Back to Journals » Nature and Science of Sleep » Volume 12
Comparison of Morning and Evening Operation Under General Anesthesia on Intraoperative Anesthetic Requirement, Postoperative Sleep Quality, and Pain: A Randomized Controlled Trial
Authors Song B, Li Y, Teng X, Li X, Yang Y, Zhu J
Received 12 April 2020
Accepted for publication 24 June 2020
Published 16 July 2020 Volume 2020:12 Pages 467—475
DOI https://doi.org/10.2147/NSS.S257896
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Steven A Shea
Bijia Song, Yang Li, Xiufei Teng, Xiuyan Li, Yanchao Yang, Junchao Zhu
Department of Anesthesiology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, People’s Republic of China
Correspondence: Junchao Zhu
Department of Anesthesiology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, People’s Republic of China
Email [email protected]
Objective: Postoperative sleep disorders can cause serious adverse effects on postoperative outcomes. The purpose of our study was to compare the effects of the timing of surgery under general anesthesia on intraoperative anesthetic drug requirements, postoperative sleep quality and pain in patients.
Materials and Methods: Eighty-four patients who underwent selective laparoscopic abdominal surgeries under general anesthesia were randomly assigned to the Day Group (8:00– 12:00) or the Night Group (18:00– 22:00). The portable sleep monitor (PSM) was used to determine sleep quality on the night before surgery (Sleep-preop), the first night after surgery (Sleep POD 1), and the third night after surgery (Sleep POD 3). The visual analog scale (VAS) was used to evaluate postoperative pain scores and the Athens Insomnia Scale (AIS) was used for assessing insomnia symptoms. The total dose of general anesthetics required and adverse effects after surgery were also assessed.
Results: Compared to Sleep-preop, patients presented with a lower sleep efficiency and a higher AIS score during Sleep POD 1 and Sleep POD 3. Furthermore, the Night Group had a significantly lower proportion of rapid eye movement sleep, stable sleep, and unstable sleep than did the Day Group at Sleep POD 1 and Sleep POD 3. The dosage of propofol and remifentanil required in the Day Group was significantly higher than that in the Night Group. Furthermore, patients in the Day Group had better pain relief, with a lower VAS score at 1, 6, 12, and 24 hours after surgery. The incidences of postoperative nausea and vomiting and dizziness were significantly higher in the Night Group than those in the Day Group.
Conclusion: Morning operations required a higher dose of anesthetic drugs than did evening operations, which may be related to the circadian rhythm. The degree of postoperative sleep disorders was greater when the operation was performed in the evening than in the morning, which was also associated with increased pain perception and increased incidence of postoperative adverse effects. Thus, our results suggest that patients with hyperalgesia and sleep disorders may benefit from operations performed in the morning.
Keywords: morning operation, evening operation, sleep quality, general anesthesia, circadian rhythm
Introduction
General anesthesia causes a drug-induced state of unconsciousness and is a non-physiological process that is similar to natural sleep.1 Its purpose is to create a state of sensory deprivation wherein patients are unresponsive to stimuli, and thus leads to explicit amnesia. Previous studies have found that general anesthesia, as an independent risk factor, may lead to desynchronization of the circadian rhythm, which could result in postoperative sleep disorders characterized by reduced rapid eye movement (REM) and slow-wave sleep (SWS).2,3 Postoperative sleep disorders could cause serious adverse effects on postoperative outcomes, such as postoperative fatigue, severe anxiety and depression, emotional detachment and delirium, and even increased pain sensitivity or postoperative pain in patients.3,4 Several studies also indicated that circadian rhythms were controlled by a main internal central clock, which is located in the anterior hypothalamus. It not only produces and regulates biological rhythms such as the sleep-wake cycle, hormones, and metabolism but also affects the dosage of general anesthetics required. This can have varying effects on the postoperative recovery and sleep quality.5–7 Previous studies have demonstrated that postoperative sleep disorders were associated with higher postoperative pain scores, changes in behavior, and poor emotional well-being, which could further aggravate postoperative sleep quality in the long term.8,9 At present, there are few studies that have assessed the effect of circadian rhythm during different timings of surgery on intraoperative anesthetic requirements, postoperative sleep quality, and pain under general anesthesia.
Based on these considerations, we sought to answer three questions in this study:
- What is the impact of morning operation and evening operation on the intraoperative anesthetic requirement under general anesthesia?
- What are the effects of different timings of surgery on the postoperative sleep quality and pain under general anesthesia?
- What is the difference between morning operation and evening operation on postoperative sleep periods such as REM, stable sleep, and unstable sleep when monitored by the portable sleep monitor (PSM)?
To this end, we aimed to compare the effects of the timing of surgery under general anesthesia on intraoperative anesthetic drug requirements, postoperative sleep quality, and pain in patients undergoing selective laparoscopic abdominal surgeries.
Materials and Methods
Participants
This study was approved by the Human Research Ethical Committee of Shengjing Hospital, Shenyang, Liaoning Province, China (IRB registration number 2017PS29K) and was compliant with the Declaration of Helsinki. Written informed consent was obtained from all subjects participating in the trial. The trial was registered prior to patient enrollment at clinicaltrials.gov (NCT04094376 Principal investigator: Bijia Song, Date of registration: 9.17.2019).
Patients who received selective laparoscopic abdominal surgeries, including cholecystectomy, appendectomy, adnexectomy and hysterectomy under general anesthesia at Shengjing Hospital of China Medical University were enrolled in this study. Inclusion criteria were as follows: age between 18 and 65 years, with an ASA physical status of I or II. Exclusion criteria were as follows: the presence of sleep disorders, pain syndrome, cardiovascular disease, sleep apnea syndrome, psychosis, history of opioid usage, history of abnormal operation or anesthesia recovery, unwillingness to provide informed consent, or a patient with a language communication disorder.
Sample Size
The sample size was calculated according to our preliminary study, and 30 patients were selected, with 15 patients in the Day group and 15 patients in the Night group. Based on the primary outcome of the Athens Insomnia Scale (AIS) score between the two groups in our preliminary study and according to the calculation of the sample size, we chose 0.6 as the estimated variability between the two groups, and 0.9 as the standard deviation. Therefore, 35 patients for each group were required, assuming a two-sided Type I error (α) of 0.05 and a power of 80%. Potential loss was expected during follow-up or due to drop out; therefore, a total of 84 patients were enrolled in this study.
Standardized Anesthesia
Eighty-four patients were assigned to either the Day Group (D Group) or the Night Group (N Group) prior to surgery by a table of computer-generated random numbers. The allocation ratio was 1:1 for the two groups. Group assignments were sealed in sequentially numbered opaque envelopes. The patients, the surgeons, the attending anesthesiologists who were responsible for anesthetic management, the anesthesiologists who were assigned with the duty of PCA management such as assessing postoperative VAS score and adjusting the PCA pump for patients according to their VAS score and the data analyst were all blinded to the group assignment. According to the timing of the operation, the nurses would guide the patients to fast for 8 hours and give patients intravenous infusion when necessary. For patients who would receive operations in the afternoon or at night, they could eat some digestible food 8 hours before surgery. The attending anesthesiologists would also give patients intravenous infusion during the operation, according to the weight of the patient, duration of the operation, intraoperative blood loss, and surgical trauma to keep intraoperative hemodynamics stable. After entering the operating room, standard monitoring such as electrocardiogram, heart rate (HR), non-invasive blood pressure (NIBP) and peripheral oxygen saturation (SpO2) were applied. General anesthesia was induced with propofol (2.0 mg/kg), sufentanil (0.2 μg/kg), and cisatracurium (0.15 mg/kg). Then, tracheal intubation was performed 3 minutes later. After intubation, mechanical ventilation was used to maintain the PETCO2 at 35–45 mmHg. Anesthesia maintenance was achieved with continuous 50–100 μg/kg/min propofol infusion and 0.15–0.2 μg/kg/min remifentanil infusion. Patients inhaled 50% oxygen and 50% air under a fresh gas flow rate of 2 L/min. Cisatracurium (0.05 mg/kg) was administered intermittently for muscle relaxation. No other opioids were administered intraoperatively. Bispectral index (BIS) is a multiprocessor EEG parameter, specially developed to measure the effects of anesthetics on the brain hypnotic state, making it possible to measure the depth of anesthesia. The anesthesiologist adjusted the intravenous speed of remifentanil and propofol to maintain the bispectral index (BIS, BIS monitor; Aspect Medical System, Newton, MA) between 40 and 55 during the operation. The patient-controlled analgesia (PCA) system was attached after surgery and the patients were instructed to use the PCA pump (100 μg sufentanil and 0.6 mg ramosetron in 100 mL saline, every pump press resulting in a 2 mL infusion, with a 15-minute lockout interval). Ramosetron (0.3 mg) was administered prophylactically at the end of the surgery. After the surgery, the patients were transferred to the single occupant room. And the postoperative monitoring such as HR, NIBP and SpO2 were applied.
Study Protocol and Measurements
All the operations for patients in the D Group started from 8:00 and finished before 12:00, and all the operations for patients in the N Group started from 18:00 and finished before 22:00. The PSM (PSM100A; Sealand Technology (Chengdu) Co. Ltd) is based on a patented, FDA cleared algorithm that utilizes a direct and repeatable process to determine sleep quality. The recording only requires a single-lead electrocardiogram or photoplethysmography and an accelerometer to produce an output that does not require specialized skills or training to operate or interpret. Thus, it can easily collect data points over time, establishing a trend line for sleep quality and identifying sleep quantity.10 The sleep process can be divided into the awake period, non-rapid eye movement (NREM), and REM. NREM includes Stage 1–4. Stages 3 and 4 are combined to represent “deep sleep” or “stable sleep” where the brain almost exclusively produces delta waves. Stage 1 occurs for a very brief period as a person is falling asleep. It is a very light sleep phase as the person is drifting in and out of sleep. Stage 2 is defined when brain waves slow down with an occasional burst of faster brain waves. Stages 1 and 2 are combined to represent “light sleep” or “unstable sleep”.11 The PSM in our study was used for three nights from 23:00 to 06:00 on the night before surgery (Sleep-preop), the first night after surgery (Sleep POD 1), and the third night after surgery (Sleep POD 3). To wear the PSM: a) stick two electrodes, respectively, at the 3rd intercostal space of the right midclavicular line and the V5 point on the left anterior axillary line; b) place the PSM on the left anterior axillary line close to the left clavicle; c) press the power button for 3 seconds to start recording; d) press power button for 3 seconds again to stop recording.
The BIS, mean arterial pressure (MAP), and HR of each patient were recorded at 5 min after entering the operation room (T0); intubation (T1); 5 min after incubation (T2); at the end of the operation (T3); extubation (T4); and 5 min after extubating (T5). The total doses of general anesthetics required were recorded for both groups during the operation.
Sleep efficiency was evaluated by the ratio of total sleep time/total recording time, the proportion of REM sleep, unstable sleep and stable sleep were recorded by a study assistant who was blinded to patient’s information. The AIS is a self-rated psychometric questionnaire quantifying sleep difficulty, based on the International Classification of Diseases-10th edition (ICD-10) criteria.12 The AIS consists of 8 items: sleep induction, waking during the night, final awakening, total sleep duration, sleep quality, well-being, functioning capacity, and sleepiness during the day, and is based on a 0–3 scale, in which “3” designates negative outcomes. Total AIS scores range from 0 to 24 points. A total score of ≥6 points indicate a diagnosis of insomnia.13,14 Postoperative pain scores were evaluated by the visual analog scale (VAS) score,15 where 0 indicates painlessness, and 10 indicates severe pain. The VAS score was measured at 1, 6, 12, and 24 hours postoperatively. If the patients stayed awake after surgery, the anesthesiologists would assess the VAS directly. Or if the patients were asleep after surgery, the anesthesiologists would check the pump press numbers within 1 hour. If the patients pressed frequently, the anesthesiologists would record a score of 3, or a score of 2. Total consumption of postoperative PCA doses and adverse effects during 24 hours after surgery such as hypotension, bradycardia, nausea and vomiting, and dizzy were recorded and treated accordingly.
Statistical Analysis
SPSS 20.0 statistical software (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 8.0 software were used for data analysis. The distribution of variables was assessed by the Kolmogorov–Smirnov test. Continuous data are represented as mean ± standard deviation and were analyzed by independent-samples Student’s t-test. Chi-square test was used to analyze differences in postoperative adverse effects between groups. Independent-samples Student’s t-test and the Wilcoxon rank-sum test were used to analyze AIS and sleep efficiency. P < 0.05 was considered to be statistically significant.
Results
We initially assessed 103 patients for eligibility to participate in this study (Figure 1), and of these, 11 patients did not meet the inclusion criteria and 8 patients declined to participate. Finally, 84 patients were enrolled in this study. Following completion of the study, three patients in the D Group were excluded from the study, since sleep monitoring failed in two patients due to detachment of the electrode, and one patient accepted conversion to laparotomy during the operation. Two patients in the N Group were excluded from the study, since one patient was unwilling to continue receiving sleep monitoring and one patient was allergic to the electrode paste. Finally, the data from 39 patients in the D Group and 40 patients in the N Group were analyzed in the present study.
 |
Figure 1 Flow diagram showing the patients that were included and excluded in this study. |
Baseline Characteristics of the Study Groups
No significant differences were found in age, weight, sex, duration of anesthesia (min), duration of surgery (min), total sleep time on the night before surgery (min), and actual blood loss (mL) among the study groups (P > 0.05, respectively) (Table 1).
 |
Table 1 Demographics of Patients in the Day Group and the Night Group |
Intraoperative Information Between the Study Groups
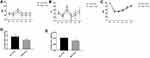
Patients in the N Group had significantly lower BIS values than those in the D Group at each time point from T1 to T5 (Figure 2C). There were no significant differences in the MAP and HR at T0 between the two groups (P > 0.05, respectively). However, there were significant increases in the MAP and HR at T2, and the MAP and HR were stably maintained at other time points. Patients in the N Group showed significantly lower levels of MAP and HR from T1 to T5 than did patients in the D Group (Figure 2A and B).
Intraoperative Dosage of Remifentanil and Propofol of the Study Groups
The total required dosage of propofol (mg/h/kg) in the N Group was statistically lower than that in the D Group (4.09±0.40 vs 5.59±0.72) (P < 0.001, Figure 2D). Furthermore, the total required dosage of remifentanil (mg/h/kg) in the N Group was also significantly lower than that in the D Group (0.008±0.0004 vs 0.01±0.001) (P < 0.001, Figure 2E).
Postoperative Sleep Quality
Compared to Sleep-preop, patients presented with a lower sleep efficiency and a higher AIS score during Sleep POD 1 and Sleep POD 3 (P < 0.001, respectively) (Figure 3A and B). Patients in the N Group had a significantly lower sleep efficiency and a higher AIS score than did patients in the D Group at Sleep POD 1 (P < 0.001, respectively) and Sleep POD 3 (P < 0.001, respectively) (Figure 3A and B). The distribution of sleep stages is shown in Figure 3. Patients in the N group experienced a significantly lower proportion of REM sleep, unstable sleep, and stable sleep than those in the D group at Sleep POD 1 (P < 0.001, respectively) and Sleep POD 3 (P < 0.001, respectively) (Figure 4A–C).
Postoperative Pain and Adverse Effects
During the first 24 hours after surgery, patients in the N Group had significantly higher VAS scores compared to the D Group at 1, 6, 12, and 24 hours after surgery (P < 0.001, respectively, Table 2). There was no significant difference between the D Group and the N Group in cumulative PCA dose (μg/kg) (0.85 ± 0.10 vs 0.90 ± 0.11, P = 0.080, respectively, Table 2). The incidences of nausea and vomiting and dizziness were also significantly higher in the N Group than those in the D Group (P < 0.05, respectively, Table 2).
 |
Table 2 Degree of Postoperative Pain and Incidence of Adverse Effects Between the Day Group and the Night Group |
Discussion
Our results confirmed that patients are more likely to experience sleep disorders after receiving general anesthesia, which was characterized by a decrease in each sleep stage distribution, a lower sleep efficiency, and a higher AIS score. The effects of evening operations on postoperative sleep disorders were more evident than those of morning operations. The medications in our study are short acting and metabolized rapidly. Propofol continuous infusion for 3 hours has a time-related half-life of 10 minutes.16 Remifentanil is a μ receptor agonist with a context-sensitive half-time within 4 minutes.17 Therefore, these two kinds of anesthetics in our study had minimally effect on postoperative sleep quality. Our data demonstrated that the total dosage of propofol and remifentanil required in patients undergoing an operation in the evening was lower than that of patients undergoing a morning operation, while the VAS scores and the incidences of postoperative nausea and vomiting and dizziness in patients undergoing an operation in the evening were higher than those of patients undergoing an operation in the morning. The mean BIS score was lower during the evening operations than during the morning operations despite these lower doses. This was consistent with the previous conclusion that the circadian clock generated circadian rhythms in all organisms, and the circadian rhythm could affect the pharmacologic sensitivity and the duration of action of general anesthetics.18 A previous study reported that postoperative sleep disturbance includes insomnia or sleep-related breathing disorder.2 The AIS is a self-rated psychometric questionnaire quantifying sleep difficulty. It only consists of 8 items, which were readily acceptable for patients to answer after surgery. Our data showed that patients in both groups experienced severe sleep disorders with a lower sleep efficiency and a higher AIS score after surgery, which manifested in a decreased percentage of each sleep stage distribution. Scarpa et al confirmed that surgical stress response and trauma are the main factors that may influence postoperative sleep.19 However, sleep disorders in patients after laparoscopic surgery were less severe (manifested as decreased N3 sleep but not REM sleep) during the night after surgery, whereas significant sleep disorders (manifested as increased N2 sleep, and decreased or lost N3 and REM sleep) occurred in patients after open operations.20 Thus, in order to reduce the effect of surgery on postoperative sleep quality, we selected patients who received laparoscopic surgery under general anesthesia. Chassard et al found that the circadian rhythm was a fundamental regulatory principle that affected the doses and duration of action of general anesthetics depending on the administration time-of-day.21 An animal study showed that the duration of propofol anesthesia exhibited a statistically significant circadian rhythm with a three-fold amplitude.5 In our study, we found that postoperative sleep disorders after the evening operation were more severe than those after the morning operation. This could be explained by the fact that general anesthetics may be administered at a relatively higher dose at night, and propofol may be more effective at night than during the day. The possible mechanisms for this are as follows: First, propofol is supposed to act mainly via GABAA receptors to induce anesthesia,22 and the activation of GABAA receptors increases at night and plays an important role in sleep regulation.23 Thus, general anesthetics like propofol that activate GABAA receptors would work better at night than during the day. Second, melatonin, which plays an important role in regulating sleep, could also regulate GABAA receptors, which causes a significant reduction in the total dose of propofol at night.24,25 In the present study, we found that patients in the N Group had higher VAS scores than did those in the D Group during the first 24 hours after surgery. The cumulative PCA dose of the N Group was higher than that of the D Group; however, no significant difference was found between the two groups. One possible reason might be the weights of the patients in the N Group are heavier comparing to the D Group. Postoperative sleep disorders could lead to hyperalgesia.26 Since postoperative sleep disorders after the evening operation were more severe than that after morning operation, the incidence of hyperalgesia may be higher in the evening than that in the morning. Furthermore, the increased proportion of REM-sleep may have heightened the threshold for pain.27 In our study, the decrease in postoperative REM sleep was more evident in the N Group than that in the D Group, which may cause more serious postoperative pain in the N Group.
There are several limitations of this study that should be noted. First, we only collected data one night before surgery, which may not adequately reflect the normal sleep state of the patients. Second, although we tried to mitigate factors that may affect postoperative sleep quality, light, noise, or interruptions due to nursing care at night might have inevitably had negative effects on sleep quality. Third, we did not collect or analyze the data that would indicate whether the patients in the D group slept between the time after surgery and before the start of their night sleep. Further studies about how sleep between the time after surgery and night sleep affects overall sleep architecture will be needed in the future. Fourth, we only collected data on the short-term sleep quality after surgery. The effect of undergoing operation under general anesthesia at different time periods on long-term sleep quality after surgery needs further study.
Conclusion
There may be a relationship between general anesthesia and the circadian rhythm. Patients undergoing an evening operation required a lower dosage of anesthetic drugs than did patients undergoing an operation in the morning. A greater degree of subsequent sleep disruption may occur when anesthesia and surgery are performed at the night than during the day. Furthermore, disrupted sleep could increase postoperative pain perception. Thus, the results of our study suggest that patients with hyperalgesia and sleep disorders may benefit from operations performed in the morning.
Data Sharing Statement
The individual deidentified participant data in our study could be shared with the readers. Readers can obtain the data by emailing the corresponding author ([email protected]). We did not include specific data and documents from other studies in our study. All the data in our study are available for 10 years.
Acknowledgments
The authors would like to thank Raymond C. Koehler, MD, Ph.D., from the Departments of Anesthesiology and Critical Care Medicine, Johns Hopkins University, Baltimore, Md, USA and Dr. Weifeng Song, MD, Ph.D., from the Department of Anesthesiology and Perioperative Medicine, School of Medicine, the University of Alabama at Birmingham, Birmingham, Alabama, USA for their discussion and advice on this study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Selvadurai S, Maynes JT, McDonnell C, et al. Evaluating the effects of general anesthesia on sleep in children undergoing elective surgery: an observational case-control study. Sleep. 2018;41(8):1093–1094. doi:10.1093/sleep/zsy094
2. Krenk L, Jennum P, Kehlet H. Sleep disturbances after fast-track hip and knee arthroplasty. Br J Anaesth. 2012;109:769–775. doi:10.1093/bja/aes252
3. Masllow GA, Lipinski WJ, Matlen LB, et al. Isoflurane anesthesia does not satisfy the homeostatic need for rapid eye movement sleep. Anesth Analg. 2010;110:1283–1289. doi:10.1213/ANE.0b013e3181d3e861
4. Pick J, Chen Y, Moore JT, et al. Rapid eye movement sleep debt accrues in mice exposed to volatile anesthetics. Anesthesiology. 2011;115:702–712. doi:10.1097/ALN.0b013e31822ddd72
5. Challet E, Gourmelen S, Pevet P, et al. Reciprocal relationships between general (propofol) anesthesia and circadian time in rats. Neuropsychopharmacology. 2007;32:728–735. doi:10.1038/sj.npp.1301081
6. Karkela J, Vakkuri O, Kaukinen S, Huang W, Pasanen M. The influence of anaesthesia and surgery on the circadian rhythm of melatonin. Acta Anaesthesiol Scand. 2002;46:30–36. doi:10.1034/j.1399-6576.2002.460106.x
7. Ram E, Vishne T, Weinstein T, Beilin B, Dreznik Z. General anesthesia for surgery influences melatonin and cortisol levels. World J Surg. 2005;29:826–829. doi:10.1007/s00268-005-7724-1
8. Kain ZN, Mayes LC, Caldwell-Andrews AA, et al. Sleeping characteristics of children undergoing outpatient elective surgery. Anesthesiology. 2002;97(5):1093. doi:10.1097/00000542-200211000-00010
9. Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002;73(2):405–417. doi:10.1111/1467-8624.00414
10. Miller JN, Kupzyk KA, Zimmerman L, et al. Comparisons of measures used to screen for obstructive sleep apnea in patients referred to a sleep clinic. Sleep Med. 2018;51:15–21. doi:10.1016/j.sleep.2018.06.007
11. Hassan AR, Bhuiyan MIH. Dual Tree Complex Wavelet Transform for Sleep State Identification from Single Channel Electroencephalogram. IEEE International conference on Telecommunications and Photonics, IEEE; 2016:1–5.
12. Okajima I, Nakajima S, Kobayashi M, Inoue Y. Development and validation of the Japanese version of the Athens insomnia scale. Psychiatry Clin Neurosci. 2013;67(6):420–425. doi:10.1111/pcn.12073
13. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens insomnia scale validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48:555–560. doi:10.1016/S0022-3999(00)00095-7
14. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the athens insomnia scale. J Psychosom Res. 2003;55:263–267. doi:10.1016/S0022-3999(02)00604-9
15. El Sherif FA, Othman AH, Abd ER, Ahmad M, Taha O. Effect of adding intrathecal morphine to a multimodal analgesic regimen for postoperative pain management after laparoscopic bariatric surgery: a prospective, double-blind, randomized controlled trial. Br J Pain. 2016;10(4):209–216. doi:10.1177/2049463716668904
16. Lundström S, Twycross R, Mihalyo M, Wilcock A. Propofol. J Pain Symptom Manage. 2010;40(3):466–470. doi:10.1016/j.jpainsymman.2010.07.001
17. Cohen J, Royston D. Remifentanil. Curr Opin Crit Care. 2001;7:227–231. doi:10.1097/00075198-200108000-00003
18. Turek FW, Pinto LH, Vitaterna MH, Penev PD, Zee PC, Takahashi JS. Pharmacological and genetic approaches for the study of circadian rhythms in mammals. Front Neuroendocrinol. 1995;16:191. doi:10.1006/frne.1995.1007
19. Scarpa M, Pinto E, Saraceni E, et al. Randomized clinical trial of psychological support and sleep adjuvant measures for postoperative sleep disturbance in patients undergoing oesophagectomy. Br J Surg. 2017;104(10):1307–1314. doi:10.1002/bjs.10609
20. Rosenberg-Adamsen S, Skarbye M, Wildschiodtz G, et al. Sleep after laparoscopic cholecystectomy. Br J Anaesth. 1996;77:572–575. doi:10.1093/bja/77.5.572
21. Warltier D, Chassard D, Bruguerolle B. Chronobiology and anesthesia. Anesthesiology 2004;100(2):413–427. doi:10.1097/00000542-200402000-00034
22. Trapani G, Altomare C, Liso G, Sanna E, Biggio G. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr Med Chem. 2000;7:249–271. doi:10.2174/0929867003375335
23. Kanterewicz BI, Rosenstein RE, Golombek DA, et al. Daily vailations in GABA receptor function in Syrian hamster cerebral cortex. Neurosci Lett. 1995;200(3):211–213. doi:10.1016/0304-3940(95)12112-H
24. Naguib M, Gottumukkala V, Goldstein PA. Melatonin and aries— thesia: a clinical perspective. J Pineal Res. 2007;42(1):12–21. doi:10.1111/j.1600-079X.2006.00384.x
25. Aguib M, Samarkandi AH, Moniem MA, et al. The effects of melatonin premedication on propofol and thiopental induction dose response curves: a prospective, randomized, double—blind study. Anesth Analg. 2006;103(6):1448–1452. doi:10.1213/01.ane.0000244534.24216.3a
26. Wang PK, Cao J, Wang H, et al. Short-term sleep disturbance–induced stress does not affect basal pain perception, but does delay postsurgical pain recovery. J Pain. 2015;16:1186–1199. doi:10.1016/j.jpain.2015.07.006
27. Kshatri AM, Baghdoyan HA, Lydic R. Cholinomimetics, but not morphine, increase antinociceptive behavior from pontine reticular regions regulating rapid-eye-movement sleep. Sleep. 1998;21(7):677–685. doi:10.1093/sleep/21.7.677
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.