Back to Journals » Patient Preference and Adherence » Volume 8
Comparison of one-year clinical outcomes between intravascular ultrasound-guided versus angiography-guided implantation of drug-eluting stents for left main lesions: a single-center analysis of a 1,016-patient cohort
Authors Gao X, Kan J , Zhang Y, Zhang J , Tian N, Ye F, Ge Z, Xiao P, Chen F, Mintz G, Chen S
Received 8 April 2014
Accepted for publication 12 June 2014
Published 23 September 2014 Volume 2014:8 Pages 1299—1309
DOI https://doi.org/10.2147/PPA.S65768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Xiao-Fei Gao,1,* Jing Kan,1,* Yao-Jun Zhang,1,2 Jun-Jie Zhang,1 Nai-Liang Tian,1 Fei Ye,1 Zhen Ge,1 Ping-Xi Xiao,1 Feng Chen,3 Gary Mintz,4 Shao-Liang Chen1
1Department of Cardiology, Nanjing First Hospital, Nanjing Medical University, Nanjing, People’s Republic of China; 2Thorax Center, Erasmus Medical Center, Rotterdam, the Netherlands; 3Department of Epidemiology and Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, People’s Republic of China; 4Division of Cardiology, Cardiovascular Research Foundation, Columbia University, New York, NY, USA
*These authors contributed equally contributed to this work
Background: The importance of intravascular ultrasound (IVUS)-guided stenting of the unprotected left main coronary artery (ULMCA) remains controversial and has not been fully studied in the subset of patients with ULMCA. This study evaluated the clinical outcome of IVUS-guided stenting using a drug-eluting stent for ULMCA.
Methods: A total of 1,016 consecutive patients with ULMCA stenosis who underwent drug-eluting stent implantation from January 2006 to December 2011 were prospectively registered. The primary endpoint of this nonrandomized registry was the rate of one-year major adverse cardiac events (MACE, including cardiac death, myocardial infarction, and target vessel revascularization). Stent thrombosis served as the safety endpoint. Propensity score matching was used to calculate the adjusted event rate.
Results: The unadjusted one-year MACE rate was 14.8% in the IVUS-guided group (n=337, 33.2%), significantly different from the 27.7% (P<0.001) in the angiography-guided group (n=679, 66.8%). After propensity score matching, 291 paired patients were matched between the two groups, and the difference in one-year MACE between IVUS-guided (16.2%) versus angiography-guided (24.4%) groups was still significant (P=0.014), mainly driven by decreased rates of cardiac death (1.7%) and target vessel revascularization (3.4%) in the IVUS-guided group when compared with 5.2% (P=0.023) and 10.0% (P=0.002) in the angiography-guided group, respectively. Although it did not reach significance (P=0.075), the adjusted one-year rate of stent thrombosis in the angiography-guided group was higher than in the IVUS-guided group.
Conclusion: Compared with angiography guidance, IVUS-guided treatment of ULMCA using a drug-eluting stent was associated with a significant reduction of one-year cardiac death and target vessel revascularization, resulting in less frequent one-year MACE after propensity score matching.
Keywords: unprotected left main, intravascular ultrasound, major adverse cardiac events
Introduction
In the modern drug-eluting stent (DES) era, percutaneous coronary intervention of unprotected left main coronary artery (ULMCA) stenosis has been increasing rapidly.1 Percutaneous coronary intervention remains a class IIa2 or IIb3 recommendation in current practice guidelines because of its higher rates of target vessel revascularization (TVR) in distal ULMCA bifurcation lesions.4,5 Intravascular ultrasound (IVUS) overcomes many of the limitations of angiography by providing more accurate quantitative information about vessel size, lesion length, and lesion sites.6–8 Previous studies have reported a reduction of unadjusted rates of cardiac death, myocardial infarction, stent thrombosis, and instent restenosis after placement of a DES in the left main artery when guided by IVUS.9,10 This reduction was consistently noted in a recent meta-analysis by Zhang et al11 when overall coronary artery lesions were included. Nonetheless, there is still a lack of definitive data regarding the importance of IVUS-guided DES implantation for a diseased left main vessel.12 Accordingly, this prospective registry is designed to address the clinical benefits of IVUS-guided stenting of ULMCA stenosis.
Materials and methods
Study design and patient population
From January 2006 to December 2011, a total of 1,016 consecutive real-world patients with ULMCA lesions (defined as diameter stenosis ≥50% by visual estimation) treated with DES implantation at our center were prospectively enrolled into this nonrandomized, open-label, single-center registry. Six of the experienced primary operators involved in this research routinely performed IVUS. For the purposes of this study, IVUS was performed at the discretion of the operators who agreed on the definitions of optimal angiographic and IVUS criteria. However, IVUS was also required if the operator needed to know the reference vessel diameter, expanding status of stent struts, instent haziness, strut fracture, or edge dissection. Patients included in the study were divided into an IVUS-guided group and a conventional angiography-guided group. The procedure was considered IVUS-guided when optimal stent implantation was achieved after IVUS assessment or post-dilation was performed after suboptimal stent placement. Patients were included in the angiography-guided group if they had stent implantation by angiography or IVUS defined suboptimal stent placement without further post-dilation (failed to achieve optimal stent implantation successfully or not thought to influence clinical outcomes based on the operator’s decision). The clinical outcomes and independent outcome predictors between these two groups were compared.
Both interventionists and surgeons agreed on the treatments of percutaneous coronary intervention. The study was approved by the ethics committee and written informed consent was obtained from all patients prior to inclusion in the study.
Procedures and periprocedural medications
All interventional procedures were performed in accordance with current standards. Use of glycoprotein IIb/IIIa inhibitors, low molecular weight heparin, type of DES, predilation, and intra-aortic balloon pump were at the operator’s discretion. A 300 mg loading dose of clopidogrel was administered before the index procedure. Post-dilation using a noncompliant balloon (1.0:1.0 ratio of balloon/stent) was recommended in both groups and upsized as necessary in patients with suboptimal expansion or stent malapposition, as shown by angiography or IVUS. IVUS was performed only if patients were not at risk of circulatory collapse. Post-procedural IVUS was recommended to further evaluate the quality of stenting and was left to the operator’s discretion. IVUS images were obtained using a commercially available imaging system with a 40 MHz mechanical transducer (Boston Scientific Corporation, Natick, MA, USA). IVUS-defined optimal results were TIMI (Thrombolysis In Myocardial Infarction) flow grade 3, minimum stent lumen cross-sectional area >6.9 mm2, full apposition of stent, and no major dissection.10,13 Angiographic success was defined as TIMI grade 3 and residual stenosis <10%. After the intervention, all patients received aspirin 100 mg/day for life and clopidogrel 75 mg/day for at least 12 months.
Study endpoints and definitions
The primary endpoint was the one-year rate of major adverse cardiac events (MACE), defined as cardiac death, myocardial infarction, and TVR. The safety endpoint was the occurrence of stent thrombosis. All deaths were considered cardiac in origin unless a noncardiac cause was confirmed clinically or at autopsy. Myocardial infarction was diagnosed in accordance with Third Universal Definition of Myocardial Infarction.14 Target lesion revascularization and TVR were defined as repeat revascularization (including percutaneous coronary intervention and coronary artery bypass grafting) for target lesions and target vessels, respectively, in the presence of symptoms or objective signs of ischemia. Stent thrombosis was then classified by the Academic Research Consortium definition as definite, probable, or possible, and as early (0–30 days post stent implantation), late (31–360 days), or very late (>360 days).15 The definition of definite stent thrombosis describes symptoms suggestive of an acute coronary syndrome and angiographic or pathological confirmation of stent thrombosis. Probable stent thrombosis included unexplained death within 30 days or target vessel myocardial infarction without angiographic confirmation of stent thrombosis. Possible stent thrombosis included any unexplained death after 30 days. Lesion specificities were defined according to American Heart Association/American College of Cardiology criteria.16 The New Risk Stratification (NERS) and Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery scores (SYNTAX) were prognostication before stenting of unprotected left main stenosis.17,18
Clinical follow-up
Clinical follow-up was performed either by telephone or through a clinical office visit. Telephone interviews were conducted at 1, 6, 9, and 12 months. Repeat coronary angiography was scheduled at 12 months after the index procedure, or earlier if clinically indicated. An independent committee that was blinded to the study assessed all clinical events.
Statistical analysis
The Kolmogorov–Smirnov test was used to assess the distribution of continuous variables. Continuous variables were expressed as the mean ± standard deviation or median and were compared using the Student’s t-test (for normal data) and Mann–Whitney U-test (for non-normally distributed variables) as appropriate. Categorical variables were presented as frequencies or percentages and compared using chi-square statistics or Fisher’s Exact test. Survival curves were generated by the Kaplan–Meier method and compared using the log-rank test. A propensity score analysis was performed to minimize any selection bias due to differences in baseline characteristics between the two treatment groups.19 Variables included in the logistic regression model to calculate the propensity score were age, sex, hypertension, diabetes, hyperlipidemia, smoking history, serum creatinine, unstable angina, acute myocardial infarction, chronic renal insufficiency, peripheral arterial disease, left ventricular ejection fraction, previous bypass surgery, previous percutaneous intervention, multivessel disease, use of glycoprotein IIb/IIIa receptor inhibitors, lesion location, lesion tortuosity, calcification or thrombus, restenotic lesion, chronic total occlusion, a transfemoral or transradial approach, and incomplete revascularization. Model discrimination was assessed with the C-statistic and model calibration with the Hosmer–Lemeshow test. The new propensity score was then incorporated into Cox proportional hazards regression models as a covariate to assess the efficacy of IVUS guidance versus angiography guidance. In addition, to reduce the effect of treatment selection bias and potential confounding in this observational study, we performed rigorous adjustment for significant differences in the baseline characteristics of patients with propensity score matching using the following algorithm: 1:1 optimal match with a ±0.03 caliper and no replacement. Multivariable Cox proportional hazards regression modeling was performed to determine independent predictors of the primary endpoint with purposeful selection of covariates. Variables associated at univariate analysis (all with a P-value ≤0.1) and those judged to be of clinical importance from previously published reports were eligible for inclusion into the multivariable model-building process. The goodness of fit of the Cox multivariable model was assessed with the Grønnesby–Borgan–May test. The results are reported as hazard ratios with associated 95% confidence intervals and P-values. All statistical analyses were performed with Stata version 12.0 (Stata Corporation, College Station, TX, USA).
Results
Of 1,016 patients with ULMCA lesions, 463 (43.4%) were in the group guided by IVUS, and 553 (54.4%) were in the group guided by angiography. In the IVUS-guided group, IVUS-defined optimal results were initially achieved in 232 patients (50.0%). Of 231 patients who initially attained suboptimal results, post-dilation was performed in 105 who were therefore included in the IVUS-guided group; the remaining 126 patients who did not receive post-dilation were included in the angiography-guided group. Thus, there were 337 patients (33.2%) in the IVUS-guided group and 679 (66.8%) in the angiography-guided group who were included in the final analysis (Figure 1).
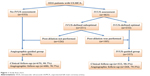  | Figure 1 Study flow chart. |
Baseline clinical characteristics
Baseline clinical characteristics are shown in Table 1. Patients in the angiography-guided group had a lower estimated glomerular filtration rate (69.2±21.6 mL/min/1.73 m2), lower left ventricular ejection fraction (56.7%±11.7%), and more frequent ST-segment elevation myocardial infarction (11.5%) when compared with the IVUS-guided group (73.3±22.3 mL/min/1.73 m2, P=0.005; 58.7%±10.1%, P=0.011; and 7.1%, P=0.029, respectively).
Lesions and procedural characteristics
Table 2 shows that patients in the angiography-guided group had more frequent downstream lesions in the left circumflex and right coronary artery, and more chronic total occlusion lesions, with more multivessel disease (57.9%) when compared with the IVUS-guided group (48.4%, P=0.004). As a result, the angiography-guided group had a higher risk score stratified by either the Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery score or New Risk Stratification method.
As reflected by the difference in lesion complexity between the two groups, the transradial approach was used less frequently in the angiography-guided group (50.2%) than in the IVUS-guided group (62.9%, P<0.001). Notably, a larger stent (3.5±0.4 mm) and noncompliant balloon (3.8±0.5 mm) for post-dilation were required in the IVUS-guided group, significantly different to those in the angiography-guided group (3.4±0.4 mm and 3.6±0.4 mm, P<0.001 and P<0.001, respectively, Table 3). Finally, there were lower rates of complete revascularization (58.3%) and angiographic success (93.5%) in the angiography-guided group when compared with 74.8% (P<0.001) and 99.7% (P<0.001) in the IVUS-guided group, respectively.
Unadjusted clinical outcomes
Clinical follow-up was available in approximately 99% of patients. Angiographic follow-up was conducted in 79.5% of patients in the IVUS-guided group and in 70.7% of those in the angiography-guided group. Unadjusted clinical outcomes are summarized in Table 4. At one-year follow-up, the incidence of cardiac death, myocardial infarction, and TVR in the IVUS-guided group was 1.8%, 11.3%, and 3.3%, respectively, which was significantly less than the 6.2% (P=0.002), 17.2% (P=0.013), and 11.8% (P<0.001) in the angiography-guided group, resulting in less frequent composite MACE in the IVUS-guided group (14.8% versus 27.7%, P<0.001). The occurrence of stent thrombosis was 2.7% in the angiography-guided group, and higher than in the IVUS-guided group (0.6%, P=0.026).
Propensity score matching
The propensity score was calculated, and indicated good predictive value (C-statistic 0.78) and calibration characteristics (Hosmer–Lemeshow statistic 9.64, P=0.29). After propensity score matching, 291 pairs of patients were matched (Supplementary material, Table S1), and there was a significant difference in composite MACE between the IVUS-guided group (16.2%) and the angiography-guided group (24.4%, P=0.013, Table 5, Figure 2), mainly driven by increased cardiac death (5.2% versus 1.7%, P=0.023) and TVR (10.0% versus 3.4%, P=0.014) in the latter group. By Cox regression multivariable analysis, the only independent predictor of MACE was IVUS guidance (hazard ratio 0.66, 95% confidence interval 0.46–0.96, P=0.024).
Discussion
The major finding of this study was that IVUS-guided stenting for ULMCA lesions was associated with dramatic reductions in both the unadjusted and adjusted one-year rate of composite MACE, mainly due to a significant reduction of cardiac death and TVR.
ULMCA stenosis is characterized by frequent distal bifurcation involvement, larger parent and daughter vessel diameters, and wider distal bifurcation angle.2,3,12 It is still unclear whether clinical outcomes of ULMCA intervention using DES could be improved by IVUS guidance. A meta-analysis by Zhang et al demonstrated that the IVUS-guided DES implantation is associated with significant reductions in death, MACE, and stent thrombosis when compared with angiographic guidance.11 Also, data from the Efficacy of Xience/promus versus Cypher in rEducing Late Loss after stENTing (EXCELLENT) trial (ClinicalTrials.gov identifier NCT00698607) showed that IVUS-guided stenting for non-left main lesions had higher release of periprocedural myocardial biomarkers, reflecting the more aggressive procedures performed with IVUS guidance.20 Furthermore, a subgroup analysis from the MAIN-COMPARE (Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty Versus Surgical Revascularization) study showed that 3-year mortality was significantly lower in the IVUS-guided group than in the angiography-guided group.12 It should be noted that the risks of myocardial infarction and TVR were not influenced by IVUS guidance in that study. Overall, the current data regarding the importance of IVUS-guided stenting of ULMCA lesions is insufficient to provide clinical advantages.
In our study, patients in the angiography-guided group had more frequent comorbidities and more complex lesions, ie, more downstream lesions, chronic total occlusion lesions, ST-segment elevation myocardial infarction, renal insufficiency/impairment, left ventricular dysfunction, and higher risk scores.16,17,21 These factors, which make this subset of patients more high risk, may have influenced the primary operators against the use of IVUS. The unadjusted difference in either composite MACE or individual endpoints between these two groups may be undermined by the discrepancies in baseline characteristics of the angiography-guided group. Nevertheless, after propensity score matching, the difference in composite MACE between the two groups was sustained and the results were still favorable towards the use of IVUS guidance. Notably, the wider unadjusted range of myocardial infarction between these two groups became narrower after propensity score matching; cardiac death and TVR were still commonly seen in the angiography-guided group. Possible reasons for the favorable results using IVUS guidance include its more accurate quantification of stent diameter, less late loss, and fewer requirements for revascularization. Moreover, the stent thrombosis rate in the angiography-guided group was eight times higher when compared with the IVUS-guided group, implying that the difference in stent thrombosis rate would be significant if the sample size was expanded further, a postulation confirmed by our previous study of patients with coronary bifurcation lesions.9 All these results strongly support IVUS guidance as being the only independent factor of MACE by multivariate analysis.
Park et al12 reported significant reduction of mortality when guided by IVUS in the MAIN-COMPARE study; this was in line with our data. In contrast with our findings, they did not identify a decrease in the rate of TVR in the IVUS-guided group. This difference might be due to the different definitions of IVUS guidance used in these two studies. In the MAIN-COMPARE registry, the procedure was considered IVUS-guided when IVUS assessment was performed to evaluate stenting status, a definition that included patients with suboptimal results but without further interventions. In contrast, patients in our study were considered angiography-guided if further intervention was not performed after IVUS assessment. We believe our definition of IVUS-guided DES implantation reflected the real grouping of IVUS guidance versus non-IVUS guidance. Furthermore, the differences in techniques and types of DES used as well as duration of follow-up may be other factors contributing to the discrepancy in clinical results between these studies, despite the fact that both demonstrated an overall significant reduction in mortality by IVUS guidance.
Study limitations
The current study has several limitations. It is underpowered because it was an open-label, nonrandomized registry consisting of a small cohort of patients. Use of a larger patient population and propensity score matching would overcome these problems. Second, quantitative IVUS and angiographic analysis were not performed. Third long-term follow-up after DES implantation was not done. Extended follow-up may be critical to assess the long-term clinical benefit of IVUS-guided ULMCA stenting. Finally, although the distal segment of the left main is commonly involved, we did not perform a subgroup analysis to elucidate the importance of IVUS guidance for distal left main lesions in this cohort of all left main lesions.
Conclusion
Our registry demonstrates that, after propensity score matching, IVUS-guided ULMCA stenting was associated with reduced one-year MACE compared with angiography-guided stenting, mainly driven by a decrease in cardiac death and TVR. However, a randomized study with a larger patient sample size is needed to further address the real advantages of IVUS over angiography guidance in this patient and lesion subset.
Acknowledgment
The study was supported by the Jiangsu Provincial Special Program of Medical Science (BL2013001).
Disclosure
The authors report no conflicts of interest in this work.
References
Capodanno D, Stone GW, Morice MC, et al. Percutaneous coronary intervention versus coronary artery bypass graft surgery in left main coronary artery disease: a meta-analysis of randomized clinical data. J Am Coll Cardiol. 2011;58:1426–1432. | ||
Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2010;31: 2501–2555. | ||
Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Catheter Cardiovasc Interv. 2009;74:E25–E68. | ||
Palmerini T, Sangiorgi D, Marzocchi A, et al. Ostial and midshaft lesions versus bifurcation lesions in 1111 patients with unprotected left main coronary artery stenosis treated with drug-eluting stents: results of the survey from the Italian Society of Invasive Cardiology. Eur Heart J. 2009;30:2087–2094. | ||
Chen SL, Zhang Y, Xu B, et al. Five-year clinical follow-up of unprotected left main bifurcation lesion stenting: one-stent versus two-stent versus double kissing crush techniques. EuroIntervention. 2012;8:803–814. | ||
Mintz GS. Features and parameters of drug-eluting stent deployment discoverable by intravascular ultrasound. Am J Cardiol. 2007; 100:26M–35M. | ||
Claessen BE, Mehran R, Mintz GS, et al. Impact of intravascular ultrasound imaging on early and late clinical outcomes following percutaneous coronary intervention with drug-eluting stents. JACC Cardiovasc Interv. 2011;4:974–981. | ||
Roy P, Steinberg DH, Sushinsky SJ, et al. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. Eur Heart J. 2008;29: 1851–1857. | ||
Chen SL, Ye F, Zhang JJ, et al. Intravascular ultrasound-guided systematic two-stent techniques for coronary bifurcation lesions and reduced late stent thrombosis. Catheter Cardiovasc Interv. 2013; 81:456–463. | ||
Hong MK, Mintz GS, Lee CW, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305–1310. | ||
Zhang Y, Farooq V, Garcia-Garcia HM, et al. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012; 8:855–865. | ||
Park SJ, Kim YH, Park DW, et al. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv. 2009;2:167–177. | ||
Choi SY, Maehara A, Cristea E, et al. Usefulness of minimum stent cross sectional area as a predictor of angiographic restenosis after primary percutaneous coronary intervention in acute myocardial infarction (from the HORIZONS-AMI Trial IVUS substudy). Am J Cardiol. 2012; 109:455–460. | ||
Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. | ||
Mauri L, Hsieh WH, Massaro JM, et al. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007; 356:1020–1029. | ||
Ryan TJ, Bauman WB, Kennedy JW, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American Heart Association/American College of Cardiology Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Committee on Percutaneous Transluminal Coronary Angioplasty). Circulation. 1993;88:2987–3007. | ||
Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. | ||
Chen SL, Chen JP, Mintz G, et al. Comparison between the NERS (New Risk Stratification) score and the SYNTAX (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) score in outcome prediction for unprotected left main stenting. JACC Cardiovasc Interv. 2010;3:632–641. | ||
D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. | ||
Park KW, Kang SH, Yang HM, et al. Impact of intravascular ultrasound guidance in routine percutaneous coronary intervention for conventional lesions: data from the EXCELLENT trial. Int J Cardiol. 2013; 167:721–726. | ||
Farooq V, Serruys PW, Garcia-Garcia HM, et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions in the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) Trial. J Am Coll Cardiol. 2013;61:282–294. |
Supplementary material
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







