Back to Journals » Infection and Drug Resistance » Volume 12
Comparison of pegylated interferon monotherapy and de novo pegylated interferon plus tenofovir combination therapy in patients with chronic hepatitis B
Authors Zheng C, Yan H, Zeng J, Cai S , Wu X
Received 19 November 2018
Accepted for publication 4 March 2019
Published 12 April 2019 Volume 2019:12 Pages 845—854
DOI https://doi.org/10.2147/IDR.S195144
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Caixia Zheng,1,* Honghong Yan,2,* Jianyong Zeng,1,* Shaohang Cai,2,3 Xiaolu Wu1
1Department of Infectious Diseases, First Affiliated Hospital of Xiamen University, Xiamen, Fujian Province, People’s Republic of China; 2Intensive Care Unit, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong Province, People’s Republic of China; 3Department of Infectious Diseases and Hepatology Unit, NanFang Hospital, Southern Medical University, Guangzhou, Guangdong Province, People’s Republic of China
*These authors contributed equally to this work
Background and aim: We aimed to evaluate the effectiveness of pegylated interferon (Peg-IFN) monotherapy (IFN group) and combination therapy with tenofovir (TDF) and Peg-IFN (IFN+TDF group) in chronic hepatitis B (CHB) patients.
Patients and methods: Data of 143 CHB patients were analyzed in this study. All patients enrolled received liver biopsy. Virologic responses were defined as hepatitis B virus (HBV) DNA <100 IU/mL, biochemical responses were defined as normalization of alanine aminotransferse (ALT) levels, and HBeAg serological response was defined as
HBeAg loss or HBeAg seroconversion to HBeAb. HBsAg serological response was defined as HBsAg loss or HBsAg seroconversion to HBsAb.
Results: We observed that a total of 16.7% (11/66) and 33.8% (26/77) patients in IFN and IFN+TDF group achieved complete viral suppression after 48 weeks treatment (P=0.02). Although HBeAg levels in CHB patients in the IFN+TDF group decreased more rapidly during the 48-week treatment, we did not observe significant differences in HBeAg serological loss or seroconversion rates between the two groups at 24 and 48 weeks. HBsAg loss was observed in 13.0% (10/77) of CHB patients in the IFN+TDF group at 48 weeks, compared with only 3% (2/66) patients in the IFN group (P=0.032). No significant difference was observed in HBsAg seroconversion rate between the two groups during 48-week treatment. The biochemical response rate was also significantly higher in the IFN+TDF group than that in the IFN group at week 48 (P=0.015). Multivariate logistic analysis showed that IFN+TDF treatment (OR=4.41, P=0.003), severe baseline hepatic inflammation (OR=4.16, P<0.001), and lower baseline serum HBV DNA levels (OR=0.98, P=0.03) were strong predictors for the virological response. Younger age (OR=0.89, P=0.01), higher baseline ALT level (OR=1.01, P=0.038), and lower baseline HBeAg level (OR=0.99, P=0.008) were independent predictors for HBeAg sero-response after 48 weeks treatment. While only severe liver fibrosis (OR=1.69, P=0.028) and lower baseline HBsAg level (OR=0.22, P=0.005) were independent factors associated with HBsAg sero-response after 48 weeks treatment.
Conclusion: Peg-IFN combined with TDF may increase the virological response rate, biochemical response rate, and HBsAg loss rate in patients with CHB infection. The combination treatment is more suitable for those patients who are likely to respond to the treatment.
Keywords: hepatitis B, virological response, pegylated interferon, tenofovir
Introduction
Chronic hepatitis B virus (HBV) infected approximately 400 million patients worldwide.1 The potential clinical terminal outcomes of chronic HBV infection include cirrhosis, liver failure, and hepatocellular carcinoma (HCC).2 The therapeutic goal in the clinical management of chronic hepatitis B (CHB) infection is to inhibit HBV replication and achieve HBeAg seroconversion in HBeAg-positive patients, so as to prevent end-stage liver diseases, such as cirrhosis and HCC, and prolong the survival of patients with CHB infection.3,4
Currently, interferon and nucleotide analogs (NUCs) are important antiviral agents for the treatment of CHB infection.5,6 Pegylated interferon (Peg-IFN) is recommended as first-line therapy in the interferon treatment of CHB infection. However, due to its low response rate and side effects, a large proportion of CHB patients receiving interferon therapy fail to achieve the desired results.5 Tenofovir (TDF) belongs to NUCs and is recommended as a first-line treatment agent.7 However, TDF treatment requires a very long time, because NUCs cannot clear closed circular covalent DNA (cccDNA).8,9 Premature discontinuation of TDF treatment can lead to poor prognosis, such as relapse or liver failure.8 The ideal drug cessation indicator recommended by the international guidelines is the clearance and seroconversion of hepatitis B surface antigen (HBsAg) in patients with CHB.3,4 However, the effect of TDF monotherapy on HBsAg levels is limited. It takes a very long time, sometimes even decades, for patients with TDF monotherapy to achieve HBsAg serological response.
Recent studies have shown that TDF combined with Peg-IFN can effectively increase the probability of HBsAg serological response.10 However, the results of these studies are still contradictory. In addition, since both TDF and Peg-IFN are expensive agents, the combination of TDF and Peg-IFN treatment in all CHB patients is not cost-effective. How to screen CHB people with higher response possibility when treated with TDF+Peg-IFN and how to improve the therapeutic effect and reduce the risk of long-term end-stage liver diseases have not been reported.
In this study, the effectiveness of Peg-IFN monotherapy and combination therapy with TDF and Peg-IFN in CHB patients was analyzed. The aim of this study was to observe the difference of efficacies between the two treatment strategies and to evaluate factors associated with virological response.
Patients and methods
Subjects
Data of 143 CHB patients were analyzed in this study (Figure S1). The patients were enrolled and treated from June 2015 and were regularly followed-up each 12 weeks. CHB infection was defined as a sero-positive of HBsAg for ≥6 months with persistent or repetitive alanine aminotransferse (ALT) elevation.11 Exclusion criteria include patients were co-infected with hepatitis C or hepatitis D virus or HIV or patients suffered from autoimmune liver disease or heavy alcoholic abuse (>30 g/day).12
 | Figure S1 Flow chart of the study.Abbreviations: CHB, chronic hepatitis B virus; HCV, hepatitis C virus; Peg-IFN, pegylated interferon; TDF, tenofovir. |
Methods
The Institutional Review Board of First Affiliated Hospital, Xiamen University approved the study. All the patients signed a written informed consent before anti-HBV treatment. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All the patients underwent liver biopsy before anti-viral treatment.
ALT levels were examined using the Olympus AU5400 biochemical analyzer. The upper limit of normal (ULN) for ALT was 40 U/L. Levels of HBV serological markers were determined using a commercially available radioimmunoassay (ARCHITECT i2000SR, Abbott Laboratories, 100 Abbott Park Road, Illinois, US) and serum HBV DNA viral load was detected using Daan real-time PCR test (Daan Gene Co, Ltd of Sun Yat-sen University, Guangdong, China, with linear range of 102–108 IU/mL).13
Virologic responses were defined as HBV DNA <100 IU/mL, biochemical responses were defined as normalization of ALT levels (the lower limits and upper limits of normal used of ALT is 4–40 U/L), and HBeAg serological response was defined as HBeAg loss or HBeAg seroconversion to HBeAb.14 HBsAg serological response was defined as HBsAg loss or HBsAg seroconversion to HBsAb.
Liver biopsy was performed with a 16-gauge Menghini biopsy needle. A minimum of 20 mm liver tissue was required for diagnosis.15–17 Liver fibrosis stages were defined according to METAVIR scoring system,18,19 while liver inflammation was staged according to Scheue score system reported by liver pathologists.20,21
Statistical analysis
The measurement units were expressed as mean ± SD for normally distributed data and median (range) for data with non-normal distribution. Categorical data were expressed as percentages. Student’s t-test analysis was used to compare the differences between the two groups. All analyses were performed using SPSS (version 13.0) with an alpha level of 0.05.
Results
Baseline demographics and clinical characteristics
A total of 143 patients were included in the study. Of these, 66 CHB patients received Peg-IFN therapy (Peg-IFN group, average age: 29.6±5.5) and 77 CHB patients received Peg-IFN combined with TDF treatment (Peg-IFN+TDF group, average age: 30.3±7.3). There were 48 males (72.7%) and 55 males (71.4%) in the Peg-IFN group and Peg-IFN+TDF group, respectively. No significant differences in the distributions of baseline demographics and clinical characteristics between the two groups were observed (P>0.05, Table 1).
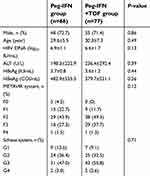 | Table 1 Clinical characteristics of patents enrolled |
Virological response after 48-weeks treatment
The viral quantification curves are shown in Figure 1A. There was no significant difference in baseline HBV DNA load between the two groups. Significant rapid decreases of DNA loads were observed in IFN+TDF groups at week 12 (3.78±2.31 vs 2.47±1.87 log10 IU/mL in IFN group and IFN+TDF group, P<0.001), week 24 (2.43±1.64 vs 1.82±1.56 log10 IU/mL, P=0.024), and week 48 (1.80±1.90 vs 1.22±1.23 log10 IU/mL, P=0.029), respectively.
Virological responses at week 24 and week 48 are shown in Figure 1B. We observed that a total of 10.6% (7/66) and 23.4% (18/77) patients in IFN group and IFN+TDF group achieved complete viral response, respectively (P=0.045). At week 48, 16.7% (11/66) and 33.8% (26/77) patients in IFN and IFN+TDF group achieved complete viral suppression, respectively (P=0.02).
Serological response after 48-week treatment
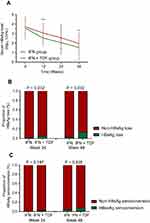
Although the HBeAg levels in CHB patients in the IFN+TDF group decreased more rapidly during the 48-week treatment (Figure 2A), we did not observe significant differences in HBeAg serological loss or seroconversion rates between the two groups at 24 and 48 weeks, as shown in Figure 2B and C.
HBsAg level in the IFN+TDF group was significantly lower than that in the IFN group at 12 weeks, 24 weeks, and 48 weeks (Figure 3A). Furthermore, HBsAg loss was observed in 13.0% (10/77) of CHB patients in the IFN+TDF group at 48 weeks, compared with only 3% (2/66) patients in the IFN group. The difference was statistically significant (P=0.032, Figure 3B). However, no significant difference was observed in HBsAg seroconversion rate between the two groups during 48-week treatment (Figure 3C).
Biochemical response after 48-week treatment
The ALT levels in both groups decreased after treatment. However, there was no difference in ALT levels between the two groups at 12 weeks and 24 weeks. At week 48, ALT level in CHB patients in the IFN+TDF group was significantly lower than that in the IFN group, as shown in Figure 4A. The biochemical response rate was also significantly higher in the IFN+TDF group than that in the IFN group at week 48, as shown in Figure 4B.
Factors associated with virological and serological response
We explored the relationships between baseline clinical characteristics and outcomes after treatment. Multivariate logistic analysis was applied to predict virological response after 48 weeks treatment (Table 2). IFN+TDF treatment (OR=4.41, P=0.003), severe baseline hepatic inflammation (OR=4.16, P<0.001), and lower baseline serum HBV DNA levels (OR=0.98, P=0.03) were strong predictors for the virological response.
 | Table 2 Factors associated with virological response at week 48 |
Multivariate analysis showed that younger age (OR=0.89, P=0.01), higher baseline ALT level (OR=1.01, P=0.038), and lower baseline HBeAg level (OR=0.99, P=0.008) were independent predictors for HBeAg sero-response after a 48-week treatment (Table 3).
 | Table 3 Factors associated with HBeAg sero-response at week 48 |
Multivariate analysis indicated that severe liver fibrosis (OR=1.69, P=0.028) and lower baseline HBsAg level (OR=0.22, P=0.005) were independent factors associated with HBsAg sero-response after 48-week treatment (Table 4).
 | Table 4 Factors associated with HBsAg sero-response at week 48 |
Adverse events during 48-week treatment
According to medical records, patients in both groups experienced varying degrees of adverse events. Among them, the most common are fever, fatigue, and alopecia. However, none of the 143 patients enrolled had terminated treatment due to adverse events. These adverse events were gradually ameliorated with the increase of treatment time.
Discussion
The end point of treatment in patients with CHB infection is to achieve HBV elimination or long-term virological suppression.22 Nucleotide antiviral drugs and interferon are the main treatment options. Numerous guidelines for diagnosis and treatment of HBV infection suggest that interferon can be used as a first-line treatment, whereas Peg-IFN is a novel interferon which has long half-life and long duration of action.1,2 In addition, during the limited course of treatment, there are less virus resistance mutations and lower recurrence rate. TDF is a nucleotide analog antiviral drug, and the therapeutic effect for patients with hepatitis B infection and AIDS has been recognized.23,24 The pharmacological mechanism of TDF is to terminate viral replication by inhibiting the activity of reverse transcriptase and inserting DNA sequences. TDF was first used in the treatment of HIV infections, and later it was found that it could also inhibit the DNA polymerase of HBV which has reverse transcriptase activity and interfere with the replication of HBV. Therefore, it was also used for the treatment of hepatitis B infection. As the drug resistance rate of TDF is low, the major guidelines recommend TDF as the first-line treatment of CHB infection.3,4 Recent studies suggest that Peg-IFN combined with TDF can further improve the response rate of CHB patients.10,25 However, the results of previous studies were not consistent. According to the results of this study, Peg-IFN combined with TDF could increase the virological response rate, biochemical response rate, and HBsAg loss rate in patients with CHB infection. Moreover, baseline liver tissue inflammation and lower viral load are independent factors associated with patients’ virological response. Younger age, higher baseline ALT levels, and lower HBeAg are independent factors associated with HBeAg serological response. Higher degree of baseline liver fibrosis and lower HBsAg levels are independent factors associated with achievement of serological response to HBsAg.
CHB patients require a treatment regimen that is well tolerated with limited treatment duration.26,27 It is well accepted that elimination of HBsAg is an ideal treatment outcome for CHB patients. Although a recent study has found that some patients can maintain a sustained virological response after a long period of treatment with NUCs, these patients are still at a high risk of recurrence.28 Since HBV still persists in infected liver cells, the risk of developing HCC is not completely eliminated.29 Due to the low rate of HBsAg elimination during NUC treatment, life-long treatment is required for most CHB patients if treated with NUC alone. Although IFN regimen has a limited duration of treatment and can effectively reduce the concentration of HBsAg, it has serious side effects and is inefficient. Therefore, not all patients can get a good response to IFN treatment. Recently, more and more clinical trials have attempted to investigate whether combination therapy with IFN and NUCs can increase response rates and provide a treatment option with limited duration of treatment.30–32 The results of this study suggest that IFN+TDF can increase virological response rate and HBsAg serological response rate, especially for those patients with lower baseline viral load, younger age, and higher degree of inflammation. Taking the fact that IFN and TDF are quite expensive agents into consideration, it is not realistic to implement this program for all CHB patients. The practice of this program is more practical for patients who are more likely to respond to the combination treatment.
Previous study suggested that levels of HBsAg, HBeAg, and HBV DNA in CHB patients treated with PEG-IFN plus entecavir (ETV) were significantly lower than those treated with ETV monotherapy.31,33 In addition, this combination therapy seemed to prevent recurrence after termination of ETV usage. However, the difference in HBsAg serological responses is not known. In addition, other studies suggested that patients with limited duration of treatment with PEG-IFN plus TDF have an increased rate of HBsAg loss compared with patients receiving monotherapy.10,30 This study was consistent with these previous studies, and we further found that higher degree of baseline liver fibrosis and lower baseline HBsAg level were favorable factors for achieving the serological response of HBsAg. However, It is a risky treatment for patients with severe liver fibrosis to receive interferon. The side effects of the patient may be more intense and therefore require close observation and careful consideration. At the same time, according to the results of this study, TDF+IFN treatment was a favorable factor for HBsAg serological response in univariate analysis. However, multivariate analysis showed that TDF+IFN was not an independent correlative factor. Therefore, further research is warranted to confirm the above results.
The current study may be biased by the small sample size and the fact that clinical data was collected from only one research center. To further validate the results, a large-scale prospective and multicenter study is warranted. In conclusion, our study reveals that Peg-IFN combined with TDF may increase the virological response rate, biochemical response rate, and HBsAg loss rate in patients with CHB. Moreover, baseline liver tissue inflammation and lower viral load are independent factors associated with virological response. Younger age, higher baseline ALT level, and lower HBeAg level are independent factors associated with HBeAg serological response. Higher degree of baseline liver fibrosis and lower HBsAg level are independent factors associated with achieving serological response to HBsAg.
Abbreviation list
ALT, alanine aminotransferse; CHB, chronic hepatitis B; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; IFN, interferon; LS, liver stiffness; TE, transient elastography; TBIL, total bilirubin, NUC, nucleos(t)ide analogs; ULN, upper limit of normal.
Ethics statements
The Institutional Review Board of First Affiliated Hospital, Xiamen University approved the study. All patients signed a written informed consent before anti-HBV treatment. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Acknowledgment
We wish to thank Hongjie Ou for his helpful assistance in the study.
Disclosure
The authors declare that they have no financial or personal relationships with other people or organizations that could inappropriately influence this work. The authors report no other conflicts of interest in this work.
References
1. Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi:10.1007/s12072-015-9675-4
2. Terrault NA, Bzowej NH, Chang K-M, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–283. doi:10.1002/hep.28156
3. European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi:10.1016/j.jhep.2017.03.021
4. European Association for the Study of the Liver.EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi:10.1016/j.jhep.2012.02.010
5. Cai S, Cao J, Yu T, Xia M, Peng J. Effectiveness of entecavir or telbivudine therapy in patients with chronic hepatitis B virus infection pre-treated with interferon compared with de novo therapy with entecavir and telbivudine. Medicine (Baltimore). 2017;96:e7021. doi:10.1097/MD.0000000000007021
6. Cai S, Yu T, Jiang Y, Zhang Y, Lv F, Peng J. Comparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week result. Clin Exp Med. 2016;16:429–436. doi:10.1007/s10238-015-0373-2
7. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi:10.1002/hep.23190
8. Chotiyaputta W, Peterson C, Ditah FA, Goodwin D, Lok AS. Persistence and adherence to nucleos(t)ide analogue treatment for chronic hepatitis B. J Hepatol. 2011;54:12–18. doi:10.1016/j.jhep.2010.06.016
9. Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39:35–46. doi:10.1111/apt.12538
10. Marcellin P, Ahn SH, Ma X, et al. Combination of tenofovir disoproxil fumarate and peginterferon alpha-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. 2016;150:134–144. doi:10.1053/j.gastro.2015.09.043
11. Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2015;10(1):1–98.
12. European Association for the Study of the Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi:10.1016/j.jhep.2012.04.004
13. Cai SH, Lv FF, Zhang YH, Jiang YG, Peng J. Dynamic comparison between Daan real-time PCR and Cobas TaqMan for quantification of HBV DNA levels in patients with CHB. BMC Infect Dis. 2014;14:85. doi:10.1186/1471-2334-14-85
14. Cai S, Li Z, Yu T, Xia M, Peng J. Serum hepatitis B core antibody levels predict HBeAg seroconversion in chronic hepatitis B patients with high viral load treated with nucleos(t)ide analogs. Infect Drug Resist. 2018;11:469–477. doi:10.2147/IDR.S163038
15. Castera L. Transient elastography and other noninvasive tests to assess hepatic fibrosis in patients with viral hepatitis. J Viral Hepat. 2009;16:300–314. doi:10.1111/j.1365-2893.2009.01087.x
16. Ou H, Cai S, Liu Y, Xia M, Peng J. A noninvasive diagnostic model to assess nonalcoholic hepatic steatosis in patients with chronic hepatitis B. Therap Adv Gastroenterol. 2017;10:207–217. doi:10.1177/1756283X16681707
17. Zeng J, Cai S, Liu J, Xue X, Wu X, Zheng C. Dynamic changes in liver stiffness measured by transient elastography predict clinical outcomes among patients with chronic hepatitis B. J Ultrasound Med. 2017;36:261–268. doi:10.7863/ultra.15.12054
18. Poynard T, Bedossa P. Age and platelet count: a simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study Groups. J Viral Hepat. 1997;4:199–208.
19. Cai S, Ou Z, Liu D, et al. Risk factors associated with liver steatosis and fibrosis in chronic hepatitis B patient with component of metabolic syndrome. United European Gastroenterol J. 2018;6:558–566. doi:10.1177/2050640617751252
20. Hong MZ, Ye L, Jin L-X, et al. Noninvasive scoring system for significant inflammation related to chronic hepatitis B. Sci Rep. 2017;7:43752. doi:10.1038/srep43752
21. Zeng X, Xu C, He D, et al. Influence of hepatic inflammation on FibroScan findings in diagnosing fibrosis in patients with chronic hepatitis B. Ultrasound Med Biol. 2015;41:1538–1544. doi:10.1016/j.ultrasmedbio.2015.01.011
22. Wu X, Cai S, Li Z, et al. Potential effects of telbivudine and entecavir on renal function: a systematic review and meta-analysis. Virol J. 2016;13:64. doi:10.1186/s12985-016-0512-8
23. Piratvisuth T, Komolmit P, Tanwandee T, et al. 52-Week efficacy and safety of telbivudine with conditional tenofovir intensification at week 24 in HBeAg-positive chronic hepatitis B. PLoS One. 2013;8:e54279. doi:10.1371/journal.pone.0054279
24. Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infe cted patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63:77–85. doi:10.1097/QAI.0b013e31828ace69
25. Moreno-Cubero E, Del Arco RTS, Peña-Asensio J, de Villalobos ES, Míquel J, Larrubia JR. Is it possible to stop nucleos(t)ide analogue treatment in chronic hepatitis B patients? World J Gastroenterol. 2018;24:1825–1838. doi:10.3748/wjg.v24.i17.1825
26. Xue X, Cai S. Assessment of liver stiffness in pediatric fontan patients using transient elastography. Can J Gastroenterol Hepatol. 2016;2016:9343960. doi:10.1155/2016/9343960
27. Xue X, Cai S, Ou H, Zheng C, Wu X. Health-related quality of life in patients with chronic hepatitis B during antiviral treatment and off-treatment. Patient Prefer Adherence. 2017;11:85–93. doi:10.2147/PPA.S127139
28. Peng J, Cao J, Yu T, et al. Predictors of sustained virologic response after discontinuation of nucleos(t)ide analog treatment for chronic hepatitis B. Saudi J Gastroenterol. 2015;21:245–253. doi:10.4103/1319-3767.161645
29. Cai SH, Lu SX, Liu LL, Zhang CZ, Yun JP. Increased expression of hepatocyte nuclear factor 4 alpha transcribed by promoter 2 indicates a poor prognosis in hepatocellular carcinoma. Therap Adv Gastroenterol. 2017;10:761–771. doi:10.1177/1756283X17725998
30. Al Ashgar H, Peedikayil MC, Al Quaiz M, et al. HBsAg clearance in chronic hepatitis B patients with add-on pegylated interferon alfa-2a to ongoing tenofovir treatment: a randomized controlled study. Saudi J Gastroenterol. 2017;23:190–198. doi:10.4103/sjg.SJG_541_16
31. Hagiwara S, Nishida N, Watanabe T, et al. Sustained antiviral effects and clearance of hepatitis surface antigen after combination therapy with entecavir and pegylated interferon in chronic hepatitis B. Antivir Ther. 2018;23:513–521. doi:10.3851/IMP3225
32. Jindal A, Vyas AK, Kumar D, Kumar G, Sharma MK, Sarin SK. Higher efficacy of pegylated interferon-alpha2b add-on therapy in hepatitis B envelope antigen-positive chronic hepatitis B patients on tenofovir monotherapy. Hepatol Res. 2018;48:451–458. doi:10.1111/hepr.13049
33. Jun DW, Ahn SB, Kim TY, et al. Efficacy of pegylated interferon monotherapy versus sequential therapy of entecavir and pegylated interferon in hepatitis B e antigen-positive hepatitis B patients: a randomized, multicenter, phase IIIb open-label study (POTENT study). Chin Med J (Engl). 2018;131:1645–1651. doi:10.4103/0366-6999.235880
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.