00003088-200544080-00003
18. Jia JY, Zhang MQ, Liu YM, et al. Pharmacokinetics and bioequivalence evaluation of two losartan potassium 50-mg tablets: a single-dose, randomized-sequence, open-label, two-way crossover study in healthy Chinese male volunteers. Clin Ther. 2010;32(7):1387–1395. doi:10.1016/j.clinthera.2010.06.018
19. Rohatagi S, Lee J, Shenouda M, et al. Pharmacokinetics of amlodipine and olmesartan after administration of amlodipine besylate and olmesartan medoxomil in separate dosage forms and as a fixed-dose combination. J Clin Pharmacol. 2008;48(11):1309–1322. doi:10.1177/0091270008322176
20. McIntyre M, Caffe SE, Michalak RA, Reid JL. Losartan, an orally active angiotensin (AT1) receptor antagonist: a review of its efficacy and safety in essential hypertension. Pharmacol Ther. 1997;74(2):181–194. doi:10.1016/S0163-7258(97)82002-5
21. Martin PD, Warwick MJ, Dane AL, Cantarini MV. A double-blind, randomized, incomplete crossover trial to assess the dose proportionality of rosuvastatin in healthy volunteers. Clin Ther. 2003;25(8):2215–2224. doi:10.1016/S0149-2918(03)80214-X
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


