Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 11
Comparison of linoleic acid-containing water-in-oil emulsion with urea-containing water-in-oil emulsion in the treatment of atopic dermatitis: a randomized clinical trial
Authors Nasrollahi SA , Ayatollahi A , Yazdanparast T, Samadi A , Hosseini H, Shamsipour M, Akhlaghi AA , Yadangi S, Abels C, Firooz A
Received 5 July 2017
Accepted for publication 29 October 2017
Published 5 January 2018 Volume 2018:11 Pages 21—28
DOI https://doi.org/10.2147/CCID.S145561
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Saman Ahmad Nasrollahi,1 Azin Ayatollahi,1 Taraneh Yazdanparast,1,2 Aniseh Samadi,1 Hamed Hosseini,3 Mansour Shamsipour,4 Ali Asghar Akhlaghi,5 Somayeh Yadangi,1 Christoph Abels,6 Alireza Firooz1,2
1Center for Research and Training in Skin Diseases and Leprosy, 2Telemedicine Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, 3Clinical Trial Center, 4Department of Research Methodology and Data Analysis, Institute for Environmental Research, Tehran University of Medical Sciences, 5Department of Epidemiology and Reproductive Health, Reproductive Epidemiology Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran; 6Dr. August Wolff GmbH & Co. KG Arzneimittel, Bielefeld, Germany
Background: Application of topical moisturizers is an essential part of the management of atopic dermatitis (AD). Linoleic acid (LA), the most abundant fatty acid in the epidermis, and its derivatives have an essential role in the structure and function of the epidermal barrier, and their defects are prominent in AD. The aim of this study was to compare the efficacy and safety of two cosmetic products containing either LA or urea in patients with AD.
Patients and methods: A total of 20 patients with AD who met the eligibility criteria and provided written informed consents were enrolled in this randomized, intra-individual split-body, single-center trial. Symmetrical lesions of patients were randomized for treatment with LA- or urea-containing water-in-oil (w/o) emulsions applied two to three times daily for 4 weeks. The efficacy of the two products was evaluated by local Scoring Atopic Dermatitis (SCORAD) of both lesions and also patient (or guardian) satisfaction. In addition, trans-epidermal water loss (TEWL), stratum corneum (SC) hydration, pH, sebum, temperature, erythema, melanin content, and ultrasonographic thickness and echo density of epidermis and dermis were measured before, and 2 and 4 weeks after, treatment.
Results: Four weeks of treatment with the LA-containing product resulted in a significant decrease in local SCORAD, TEWL, erythema, and echo density of dermis, as well as an increase in SC hydration compared to baseline. The urea-containing product also reduced the local SCORAD and echo density of dermis and increased SC hydration. In contrast to the LA-containing product, changes in TEWL and erythema were not significant. Moreover, the reduction of erythema was significantly higher in the LA-containing product-treated side compared to the urea-containing product-treated side (p = 0.006).
Conclusion: Both LA- or urea-containing w/o emulsions can significantly improve barrier dysfunction and clinical severity of AD. In agreement with literature, it was confirmed that an LA-containing w/o emulsion exhibited erythema-reducing effects. Since emollients should be used on a regular basis, patients should choose a product by individual preference following recommendation by their dermatologists.
Keywords: emollient, moisturizer, humectants, epidermal barrier, erythema
Introduction
Atopic dermatitis (AD) is a chronic inflammatory pruritic skin disorder characterized by increased trans-epidermal water loss (TEWL) which requires appropriate skin care on a regular basis.1–3
The first line of skin protection against the environmental hazardous effects, water loss, and conservation of electrolyte balance is the epidermal barrier (EB). The stratum corneum (SC), a unique differentiation end product of the epidermis, produces a set of protective/defensive functions.4
Inherited barrier abnormalities, exogenous and endogenous stressors with additional exacerbation of barrier dysfunction, and compromised antimicrobial defense with further impairment of barrier function are the main causes of barrier dysfunction in AD.4–6
One of the most important clinical features of AD which results from a dysfunctional EB is very dry skin (xerosis). Moisturizers with different agents containing varying amounts of emollients, occlusives, and/or humectants are used to reduce TEWL, increase skin hydration, and thus improve xerosis in AD patients.7 Emollients soften and smooth the skin by filling the spaces between desquamating corneocytes and provide increased cohesion leading to a smoother surface with less friction.8
Occlusive agents (such as petrolatum, mineral oil, and lanolin) retard evaporation of water by coating the SC. By decreasing TEWL, these agents are one of the best choices for treating xerosis.9
Humectants (such as glycerol, lactic acid, and urea) are water-soluble agents with the capacity to complex and hold water.10 Urea, as a humectant, decreases TEWL in normal skin and dry skin of patients with AD.11–14
Linoleic acid (LA), the most abundant fatty acid in the epidermis, and its derivatives have an essential role in the structure and function of the SC permeability barrier, and their defects are most prominent in AD.15,16 Moreover, it has also been shown that LA has anti-inflammatory effects.17,18
In this study, we compared the efficacy and safety of a water-in-oil (w/o) emulsion containing 1.5% LA with a different w/o emulsion containing 5% urea in patients with AD.
Patients and methods
This study was an open, randomized, intra-individual split-body, single-center trial. The study protocol as well as other essential documents was approved by the Ethics Committee of Tehran University of Medical Sciences and was registered in Iran Randomized Controlled Trial (IRCT; IR.TUMS.REC.1394.32) Registry with registration number IRCT2015062017994N1.
A total of 20 patients with AD who were referred to the outpatient skin clinic of the Center for Research and Training in Skin Diseases and Leprosy, met the eligibility criteria, and provided written informed consent were enrolled in this study. The inclusion criteria were female or male patients at least 2 years old and with mild-to-moderate AD (defined as Scoring Atopic Dermatitis [SCORAD] between 4 and 12, erythema and pruritus score of at least 1) without any signs of infection having at least two symmetrical lesions on arms or legs with similar local SCORAD.
Patients with severe AD (SCORAD > 12), pregnancy or lactation, drug addiction and alcoholism, AIDS or other infectious diseases (such as hepatitis), active skin disease (other than AD) at test area, documented allergies to any ingredients of study products, use of other skin care products (e.g., creams, lotions, and sunscreens) at the treatment areas throughout the course of the study, poor compliance, or enrollment in any clinical trial within the past 3 months were excluded from our study.
Symmetrical lesions of patients were randomized using a software generated randomization list for treatment with a 1.5% LA-containing w/o emulsion (aluminum stearate, aqua, beta-carotene, canola oil, cera alba, cetearyl alcohol, decyl oleate, Helianthus annuus, hydrogenated coco-glycerides, lanolin, lanolin alcohol, isomerized safflower acid, magnesium stearate, paraffin, paraffinum liquidum, petrolatum, sorbitan stearate; “Linola-F” cream; Dr. August Wolff GmbH & Co. KG Arzneimittel, Bielefeld, Germany) or a 5% urea-containing eucerin (cetylstearyl alcohol, white vaseline, and wool wax alcohols), purified water, and urea in w/o emulsion system (Samin Co., Tehran, Iran) applied two to three times daily for 4 weeks.
The efficacy of the products was evaluated by local SCORAD of both lesions and, in addition, by patient (or guardian) satisfaction. Moreover, TEWL, SC hydration, pH, sebum, temperature, erythema, melanin content, and ultrasonographic characteristics of skin (thickness and echo density of epidermis and dermis) were measured using the corresponding probes of Tewameter, Corneometer, pH meter, Sebumeter, Thermometer, and Mexameter of Cutometer® MPA 580 (CK Company, Cologne, Germany) and 22 MHz probe of high-frequency skin ultrasonography (DUB Skin Scanner; TPM, Luneburg, Germany) in standardized conditions of temperature and humidity. All the assessments were performed at baseline and 2 and 4 weeks after beginning the treatment.
Furthermore, any local adverse events at the site of applications were recorde, and the participants answered a questionnaire regarding tolerability and acceptance of each product (Figure S1).
Each subject was asked to rate the severity of itching and burning in both treatment areas from 0 (none) to 5 (severe). Patients were also asked to rate the level of satisfaction from treatment, on a 5-grade scale (5 = very satisfied, 4 = somewhat satisfied, 3 = indifferent, 2 = somewhat dissatisfied, 1 = very dissatisfied). In case of younger children and infants who were not capable of answering/filling the questionnaire, patients’ parents were asked to fill it.
Results were presented as median (quartile 1 – quartile 3), and differences were compared between two treatment groups using Wilcoxon signed-rank test.
The SPSS software version 18 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Data are presented as mean ± SD or median (quartile 1 – quartile 3) unless stated otherwise and analyzed by the Wilcoxon signed-rank test. The statistical significance level was defined as p < 0.05.
Results
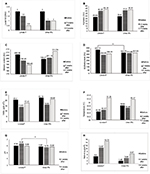
In this pilot study, 20 patients (12 females and 8 males) with a mean age of 16.75 years (SD = 13.67, range 2–45 years) and a mean SCORAD of 10.46 (SD = 1.02, range 8.7–11.9) were included, and 16 patients completed the study. The local SCORAD and the biophysical measurements at baseline and after 2 and 4 weeks of treatment on both sides are shown in Figure 1.
At baseline, there was no significant difference in local SCORAD and in any of the measured variables, except for the mean erythema level which was significantly higher in site designated to be treated by LA-containing product.
There was no significant differences between the two treated sides in any parameter, except for a higher pH in LA-containing product-treated side after 2 weeks of treatment (p-value = 0.003).
Four weeks of treatment with a LA-containing product resulted in a significant decrease in local SCORAD, TEWL, and erythema as well as an increase in SC hydration compared to baseline (Figure 1).
Treatment with a urea-containing product cream resulted in a significant decrease in local SCORAD and an increase in SC hydration. TEWL and erythema reduction after application of the urea-containing product, however, was not significant. The erythema was significantly reduced after the application of the LA-containing product-treated side compared to the side treated with the urea-containing product (p = 0.006). There was a clear, but not statistically significant trend (p = 0.098) regarding the reduction in skin melanin content in the LA-containing product-treated side, whereas skin melanin content even increased in the urea-containing product-treated side (Figure 1).
The ultrasonographic measurements showed a decrease at week 4 for both products; however, there was no statistically significant difference (Table 1).
Moreover, patients’ satisfaction with treatment was higher for the LA-containing product, and this difference was statistically significant (p = 0.046).
No statistically significant differences were found in the severity of itching and burning between two groups (p-value= 0.912 and 0.961, respectively). No other adverse reactions were reported or observed in any of the two treatment groups.
Discussion
It is well known to dermatologists and just recently confirmed by a Cochrane Review that topical application of emulsions prolongs the time to flare, decrease the number of flares, and reduce the amounts of topical corticosteroids needed.19 Therefore, the current available guidelines recommend the use of emulsions as a key and basic step in the treatment of AD,20–23 in particular, since several recent studies showed that preventing degradation and repairing the barrier dysfunctions are critical strategies for reducing the risk of relapse in AD.4,24,25
In this study, we assessed the effects of two w/o emulsions in AD lesions in a randomized, intra-individual, controlled clinical trial. One of the products contained 1.5% LA as cosmetic active ingredient, whereas the other one contained 5% urea. After 4 weeks of treatment, both products significantly decreased local SCORAD and significantly increased SC hydration, although reduction in skin erythema and TEWL was only significant in the lesions treated with the LA-containing product. An impaired skin barrier function and clinical signs of dryness are usually expected to be improved after use of the appropriate topical emulsion. Unfortunately, clinical improvement in xerosis and eczematous lesions may not necessarily induce normalization of TEWL.26
The beneficial effects of the two products, both w/o emulsions (appropriate pharmaceutical formulation for xerosis), can be explained by the measured increase in skin hydration and through possible effects on the barrier function, although only for the LA-containing product, a statistically significant change was observed for the TEWL. Components of the LA-containing product, such as complex mixture of esters, diesters and hydroxy esters of high molecular weight, lanolin alcohols, and lanolin acids, form an inert layer on the skin and can also penetrate the damaged skin and repair the EB leading to a reduction in TEWL.27–31 Thus, occlusion is the most predictable mechanism by which water loss is reduced from the skin.32 However, since the two products tested in this clinical study are not identical in their composition, they exhibit different effects on the skin (as shown).
Moreover, different effects of products used for the treatment of xerosis in AD patients are well described in the literature.12,33–35 Patients with similar disease characteristics have been considered to benefit from different topically applied emulsions with different ingredients.36 Unfortunately, up to now, there is no unifying clinical classification system available to decide which type of products are best suited for different degrees of xerosis due to different AD phenotypes.36 Humectants such as urea in emulsions are absorbed into the SC and can increase skin hydration by attracting water. Although, in our study, the TEWL following application of urea-containing product decreased from baseline, the difference was not statistically significant, whereas skin hydration increased, which can be explained by the same mechanism mentioned earlier.
Essential fatty acids (EFAs) such as LA are necessary for the synthesis of ceramides, e.g., CER[EOH], CER[EOS] and CER[EOP], which play a critical role in barrier function of the epidermis.14,15 In contrast to the urea-containing product, the LA-containing product significantly decreased erythema (one of the signs of inflammation) in this clinical study. According to the experimental literature, this is not surprising knowing that LA has anti-inflammatory effects.17,18,37 Since LA is a potent naturally occurring ligand and activator of PPRAα,38 skin inflammation of irritant contact dermatitis is reduced by the topical application of LA in a mice model.39 Moreover, an erythema-reducing effect was already reported in a previous clinical study in an irritative contact dermatitis model using a different LA-containing product. The effect of LA-containing product in that study was comparable to a 0.25% hydrocortisone-containing formulation.40
Although skin pigmentation was not in the focus of our study, a clear trend toward a reduction in the melanin content in the LA-containing product-treated side was observed. This effect of the LA-containing product on melanin content may be explained via the abovementioned anti-inflammatory properties of LA leading to a reduction in post-inflammatory hyperpigmentation.41 Of course, anti-pigmentary effect of other components in the LA-containing product cannot be ruled out.
Furthermore, unsaturated fatty acids such as oleic acid and LA can decrease tyrosinase activity (via posttranscriptional events and acceleration of the proteolysis of tyrosinase) and thus subsequently reduce melanin synthesis.42
In this study, we also used high-frequency ultrasonography to assess dermal changes after treatment with the w/o emulsions. Both creams decreased dermal echo density significantly, which might be the result of a decrease in inflammation in the dermis. In contrast to another study that compared the results of ultrasound images with pathologic findings in AD, the echogenicity of dermis had a strong negative correlation with the intensity of inflammation.43
It is worth to mention that, in this study, patients were more satisfied using the LA-containing w/o emulsion compared to the urea-containing product. This may be explained by the stinging and burning sensation of urea immediately after its application, which has been reported in previous studies.14,44
Finally, we are aware of limitations of our study. First, this study was a small pilot study and exploratory in nature with low number of patients (n = 20). Second, we used split-body design to compare two products. Third, although we focused on urea and LA, we cannot and are not excluding the effect of whole product on seen clinical improvements. On the other hand, strengths of this study are direct comparison of products with objective as well as subjective assessment of efficacy.
Conclusion
Both products, the LA- and the urea-containing w/o emulsion, increased skin hydration and thus improved the clinical severity of AD in this clinical study. However, only the LA-containing product due to the known anti-inflammatory effects of LA reduced erythema significantly after treatment. In general, the choice of moisturizers can be determined by individual preference, safety, and efficacy, and the absence of fragrances, additives, or other sensitizing agents.45
Acknowledgments
This study was supported by research grant from Sina Tejarat Pishgam Co., Tehran, Iran.
Disclosure
Christoph Abels, MD, PhD, a board-certified dermatologist, is an employee of Dr. August Wolff GmbH & Co. KG Arzneimittel, Bielefeld, Germany. The other authors report no conflicts of interest in this work.
References
Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22(2):125–137. | ||
Firooz A, Gorouhi F, Davari P, et al. Comparison of hydration, sebum and pH values in clinically normal skin of patients with atopic dermatitis and healthy controls. Clin Exp Dermatol. 2007;32(3):321–322. | ||
Wollenberg A, Ehmann LM. Long term treatment concepts and proactive therapy for atopic eczema. Ann Dermatol. 2012;24(3):253–260. | ||
Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121(6):1337–1343. | ||
Elias PM. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Ann Dermatol. 2010;22(3):245–254. | ||
Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134(4):792–799. | ||
Rawlings AV, Canestrari DA, Dobkowski B. Moisturizer technology versus clinical performance. Dermatol Ther. 2004;17(Suppl 1):49–56. | ||
Draelos ZK. Atlas of Cosmetic Dermatology. New York: Churchill Livingstone; 2000. | ||
Spruit D. The interference of some substances with the water vapour loss of human skin. Dermatologica. 1971;142(2):89–92. | ||
Idson B. Dry skin: moisturizing and emolliency. Cosmet Toiletr. 1992;107:10. | ||
Grice K, Sattar H, Baker H. Urea and retinoic acid in ichthyosis and their effect on transepidermal water loss and water holding capacity of stratum corneum. Acta Derm Venereol. 1973;53(2):114–118. | ||
Loden M. Urea-containing moisturizers influence barrier properties of normal skin. Arch Dermatol Res. 1996;288(2):103–107. | ||
Loden M, Andersson AC, Lindberg M. Improvement in skin barrier function in patients with atopic dermatitis after treatment with a moisturizing cream (Canoderm). Br J Dermatol. 1999;140(2):264–267. | ||
Serup J. A double-blind comparison of two creams containing urea as the active ingredient. Assessment of efficacy and side-effects by non-invasive techniques and a clinical scoring scheme. Acta Derm Venereol Suppl (Stockh). 1992;177:34–43. | ||
Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130(10):2511–2514. | ||
Janssens M, van Smeden J, Gooris GS, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131(10):2136–2138. | ||
Liu KL, Belury MA. Conjugated linoleic acid reduces arachidonic acid content and PGE2 synthesis in murine keratinocytes. Cancer Lett. 1998;127(1–2):15–22. | ||
Wertz PW. Biochemistry of human stratum corneum lipids. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor & Francis; 2006:10. | ||
van Zuuren EJ, Fedorowicz Z, Arents BWM. Emollients and moisturisers for eczema: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol. Epub 2017 Apr 22. | ||
National Institute for Health and Care Excellence [webpage on the Internet]. NICE Pathways – Mapping Our Guidance. London: National Institute for Health and Care Excellence; c2016. [cited Jan 28, 2016]. Available from: https://pathways.nice.org.uk/pathways/eczema#path=view%3A/pathways/eczema/treating-atopic-eczema-in-children-aged-12-and-under.xml&content=view-index. Accessed November 21, 2017. | ||
Australasian Society of Clinical Immunology and Allergy [webpage on the Internet]. Atopic Dermatitis (Eczema). Brookvale, AU: Australasian Society of Clinical Immunology and Allergy; c2016. [cited Jan 29, 2016]. Available from: https://www.allergy.org.au/images/pcc/ASCIA_PCC_Eczema_2015.pdf. Accessed November 25, 2017. | ||
Rubel D, Thirumoorthy T, Soebaryo RW, et al. Consensus guidelines for the management of atopic dermatitis: an Asia-Pacific perspective. J Dermatol. 2013;40(3):160–171. | ||
Sinclair W, Aboobaker J, Green R, et al. Guidelines on the Management of Atopic Dermatitis in South Africa. London: Dermatology; c2015 [cited Jan 28, 2016]. Available from: https://www.mm3admin.co.za/documents/docmanager/8e7be0a4-2b8d-453f-875e-cd1e5132b829/ 00079177.pdf. | ||
Szczepanowska J, Reich A, Szepietowski JC. Emollients improve treatment results with topical corticosteroids in childhood atopic dermatitis: a randomized comparative study. Pediatr Allergy Immunol. 2008;19(7):614–618. | ||
Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134(4):818–823. | ||
Vilaplana J, Coll J, Trullas C, Azon A, Pelejero C. Clinical and non-invasive evaluation of 12% ammonium lactate emulsion for the treatment of dry skin in atopic and non-atopic subjects. Acta Derm Venereol. 1992;72(1):28–33. | ||
Feingold KR, Brown BE, Lear SR, Moser AH, Elias PM. Effect of essential fatty acid deficiency on cutaneous sterol synthesis. J Invest Dermatol. 1986;87(5):588–591. | ||
Loden M. The increase in skin hydration after application of emollients with different amounts of lipids. Acta Derm Venereol. 1992;72(5):327–330. | ||
Mao-Qiang M, Brown BE, Wu-Pong S, Feingold KR, Elias PM. Exogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol. 1995;131(7):809–816. | ||
Rawlings AV, Scott IR, Harding CR, Bowser PA. Stratum corneum moisturization at the molecular level. J Invest Dermatol. 1994;103(5):731–741. | ||
Wertz PW, Downing DT. Metabolism of topically applied fatty acid methyl esters in BALB/C mouse epidermis. J Dermatol Sci. 1990;1(1):33–37. | ||
Loden M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71(1):79–82. | ||
Held E, Sveinsdottir S, Agner T. Effect of long-term use of moisturizer on skin hydration, barrier function and susceptibility to irritants. Acta Derm Venereol. 1999;79(1):49–51. | ||
Loden M. Barrier recovery and influence of irritant stimuli in skin treated with a moisturizing cream. Contact Dermatitis. 1997;36(5):256–260. | ||
Loden M, Olsson H, Skare L, Axéll T, Hud Ab A. Instrumental and sensory evaluation of the frictional response of the skin following a single application of five moisturizing creams. J Soc Cosmet Chem. 1992;43:8. | ||
Moncrieff G, Cork M, Lawton S, Kokiet S, Daly C, Clark C. Use of emollients in dry-skin conditions: consensus statement. Clin Exp Dermatol. 2013;38(3):231–238. quiz 238. | ||
Rhodes LE, Storey A. Essential fatty acids: biological functions and potential applications in the skin. In: Maibach HI, Loden M, editors. Dry Skin and Moisturizers. Boca Raton, FL: CRC Press; 2006:319–340. | ||
Moya-Camarena SY, Vanden Heuvel JP, Blanchard SG, Leesnitzer LA, Belury MA. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalpha. J Lipid Res. 1999;40(8):1426–1433. | ||
Sheu MY, Fowler AJ, Kao J, et al. Topical peroxisome proliferator activated receptor-alpha activators reduce inflammation in irritant and allergic contact dermatitis models. J Invest Dermatol. 2002;118(1):94–101. | ||
Proksch E, Abels C. Effects of linola −emulsion in comparison to a hydrocortisone-containing emulsion in the model of an irritant. Akt Dermatol. 2007;33(7):7. | ||
Verallo-Rowell VM, Katalbas SS, Pangasinan JP. Natural (mineral, vegetable, coconut, essential) oils and contact dermatitis. Curr Allergy Asthma Rep. 2016;16(7):51. | ||
Ando H, Watabe H, Valencia JC, et al. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: a new aspect of ubiquitin-proteasome function. J Biol Chem. 2004;279(15):15427–15433. | ||
Wozniak AW, Dańczak-Pazdrowska AA, Polańska AA, Janicka-Jedyńska MM, Maksin KK, Jenerowicz DD. Ultrasonographic and histopathologic images of atopic dermatitis are closely related. Pathology. 2014;46:1. | ||
Frithz A. Investigation of Cortesal®, a hydrocortisone cream and its water-retaining cream base in the treatment of xerotic skin and dry eczemas. Curr Ther Res. 1983;33:5. | ||
Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132. |
Supplementary material
  | Figure S1 Patient questionnaire. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.