Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Comparison of Hospital Length of Stay and Supportive Care Utilization Between Patients Treated with CPX-351 and 7+3 for Therapy-Related Acute Myeloid Leukemia or Acute Myeloid Leukemia with Myelodysplasia-Related Changes
Authors Price K, Cao Z, Lipkin C, Profant D , Robinson S
Received 21 October 2021
Accepted for publication 22 December 2021
Published 8 January 2022 Volume 2022:14 Pages 21—34
DOI https://doi.org/10.2147/CEOR.S342303
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Kwanza Price,1 Zhun Cao,2 Craig Lipkin,2 Deb Profant,1 Scott Robinson2
1Jazz Pharmaceuticals, Palo Alto, CA, USA; 2Premier Inc., Charlotte, NC, USA
Correspondence: Deb Profant
Jazz Pharmaceuticals, 3170 Porter Dr., Palo Alto, CA, 94304, USA
Tel/Fax +1 971 409 6166
Email [email protected]
Purpose: CPX-351 is dual-drug liposomal encapsulation of daunorubicin and cytarabine at a fixed synergistic 1:5 molar ratio. This study determined current real-world use of CPX-351 versus conventional 7+3 (cytarabine+daunorubicin) therapy and evaluated hospital length of stay (LOS) and supportive care utilization in t-AML and AML-MRC.
Patients and Methods: This retrospective, observational study utilized the Premier Healthcare Database and included patients who were aged ≥ 18 years with t-AML or AML-MRC and treated with CPX-351 or 7+3 between August 1, 2017 and February 28, 2019. All patients treated with 7+3 were required to be eligible for CPX-351 based on its FDA-approved indication. Outcome variables were annualized and adjusted for patient, hospital, and clinical confounding factors. The primary outcome was inpatient LOS. Secondary outcomes included use of blood products and use of anti-infectives.
Results: The study included 195 qualifying patients treated with CPX-351 and 160 patients treated with 7+3 who were eligible for CPX-351. Approximately one-third of the patients treated with CPX-351 were administered therapy in a hospital-based outpatient setting, and all patients treated with 7+3 received it in the inpatient setting. The regression-adjusted annualized inpatient LOS was shorter with CPX-351 than 7+3 (mean of 183.7 vs 197.1 days, p< 0.001). The difference in mean-adjusted LOS was most pronounced for t-AML, with a mean-adjusted LOS of 168.9 versus 192.5 days for CPX-351 versus 7+3, respectively (nominal p< 0.001). Supportive care utilization, including the number of administrations of red blood cells, the number of administrations of platelets, and the number of days on anti-infectives, was similar between treatment groups.
Conclusion: CPX-351 was associated with a shorter inpatient LOS than 7+3. Supportive care use, including blood products and anti-infectives, was similar for CPX-351 and 7+3. These findings suggest CPX-351 conveys resource advantages over 7+3 in patients with newly diagnosed t-AML and AML-MRC.
Keywords: acute myeloid leukemia, chemotherapy, cytarabine, daunorubicin, anthracycline, healthcare resource utilization
Introduction
Acute myeloid leukemia (AML) is one of the most common types of leukemia in adults. The number of new AML cases and deaths in the United States in 2020 was estimated to be 19,940 and 11,180, respectively.1 Secondary AML, an AML subtype with distinct cytogenetics, evolves from early or late complications of prior chemotherapy or ionizing radiation, or from an antecedent hematologic disorder such as myelodysplastic syndrome (MDS).2 Secondary AML, including therapy-related AML (t-AML) and AML with myelodysplasia-related changes (AML-MRC), accounts for 10% to 30% of all AML cases,2,3 occurs more frequently with advancing age, and is associated with a poorer prognosis compared to de novo AML.3
Intensive chemotherapy using the 7+3 regimen, which consists of cytarabine administered by 7-day continuous infusion and short infusions of an anthracycline, such as daunorubicin or idarubicin on each of the first 3 days, has been the standard of care for AML for decades. Treatment of AML is associated with significant health care resource utilization (HRU). A study of newly diagnosed AML patients found that hospital inpatient costs account for >85% of total expenditures,4 with intensive care unit stays accounting for >40% of overall hospital costs.5
In August 2017, the US Food and Drug Administration (FDA) approved CPX-351 (Vyxeos®, daunorubicin and cytarabine liposome for injection; Jazz Pharmaceuticals, Inc., Palo Alto, CA) for the treatment of adults with newly diagnosed t-AML or AML-MRC. CPX-351 is a fixed combination of daunorubicin and cytarabine with a synergistic 1:5 molar ratio encapsulated in a liposome. In clinical trials, CPX-351 has been found to have longer overall survival (OS) and higher complete remission rates versus 7+3 with a similar safety profile,6–11 with significant improvements in OS and remission rates noted in a phase 3 randomized study of older patients with newly diagnosed t-AML and AML-MRC.6
Due to its relatively recent approval, there is limited literature on the HRU of AML patients receiving CPX-351. A study using data from the phase 3 randomized clinical trial assessed the HRU in patients between the ages of 60 and 75 years with newly diagnosed t-AML and AML-MRC,12 but real-world evidence of HRU in AML patients receiving CPX-351 is lacking. This study sought to evaluate real-world resource utilization of CPX-351 versus conventional 7+3 in adults with t-AML and AML-MRC based on evidence from a US hospital administrative database.
Methods
Study Design and Data Source
A retrospective cohort study was conducted utilizing the Premier Healthcare Database (PHD). The study assessed the length of stay (LOS) and supportive care utilization in t-AML and AML-MRC patients treated with CPX-351 versus 7+3.
The PHD is a large geographically diverse hospital-based, service-level, all-payer database containing discharge information from inpatient and hospital-based outpatient visits.13 It represents ~25% of all inpatient admissions in the United States. Patients can be tracked across the inpatient and hospital-based outpatient settings, as well as across visits, through a unique identifier. During the study period, more than 146 million inpatient and outpatient encounters were reported by 813 hospitals. The PHD contains data from standard hospital discharge files, including patient demographics and disease states. A day of service–stamped log of billed items, including procedures, medications, laboratory evaluations, and diagnostic and therapeutic services can be captured at the individual patient level. Drug utilization information is available by day of stay and includes quantity, dosing, strength, and cost. In addition, information on hospital characteristics of geographic location, rural/urban populations served, teaching status, and bed capacity are available. All data are statistically de-identified and compliant with the Health Insurance Portability and Accountability Act according to 45 CRF 46.101(b)(4) and 45 CRF 164.506(d)(2)(ii)(B). This study is secondary research using a de-identified database. It is not human subjects research and is exempt from Institutional Review Board review.
Study Population
Patients aged ≥18 years with at least one inpatient or hospital-based outpatient visit with a principal or secondary discharge diagnosis of AML (International Classification of Diseases, 10th revision, Clinical Modification [ICD-10-CM] diagnosis codes C92.0x, C92.5x, or C92.x) and who had received either CPX-351 or 7+3 treatment between August 1, 2017 and February 28, 2019 were identified for inclusion in this study (Figures 1 and 2). Patients treated with CPX-351 were identified from the PHD using the hospital charge master description. Patients treated with 7+3 were identified as those with a charge master description indicating cytarabine use for 5 to 9 days, with no more than 4 days between uses, and evidence of 2 or more days of anthracycline (daunorubicin, idarubicin, or mitoxantrone) use within 3 days prior to or 4 days following the first day on cytarabine. Patients receiving adjunct therapy with gemtuzumab ozogamicin, midostaurin, sorafenib, venetoclax, quizartinib, or gilteritinib, and patients with <30 days of follow-up were excluded.
 |
Figure 2 Study period. |
According to the FDA indications at the time of this study, CPX-351 should be used for the treatment of adults with newly diagnosed t-AML or AML-MRC. Therefore, all patients treated with CPX-351 were assumed to have newly diagnosed t-AML or AML-MRC. However, since 7+3 could be used in the treatment of other AML subtypes, 7+3–treated patients who had no evidence of newly diagnosed t-AML or AML-MRC in the 12-month pre-index period were excluded from the control cohort. Patients in the 7+3 cohort who received CPX-351 in the 12-month pre-index period were also excluded. The index visit date was defined as the first hospital admission/outpatient visit with CPX-351 or 7+3 use. A variable-length follow-up period was defined as the number of days between the index admission date and the last available inpatient discharge date on or before February 28, 2019, whichever occurred first. As an exception, for patients treated in the hospital-based outpatient setting who had a treatment cycle that started before February 28, 2019 and extended beyond February 28, 2019, the last day of the treatment cycle was used as the end date of the follow-up period.
Definition and Identification of t-AML and AML-MRC
As stated above, it was assumed that all CPX-351–treated patients had newly diagnosed t-AML or AML-MRC, based on the FDA-approved treatment indication. For 7+3–treated patients, the cohort was restricted to those with evidence of t-AML or AML-MRC.
The World Health Organization (WHO) defines t-AML as AML caused by previous treatment with chemotherapy or radiation therapy.14 The definition of newly diagnosed t-AML was based on the following three conditions: (1) with an ICD-10-CM diagnosis code of C92.0x, C92.5x, C92.6x, or C92.Ax; (2) with any diagnosis of another type of cancer (ICD-10-CM diagnosis C00-D49, except for C92.0x, C92.5x, C92.6x, or C92.Ax) or received chemotherapy/radiation therapy within 12 months prior to the first date of the hospitalization/visit with an AML diagnosis; (3) without a previous diagnosis of AML (C92.0x, C92.5x, C92.6x, or C92.Ax) within the 12-month period prior to the first hospital admission/visit date with the diagnosis of AML. The WHO defines AML-MRC as AML with ≥20% blasts and additionally one of the following three criteria: (1) history of MDS or MDS/myeloproliferative neoplasm (MPN); (2) MDS-related cytogenetic abnormalities; or (3) multilineage dysplasia (≥50% dysplastic cells in two or more hematopoietic lineages) in the absence of Nucleophosmin (NPM1) or biallelic CCAAT/enhancer-binding protein alpha (CEBPA) mutations.14 In this study, AML-MRC patients included those with a principal or secondary diagnosis of C92.Ax (AML with multilineage dysplasia), or with any diagnosis of AML and evidence of MDS (ICD-10-CM diagnosis code D46x) or MPN (ICD-10-CM D47.1x), within 12 months prior to the first AML diagnosis. Based on the definitions and inherent code limitations, newly diagnosed t-AML and AML-MRC were not mutually exclusive.
Study Variables
Demographics, Hospital, and Clinical Characteristics
Patient, hospital, cancer history, and clinical characteristics were examined at the time of the index visit for the first chemotherapy treatment. Patient demographics included age, sex, race, ethnicity, and health care coverage. Hospital characteristics included urban and rural populations served, teaching status, US census geographic divisions, and bed size. Patient history of prior MDS, type of AML (newly diagnosed t-AML, AML-MRC, or undetermined), and factors that were likely to contribute to longer LOS or higher supportive care utilization, such as pre-index and concomitant treatment with any hypomethylating agent (HMA; azacitidine and decitabine)15 or anthracycline, were also reported. The Charlson-Deyo Comorbidity Index (CCI) was determined at the time of first treatment to assess overall health status of AML patients.16,17 Each individual chronic comorbidity was captured.
Outcomes
The primary outcome of the study was total inpatient LOS. Other outcomes included supportive care utilization, such as the total number of administrations of platelets and/or red blood cell (RBC) transfusions and the number of days on anti-infectives and/or white blood cell colony-stimulating factors (WBC-CSF). All outcomes were evaluated from the index admission date through the end of the follow-up period. As the length of follow-up varied across patients, all outcomes were annualized18 in the analyses. Using standard methodology from studies with similar designs to annualize the data,19–23 the actual outcomes were divided by the length of follow-up, in days, and multiplied by 365.
Statistical Methods
Descriptive statistics were performed on each treatment cohort. Continuous data were expressed as mean (with standard deviation) or median (with 25th and 75th percentiles) values. Categorical variables were expressed as counts and percentages. A bivariate analysis compared the treatment cohorts. Since LOS and number of days were not normally distributed, the Wilcoxon rank-sum test was used to compare the distribution of outcomes between the treatment cohorts. Chi-square tests were used for dichotomous or categorical variables. The analysis was powered for inpatient LOS using a Wilcoxon test at α=0.05. The p-values for all outcomes except LOS were nominal. A data analysis was implemented using SAS software version 9.4 (©SAS Institute Inc.).
An adjusted analysis was performed on LOS and supportive care utilization outcomes. Multivariable regression models were used to adjust for differences in patient, hospital, and clinical characteristics. For the outcomes of annualized total inpatient LOS and total number of days on anti-infectives, generalized linear models (GLMs) with Poisson variance and log link functions were used. For outcomes with significant proportion of zero values, such as annualized total numbers of administrations of platelets or RBC transfusions, and annualized number of days on WBC-CSF, zero-inflated Poisson regressions were applied. The key independent variable was a dichotomous variable indicating the receipt of CPX-351 versus 7+3. The list of covariates started from an extensive list of variables, including patient demographics (age group, sex, race, and ethnicity); health insurance primary payer; admission type; hospital characteristics (urbanicity, region, bed size, and teaching status); and clinical conditions (CCI and individual chronic comorbidities, newly diagnosed t-AML, AML-MRC, any pre-index HMA use, and any pre-index anthracycline use). In addition, the number of days from index admission to the end of the study period was used as a covariate, since annualization may not completely remove the effect of time.22 The final list of covariates was determined based on backward selection methods with a Bonferroni adjustment; therefore, covariates included in the regression of different outcomes could vary.
Recycled prediction24,25 was used to calculate the adjusted outcomes for each of the treatment groups following the regressions. The recycled prediction method calculates the predicted outcomes based on regression estimates and facilitates interpretation of regression results. First, all patients were assumed to receive CPX-351, and the adjusted outcomes were predicted based on the regression coefficients, holding all covariates at their actual values. Second, a prediction was made assuming all patients received 7+3. The means for the predicted values for the two hypothetical groups (CPX-351 vs 7+3) were examined, and the 95% confidence intervals (CIs) were calculated using a bootstrap method for 1000 iterations.
Exploratory Analysis of Treatment Cycles
Treatment cycles were examined as part of an exploratory analysis. The number of induction-strength treatment cycles were examined for CPX-351 and 7+3, respectively. An induction-strength CPX-351 treatment cycle was defined by the evidence for 2 or more days with CPX-351 use, with no more than 4 days between uses. An induction-strength 7+3 treatment cycle was defined by cytarabine use for 5 to 9 days, with no more than 4 days between uses, and evidence of 2 or more days of anthracycline (daunorubicin, idarubicin, or mitoxantrone) use within 3 days prior to or 4 days following the first day on cytarabine. In addition, consolidation-strength treatment cycles were also examined. A consolidation-strength cycle included CPX-351 use for at least 1 day, or cytarabine use for at least 5 days. However, some patients may have received an alternate consolidation regimen.
Results
Descriptive Analysis
The study assessed 195 patients (55%) who received CPX-351 alone without any adjunct therapy and with a length of follow-up of ≥30 days, and 160 patients (45%) who received 7+3 alone without CPX-351 or any adjunct therapy and with a length of follow-up of ≥30 days (Figure 1). The median (25th–75th percentiles) length of follow-up was 136 days (68–232) and 126 days (54.5–241.5) for patients who received CPX-351 and 7+3 (p=0.648), respectively.
The median (25th–75th percentiles) age was 68 (61–71) years for patients receiving CPX-351 versus 61 (52.5–81) years for those receiving 7+3 (nominal p<0.001; Table 1). While Medicare was the primary payer for both cohorts, there was a higher proportion of patients enrolled in Medicare in the CPX-351 cohort (59.0%) versus the 7+3 cohort (45.6%; nominal p=0.012).
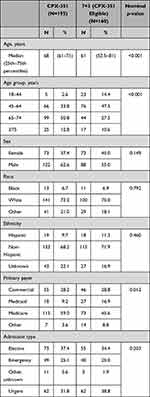 |
Table 1 Patient and Visit Characteristics |
The proportions of patients served by urban hospitals were over 90% in both cohorts (nominal p=0.086; Table 2). In the CPX-351 cohort, 87.7% of patients were treated in teaching hospitals versus 70.6% in the 7+3 cohort (nominal p<0.001). In both cohorts, most patients were treated in large hospitals with bed capacities of ≥500 (CPX-351: 80.5%; 7+3: 71.3%; nominal p=0.054).
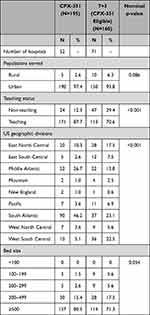 |
Table 2 Hospital Characteristics |
The percentage of patients with evidence of prior MDS was 20.0% in the CPX-351 cohort and 40.0% in the 7+3 cohort (nominal p<0.001; Table 3). In the CPX-351 cohort, although all patients should have newly diagnosed t-AML and/or AML-MRC per the FDA indications, only 32.8% of patients had identifiable t-AML and 20.5% had identifiable AML-MRC, while the AML subtype was undetermined for 65.1% of patients due to a lack of diagnosis codes for AML-MRC in the pre-index period. Since patients could have evidence of both newly diagnosed t-AML and AML-MRC, the percentages in each treatment cohort exceed 100%. In the 7+3 cohort, since additional selection criteria were applied requiring that all patients must have evidence of t-AML and/or AML-MRC, 90.0% of patients had evidence of newly diagnosed t-AML and 42.5% had evidence of AML-MRC. Prior HMA exposure in the pre-index period was reported for 12.8% of patients in the CPX-351 cohort and 13.8% in the 7+3 cohort (nominal p=0.875). Prior anthracycline use was reported for 2.6% of patients in the CPX-351 cohort versus 10.0% in the 7+3 cohort (nominal p=0.005), which could be related to the lower frequency of t-AML in the CPX-351 cohort. Most patients had a CCI of 1 to 3 (CPX-351: 74.9%; 7+3: 66.3%; nominal p=0.075).
 |
Table 3 Clinical Characteristics |
As shown in Table 4, unadjusted and unannualized median LOS was 49 days (25th–75th percentiles: 35–69; mean: 54.0; SD: 29.6) in the CPX-351 cohort versus 51.5 days in the 7+3 cohort (25th–75th percentiles: 37–73; mean: 60.7; SD: 32.9; nominal p=0.170). The median numbers of platelet administrations, RBC transfusion administrations, days on anti-infectives, and days on WBC-CSFs were similar for patients treated with CPX-351 versus 7+3, with nominal p-values >0.05.
 |
Table 4 Unadjusted LOS and Supportive Care Utilization Before Annualization |
Exploratory Analysis of Treatment Cycles
On average, 1.5 induction-strength CPX-351 treatment cycles were captured per patient in the CPX-351 cohort, with 36.3% (107/295) of treatment cycles occurring in the hospital-based outpatient setting and 63.7% (188/295) in the inpatient setting. The 7+3 cohort had an average of 1.2 induction-strength 7+3 treatment cycles per patient, with 100% of treatment cycles performed in the inpatient setting. When consolidation-strength treatment cycles were included, the median number of total treatment cycles received was 1 (25th–75th percentiles: 1–2) for both the CPX-351 and 7+3 cohorts.
Adjusted LOS and Supportive Care Utilization Outcomes
The regression-adjusted outcomes are reported in Table 5. As backward selection was used, the final list of covariates differed across different outcomes and are appropriately footnoted.
 |
Table 5 Regression-Adjusted LOS and Supportive Care Utilization |
LOS: After backward selection, the final GLM regression model for annualized LOS included the key independent variables of treatment (CPX-351 vs 7+3), newly diagnosed t-AML, and the interaction term between newly diagnosed t-AML and treatment. The covariates included in the final models are: age, gender, race, ethnicity, primary payer, hospital census division, hospital bed size, type of admission (emergent, urgent, or elective), CCI, AML-MRC, prior HMA use, and number of days from the index admission date to the end of the study period. The adjusted annualized inpatient LOS per patient was 13.4 days shorter for patients treated with CPX-351 versus 7+3 (183.7 [95% CI: 180.1–187.1] vs 197.1 [95% CI: 193.3–200.8] days; p<0.001). Patients with identifiable newly diagnosed t-AML had an adjusted annualized inpatient LOS that was 23.6 days shorter if treated with CPX-351 versus 7+3 (168.9 vs 192.5 days; p<0.001). For patients without evidence of newly diagnosed t-AML, the adjusted annualized inpatient LOS was 9.1 days longer for CPX-351 versus 7+3 (190.4 vs 181.3 days; p=0.025).
Platelets: The covariates list in the final zero-inflated Poisson regression of the annualized number of administrations of platelets included: race, ethnicity, hospital census division, hospital bed size, type of admission, AML-MRC, and prior HMA use. The adjusted annualized number of administrations of platelets was 4.8 and 4.5 in the CPX-351 and 7+3 cohorts, respectively (nominal p=0.368).
RBC transfusions: The zero-inflated Poisson regression of the annualized number of RBC transfusion administrations included race, ethnicity, hospital census division, hospital bed size, type of admission, AML-MRC, and newly diagnosed t-AML as covariates. The adjusted annualized number of RBC transfusion administrations was 5.0 and 4.9 in the CPX-351 and 7+3 cohorts, respectively (nominal p=0.512).
Anti-infectives: Race, ethnicity, primary payer, hospital census division, hospital bed size, type of admission, CCI, prior HMA use, and prior anthracycline use were included as covariates in the final regression model for the annualized number of days on anti-infectives. The adjusted annualized number of days on anti-infectives was 22.4 and 22.9 days for the CPX-351 and 7+3 cohorts, respectively (nominal p=0.408).
WBC-CSF: The final zero-inflated Poisson regression model for number of days on WBC-CSF included covariates of primary payer, hospital census division, teaching hospital, and number of days from the index admission date to the end of the study period. The adjusted annualized number of days on WBC-CSF was 2.0 and 3.0 for the CPX-351 and 7+3 cohorts, respectively (nominal p=0.053).
Discussion
This study primarily focused on the LOS and supportive care utilization associated with real-world use of CPX-351 immediately following its approval to treat adults with newly diagnosed t-AML and AML-MRC. The key finding of the study was patients treated with CPX-351 had a shorter adjusted annualized inpatient LOS (183.7 vs 197.1 days for 7+3), despite patients treated with CPX-351 being older than those treated with 7+3. In an open-label, multicenter, randomized, phase 3 trial, the estimated duration of hospitalization per patient-year was 198.4 days with CPX-351 (n=153) and 240.5 days with 7+3 (n=151).12 Both the clinical trial and this observational database study demonstrated reduced LOS for the CPX-351 cohort (by 13.4 days and 42.1 days, respectively). Noted differences in LOS may, at least in part, reflect patients’ demographics and clinical characteristics, including age, comorbid conditions, and disease severity; however, although the difference in LOS was smaller in this study after adjustment for patient, hospital, and clinical characteristics, it remained significant (p<0.001). The differences in LOS likely also reflect the study design and treatment administration schedules. Whereas 7+3 is administered as a 7-day continuous infusion of cytarabine with individual intravenous bolus doses of daunorubicin over the first 3 days of the first induction cycle, CPX-351 is administered as a 90-minute infusion on Days 1, 3, and 5 for the first induction and Days 1 and 3 for the second induction and consolidation, allowing for outpatient administration in appropriate patients particularly during consolidation, which may shorten the overall LOS with CPX-351.26
As demonstrated by Lancet et al,6 CPX-351 treatment led to better median OS in patients with t-AML (12.17 months) versus AML with antecedent MDS or chronic myelomonocytic leukemia (CMML; 7.38 months). In comparison, among patients receiving 7+3, the median OS was the same (5.95 months) between t-AML and AML with antecedent MDS or CMML subgroups.6 The current study, which focuses on HRU outcomes of patients with secondary AML, found that, while CPX-351 treatment was associated with shorter inpatient LOS overall, the effect of treatment varied across different AML subtypes. In particular, patients with newly diagnosed t-AML treated with CPX-351 had significantly shorter LOS versus those treated with 7+3. However, among patients without evidence of newly diagnosed t-AML, CPX-351 treatment was associated with a longer inpatient LOS versus those treated with 7+3. Findings from this study and Lancet et al6 comparing CPX-351 to 7+3 suggest CPX-351 may have a more favorable effect on both clinical and HRU outcomes when used for the treatment of t-AML. However, further studies are needed to confirm this finding, especially given limitations in identifying clinical characteristics of patients in the Premier database and, in particular, the AML-MRC population.
An additional HRU analysis assessed hematologic support and the need for anti-infectives. Hematologic and oncologic adverse events associated with CPX-351 include anemia, thrombocytopenia, hemorrhage, neutropenia, and febrile neutropenia.27 CPX-351 also can be associated with more frequent bleeding in the elderly.27 Safety evaluation from the phase 3 clinical study comparing CPX-351 to 7+3 demonstrated that the adverse event profiles of CPX-351 and 7+3 were similar except for more prolonged myelosuppression with CPX-351.6,28 Persistence of CPX-351 liposomes in the plasma prolongs drug exposure, which can result in bone marrow suppression with longer recovery times for platelets and neutrophils,6 necessitating the use of supportive care for the management of some patients. Despite the prolonged myelosuppression previously reported with CPX-351, the utilization of platelets, RBCs, and anti-infectives was similar between CPX-351 and 7+3 in this study. The mean adjusted number of days on WBC-CSF was slightly numerically less with the CPX-351 versus 7+3 cohorts in this study (nominal p=0.053). Our findings thus demonstrated that the use of CPX-351 is associated with shorter LOS without increasing the need for supportive care. In Villa et al,12 respective utilization of RBC and platelet transfusions, usage of anti-infective agents, and administrations of WBC-CSF were also similar between the CPX-351 and 7+3 cohorts.
Although costs of the 7+3 drugs are relatively low, there are significant additional costs associated with the required inpatient hospital stay.29,30 In the current study, the estimated reduction of inpatient stay by 13.4 days for the CPX-351 cohort could potentially translate to savings to hospitals. In the literature, using patient data from the phase 3 trial, a budget impact analysis revealed that, in a hypothetical 1 million member health plan, adoption of CPX-351 would result in an estimated increase in the number of patients with a complete response, resulting in a 3-year incremental cost decrease of almost $180,000 per responding patient versus 7+3.31
Based on findings in Villa et al,12 the median number of cycles administered was 2 (25th–75th percentiles: 1–4 cycles) in the CPX-351 cohort versus 1 (25th–75th percentiles: 1–4 cycles) in the 7+3 cohort. In comparison, the median number of treatment cycles was 1 (25th–75th percentiles: 1–2 cycles) for both the CPX-351 and 7+3 cohorts in the current study. The difference in median number of treatment cycles between Villa et al and this study may reflect the treatment decisions made regarding consolidation cycles.
The treatment paradigm of AML for older patients is rapidly evolving. In recent years, a plethora of new agents have emerged, expanding the therapeutic landscape for this difficult-to-treat population. In addition to CPX-351, agents recently approved in the United States include lower-intensity regimens, such as venetoclax in combination with HMAs or low-dose cytarabine, and targeted agents, such as midostaurin (FLT3 inhibitor), gilteritinib (FLT3 inhibitor), ivosidenib (IDH1 inhibitor), enasidenib (IDH2 inhibitor), glasdegib (Hedgehog signaling pathway inhibitor), or gemtuzumab ozogamicin (anti-CD33 antibody conjugate).32,33 Together, these newer agents are associated with good tolerability profiles and/or improved efficacy for segments of the AML population and permit more personalized treatment plans. Additional clinical data are needed to further refine the optimal treatment paradigm.
Recently, 3 large real-world evidence studies have evaluated the safety and efficacy of CPX-351.34–36 In an Italian compassionate use program of CPX-351 in older adults with newly diagnosed t-AML or AML-MRC, treatment with CPX-351 resulted in a median OS of 16.1 months.34 In a French retrospective, multicenter analysis in patients with newly diagnosed t-AML or AML-MRC, median OS was not reached with CPX-351 treatment, with a 1-year OS rate of 69%.35 In a German retrospective, multicenter analysis in patients with newly diagnosed t-AML or AML-MRC, treatment with CPX-351 resulted in a median OS of 21 months.36 In all 3 studies, CPX-351 was associated with an acceptable safety profile, consistent with results from the phase 3 trial. However, these studies did not address the issue of HRU.
Several limitations of this real-world study warrant mention. As in all retrospective observational studies, patients were not randomly assigned into treatment cohorts; therefore, there could be selection bias in the estimated treatment effects. Nonetheless, the multivariable regressions were used to control for differences in patient, hospital, and clinical characteristics and to offset potential bias. Patients could be tracked across inpatient visits as well as outpatient and follow-up visits in hospital-affiliated centers; however, if a patient transferred out of the hospital system during treatment or follow-up, they may have been subsequently lost to follow-up, resulting in incomplete data for that patient. Also, when a patient was treated in a different hospital before index admission, previous diagnoses were not captured. The respective designations of t-AML and AML-MRC were defined according to the National Cancer Institute and WHO. Due to limitations of clinical data from the PHD, such as the lack of laboratory findings (ie, complete blood counts, bone marrow aspirations), identification of AML relied solely on diagnosis codes reported by the hospitals. The lack of this clinical detail, in combination with not all previous diagnoses being captured in the database, resulted in an “undetermined” cohort of AML patients who could not be assigned to t-AML or AML-MRC. Since patients in the 7+3 cohort were required to have an identifiable diagnosis of t-AML or AML-MRC, not all CPX-351–eligible patients treated with 7+3 could be identified and included in this study. In addition, given the limited sample size of patients in the CPX-351 cohort with evidence of newly diagnosed t-AML or AML-MRC captured in the database, it was assumed all CPX-351–treated patients had on-label use of the medication even if their AML subtype could not be specifically determined.
Conclusions
This study provided a real-world assessment of the current use of CPX-351 for the treatment of t-AML and AML-MRC in clinical oncology practice in the immediate 19 months following FDA approval. LOS and supportive care utilization from real-world data were consistent with the pivotal clinical trial data for patients treated with CPX-351. Inpatient LOS associated with the treatment of t-AML and AML-MRC was favorable for CPX-351, with 36.3% of the CPX-351 induction-strength treatment cycles occurring in the hospital-based outpatient setting. Regression analyses that were adjusted for patient, hospital, and clinical covariates revealed that treatment with CPX-351 compared to conventional 7+3 was associated with shorter inpatient days and comparable supportive care utilization. Future health economics and outcomes research should further investigate the clinical details and characterize the patients who received CPX-351 in the outpatient setting, as well as the effectiveness of CPX-351 treatment in the outpatient setting for both induction and consolidation cycles, specifically.
Abbreviations
AML, acute myeloid leukemia; AML-MRC, acute myeloid leukemia with myelodysplasia-related changes; CCI, Charlson-Deyo Comorbidity Index; CI, confidence interval; CMML, chronic myelomonocytic leukemia; FDA, US Food and Drug Administration; GLM, generalized linear model; HMA, hypomethylating agent; HRU, health care resource utilization; ICD-10-CM, International Classification of Diseases, 10th revision, Clinical Modification; LOS, length of stay; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; PHD, Premier Healthcare Database; OS, overall survival; RBC, red blood cell; t-AML, therapy-related acute myeloid leukemia; WBC-CSF, white blood cell colony-stimulating factor; WHO, World Health Organization.
Data Sharing Statement
Data from the Premier Healthcare Database are proprietary and cannot be shared externally.
Research Ethics and Patient Consent
This study is secondary research using a de-identified database (Premier Healthcare Database). It is not human subjects research and is considered exempt from Institutional Review Board oversight, as dictated by Title 45 Code of Federal Regulations, Part 46 of the United States, specifically 45 CFR 46.101(b)(4). In accordance with the HIPAA Privacy Rule, disclosed data from the PHD are considered de-identified per 45 CFR 164.506(d)(2)(ii)(B) through the “Expert Determination” method.
Acknowledgments
Ms. Carol Cohen, a senior medical writer formerly employed by Premier Applied Sciences, Premier Inc., provided writing, editing, and publication support. Dylan Supina of Jazz Pharmaceuticals provided comments and suggestions to the revision of the manuscript. Additional editorial assistance was provided by Cello Health Communications/SciFluent, and was financially supported by Jazz Pharmaceuticals. This work was presented in part at the American Society of Hematology 61st Annual Meeting and Exposition; December 7–10, 2019; Orlando, FL and the Hematology/Oncology Pharmacy Association 16th Annual Conference; March 11–14, 2020; Tampa, FL.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was funded by Jazz Pharmaceuticals.
Disclosure
Kwanza Price was an employee of Jazz Pharmaceuticals at the time of the study. Zhun Cao and Craig Lipkin are employees of Premier Applied Sciences, Premier Inc., which received payment from Jazz Pharmaceuticals to conduct the study. Deb Profant is an employee of Jazz Pharmaceuticals. Scott Robinson was an employee of Premier Inc. at the time of the study. The authors report no other conflicts of interest in this work.
References
1. American Cancer Society. Key statistics for acute myeloid leukemia (AML). Available from: https://www.cancer.org/cancer/acute-myeloid-leukemia/about/key-statistics.html.
2. Kuykendall A, Duployez N, Boissel N, Lancet JE, Welch JS. Acute myeloid leukemia: the good, the bad, and the ugly. Am Soc Clin Oncol Educ Book. 2018;38:555–573. doi:10.1200/EDBK_199519
3. Granfeldt Østgård LS, Medeiros BC, Sengeløv H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641–3649. doi:10.1200/JCO.2014.60.0890
4. Irish W, Ryan M, Gache L, Gunnarsson C, Bell T, Shapiro M. Acute myeloid leukemia: a retrospective claims analysis of resource utilization and expenditures for newly diagnosed patients from first-line induction to remission and relapse. Curr Med Res Opin. 2017;33(3):519–527. doi:10.1080/03007995.2016.1267615
5. Halpern AB, Culakova E, Walter RB, Lyman GH. Association of risk factors, mortality, and care costs of adults with acute myeloid leukemia with admission to the intensive care unit. JAMA Oncol. 2017;3(3):374–381. doi:10.1001/jamaoncol.2016.4858
6. Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–2692. doi:10.1200/JCO.2017.77.6112
7. Maakaron JE, Mims AS. Daunorubicin-cytarabine liposome (CPX-351) in the management of newly diagnosed secondary AML: a new twist on an old cocktail. Best Pract Res Clin Haematol. 2019;32(2):127–133. doi:10.1016/j.beha.2019.05.005
8. Chen EC, Fathi AT, Brunner AM. Reformulating acute myeloid leukemia: liposomal cytarabine and daunorubicin (CPX-351) as an emerging therapy for secondary AML. Onco Targets Ther. 2018;11:3425–3434. doi:10.2147/OTT.S141212
9. Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123(21):3239–3246. doi:10.1182/blood-2013-12-540971
10. Talati C, Lancet JE. CPX-351: changing the landscape of treatment for patients with secondary acute myeloid leukemia. Future Oncol. 2018;14(12):1147–1154. doi:10.2217/fon-2017-0603
11. Kolitz JE, Strickland SA, Cortes JE, et al. Consolidation outcomes in CPX-351 versus cytarabine/daunorubicin-treated older patients with high-risk/secondary acute myeloid leukemia. Leuk Lymphoma. 2020;61(3):631–640. doi:10.1080/10428194.2019.1688320
12. Villa KF, Ryan RJ, Chiarella M, Louie AC. Healthcare resource utilization in a phase 3 study of CPX-351 in patients with newly diagnosed high-risk/secondary acute myeloid leukemia. J Med Econ. 2020;23(7):714–720. doi:10.1080/13696998.2020.1744613
13. Premier Applied Sciences®. Premier Inc. Premier Healthcare Database: data that informs and performs. Available from: https://learn.premierinc.com/white-papers/premier-healthcare-database-whitepaper.
14. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544
15. Stahl M, DeVeaux M, Montesinos P, et al. Hypomethylating agents in relapsed and refractory AML: outcomes and their predictors in a large international patient cohort. Blood Adv. 2018;2(8):923–932. doi:10.1182/bloodadvances.2018016121
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi:10.1016/0895-4356(92)90133-8
18. Diehr P, Yanez D, Ash A, Hornbrook M, Lin DY. Methods for analyzing health care utilization and costs. Annu Rev Public Health. 1999;20:125–144. doi:10.1146/annurev.publhealth.20.1.125
19. Engel-Nitz NM, Alemayehu B, Parry D, Nathan F. Differences in treatment patterns among patients with castration-resistant prostate cancer treated by oncologists versus urologists in a US managed care population. Cancer Manag Res. 2011;3:233–245. doi:10.2147/CMR.S21033
20. DaCosta Byfield S, Nash Smyth E, Mytelka D, Bowman L, Teitelbaum A. Healthcare costs, treatment patterns, and resource utilization among pancreatic cancer patients in a managed care population. J Med Econ. 2013;16(12):1379–1386. doi:10.3111/13696998.2013.848208
21. Bell JA, Galaznik A, Farrelly E, et al. Economic burden of elderly patients with acute myeloid leukemia treated in routine clinical care in the United States. Leuk Res. 2018;71:27–33. doi:10.1016/j.leukres.2018.06.010
22. Meyer N, Hao Y, Landsman-Blumberg P, Johnson W, Juneau P, Willemann Rogerio J. Health care costs and utilization of a large insured female population with advanced or metastatic breast cancer by receipt of HER2-targeted agents. Comp Eff Res. 2015;5:21–34. doi:10.2147/CER.S62090
23. Weichle T, Hynes DM, Durazo-Arvizu R, Tarlov E, Zhang Q. Impact of alternative approaches to assess outlying and influential observations on health care costs. Springerplus. 2013;2:614. doi:10.1186/2193-1801-2-614
24. Basu A, Rathouz PJ. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6(1):93–109. doi:10.1093/biostatistics/kxh020
25. Li Z, Mahendra G. Using “recycled predictions” for computing marginal effects. SAS Global Forum 2010, Statistics and Data Analysis. Paper 272-2010. Available from: https://support.sas.com/resources/papers/proceedings10/272-2010.pdf.
26. Vyxeos dosing and administration information. Available from: https://vyxeospro.com/files/Vyxeos-Dosing-Brochure.pdf.
27. UpToDate®. Liposomal daunorubicin and cytarabine: drug information. Available from: https://www.uptodate.com/contents/liposomal-daunorubicin-and-cytarabine-drug-information?search=liposomal%20daunorubicin-cytarabine&source=panel_search_result&selectedTitle=1~7&usage_type=panel&kp_tab=drug_general&display_rank=1.
28. Krauss AC, Gao X, Li L, et al. FDA approval summary: (daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin Cancer Res. 2019;25(9):2685–2690. doi:10.1158/1078-0432.CCR-18-2990
29. Fedele PL, Avery S, Patil S, Spencer A, Haas M, Wei A. Health economic impact of high-dose versus standard-dose cytarabine induction chemotherapy for acute myeloid leukaemia. Intern Med J. 2014;44(8):757–763. doi:10.1111/imj.12478
30. Vaughn JE, Shankaran V, Walter RB. Trends in clinical benefits and costs of novel therapeutics in AML: at what price does progress come? Curr Hematol Malig Rep. 2019;14(3):171–178. doi:10.1007/s11899-019-00510-2
31. Jensen IS, Wu E, Sacks NC, Cyr PL, Chung KC. Budget impact analysis of using daunorubicin-cytarabine liposome in patients with newly diagnosed therapy-related AML or AML and myelodysplasia-related changes. Am Health Drug Benefits. 2018;11(7):380–386.
32. Urbino I, Secreto C, Olivi M, et al. Evolving therapeutic approaches for older patients with acute myeloid leukemia in 2021. Cancers (Basel). 2021;13(20):5075. doi:10.3390/cancers13205075
33. Saxena K, Konopleva M. New treatment options for older patients with acute myeloid leukemia. Curr Treat Options Oncol. 2021;22(5):39. doi:10.1007/s11864-021-00841-4
34. Chiche E, Rahmé R, Bertoli S, et al. Real-life experience with CPX-351 and impact on the outcome of high-risk AML patients: a multicentric French cohort. Blood Adv. 2021;5(1):176–184. doi:10.1182/bloodadvances.2020003159
35. Guolo F, Fianchi L, Minetto P, et al. CPX-351 treatment in secondary acute myeloblastic leukemia is effective and improves the feasibility of allogeneic stem cell transplantation: results of the Italian compassionate use program. Blood Cancer J. 2020;10(10):96. doi:10.1038/s41408-020-00361-8
36. Rautenberg C, Stolzel F, Rollig C, et al. Real-world experience of CPX-351 as first-line treatment for patients with acute myeloid leukemia. Blood Cancer J. 2021;11(10):164. doi:10.1038/s41408-021-00558-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

