Back to Journals » Clinical Ophthalmology » Volume 15
Comparison of Femtosecond Laser-Assisted Cataract Surgery and Conventional Phacoemulsification on Endothelial Cell Density When Using Torsional Modality
Authors Oka Y, Sasaki N, Injev VP
Received 31 July 2021
Accepted for publication 23 September 2021
Published 20 October 2021 Volume 2021:15 Pages 4227—4237
DOI https://doi.org/10.2147/OPTH.S329935
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract of "LenSx versus conventional cataract surgery" [ID 329935].
Views: 2694
Yoshitaka Oka,1 Noriyuki Sasaki,2 Valentine P Injev3
1Senshinkai Eye Institute, Iizuka-shi, Japan; 2Alcon Japan Ltd., Tokyo, Japan; 3Alcon Vision LLC, Lake Forest, CA, USA
Correspondence: Yoshitaka Oka
Senshinkai Eye Institute, 371-1 Kawazu, Iizuka-shi, Fukuoka-ken, 820-0067, Japan
Tel +81-948-22-5155
Email [email protected]
Purpose: To compare the effects of femtosecond laser assisted cataract surgery (FLACS) and manual phacoemulsification on cumulative dissipated energy (CDE), torsional amplitude, and endothelial cell density (ECD).
Patients and Methods: This prospective, randomized study was conducted at Oka Eye Clinic (Fukuoka, Japan). Surgeries were performed using FLACS (with LenSx) or conventional technique in adults ≥ 20 years with grade 2– 4 cataracts. Visits included preoperative, surgery day, and 5 postoperative visits (days 1, 4– 10, 20– 40, 60– 120, and 150– 210). Primary endpoint was CDE. Secondary endpoints included ECD percent change at day 150– 210 versus preoperative visit and average torsional amplitude on surgery day. Exploratory endpoints included central corneal thickness and corrected distance visual acuity (CDVA). Superiority of FLACS to conventional technique was evaluated using t-tests based on a mixed model for repeated measures.
Results: Full analysis set included 53 eyes per group. Mean cataract grade was 2.92± 0.58 in FLACS and 2.94± 0.57 in conventional group. FLACS versus conventional method had significantly lower mean CDE (0.213± 0.334 versus 1.718± 0.898%-seconds, respectively; P< 0.0001), demonstrating superiority of FLACS. Low endothelial cell loss (ECL) was achieved with both FLACS and conventional methods (1.5± 5.6% and 2.7± 5.2%; P=0.260). Torsional amplitude was significantly lower for FLACS versus conventional method (19.6± 16.0% versus 31.1± 6.6%; P< 0.0001). Central corneal thickness was comparable for both methods at all visits except day 1; CDVA was comparable for both methods at all postoperative visits.
Conclusion: FLACS achieved significantly lower CDE compared with the conventional surgical method (P< 0.0001). Low ECL was achieved with both FLACS (1.5%) and conventional (2.7%) methods.
Keywords: cumulative dissipated energy, endothelial cell loss, LenSx, torsional phaco
Introduction
Cataract is the leading cause of blindness worldwide; in 2010, cataract was reported to cause blindness in approximately 11 million people and visual impairment in 35 million people.1 An aging population and improved access to healthcare may contribute to an increase in the number of cataract surgeries performed each year. In some countries, the cataract surgery rate was up to 10,000 surgeries per million population in 1 year.2 There is a need to continuously improve cataract surgery performance to achieve optimal visual outcomes and reduce intraoperative trauma resulting from the surgical intervention.
Femtosecond laser assisted cataract surgery (FLACS) can improve surgical accuracy and efficiency by standardizing corneal incisions and improving centration, circularity, and intended diameter of the capsulotomy.3–5 Furthermore, lens fragmentation using FLACS resulted in lower effective phacoemulsification time compared with the conventional method.6 Indications for LENSX Laser System (Alcon Vision LLC), one of the commercially available FLACS surgery platforms, include anterior capsulotomy, nucleus fragmentation, and corneal incision. The LenSx system was demonstrated to be a safe and efficient method of performing corneal incisions, capsulorhexis, and lens fragmentation.7 In comparative studies, femtosecond lasers produced more precise and reproducible capsulorhexis and significantly lower IOL power calculation errors compared with manual procedures.8,9 Less energy and time were required for phacoemulsification using FLACS compared with conventional phacoemulsification.10−12 Additionally, better visual and refractive outcomes were reported with femtosecond lasers.13
The CENTURION Vision System (Alcon Vision LLC) is a widely used phacoemulsification system for cataract surgery. Lower phacoemulsification time and improved followability have been reported with torsional ultrasound compared with a phacoemulsification system operated in longitudinal mode.14 When using the torsional ultrasound, lower cumulative dissipated energy (CDE), shorter aspiration time, and lower use of aspiration fluid have been reported with an active-fluidics system compared with a gravity-fluidics system.15 CDE is the measurement of ultrasound energy at the incision site during phacoemulsification; lower CDE has been linked to better outcomes for endothelial cells after cataract surgery and may lead to better cornea recovery.16
Endpoints such as surgery-related endothelial cell loss (ECL) and increase in central corneal thickness can be assessed to measure the degree of traumatic effects from cataract surgery on the eye. ECL after cataract surgery can vary; some studies reported ECL between 1.2% and 17%,17 while in other studies ECL ranged from 4% to 25%.18 ECL correlated with a number of phacoemulsification parameters, including CDE, average phacoemulsification power and time, aspiration time, aspiration volume, and volume used of balanced salt solution.16 Increased risk of ECL and corneal edema associated with CDE highlight the importance of reducing CDE during phacoemulsification. FLACS has been shown to reduce the amount of required ultrasound energy, which may have an effect on ECL.12,19,20
This study assessed CDE and torsional amplitude on the cataract surgery day, postoperative outcomes after cataract surgery (ie, ECL), and compared FLACS capsulotomy and lens fragmentation using the FLACS versus manual cataract surgery (ie, conventional technique). The goal of the study was to examine if the use of FLACS improved CDE, torsional amplitude, and endothelial cell density (ECD) outcomes compared with manual phacoemulsification with torsional modality. The Centurion Vision System with identical phacoemulsification parameters was used for both methods.
Methods
Study Design
This was a prospective, randomized, observer-masked (specular microscope only) clinical trial (ClicalTrials.gov, NCT03479944) conducted at a single study site in Japan, August 22, 2018 to May 17, 2019, in accordance with the Declaration of Helsinki. An independent ethics committee, Non-Profit Organization MINS Institutional Review Board, reviewed and approved the clinical study protocol.
Included in the study were adults ≥20 years old with grade 2–4 cataracts per Emery-Little Classification and with planned cataract removal by phacoemulsification in both eyes. Subjects had potential Corrected Distance Visual Acuity (CDVA) of 0.7 decimal or better in the study eye. Excluded from the study were subjects with corneal opacity; hypotony or presence of a corneal implant; ocular or eyelid disease; corneal disease that precluded applanation of the cornea or transmission of laser light at 1030 nm; blood or other material in the anterior chamber, poorly dilating pupil, contraindications to cataract surgery, corneal ECD <2000 cells/mm2; peripheral iridotomy; two different grades of cataract in both eyes; expected ocular surgical treatment other than Nd:YAG capsulotomy; systemic or ophthalmic disease. Additionally, subjects could be excluded from the study during surgery if there were any additional procedures or interventions (ie, posterior capsule rupture, vitreous loss); incomplete continuous curvilinear capsulorhexis by LenSx; significant anterior chamber bleeding; uncontrolled intraocular pressure; or at the discretion of the surgeon because of clinical reasons.
The study was done in paired eyes from the same subject. Subjects were randomized using random assignment by the permutated block method, with 1 eye assigned to FLACS and the other eye to conventional techniques. Randomization information was masked to the specular microscope observer other than the surgeon.
Surgery
All surgeries were performed by the same experienced surgeon (Y.O.), using either FLACS or conventional surgical technique. For FLACS, the LenSx Laser System was used for the capsulorhexis and lens fragmentation, followed by manual corneal incision and phacoemulsification (Table 1). For the conventional surgical technique, initial corneal incision was followed by continuous curvilinear capsulorhexis and lens fragmentation using a manual stop and chop technique (Nagahara Phaco Chopper, ASICO) and phacoemulsification. Corneal incisions were performed manually in both groups to match conditions and minimize the differences in methods other than removal of the lens during phacoemulsification. Phacoemulsification after both FLACS and conventional techniques was performed using the Centurion Vision System with a balanced ultrasound tip (phaco power, 0%; torsional power, 50%; vacuum, 600 mmHg; aspiration flow, 30 mL/min; ultrasonic mode, continuous). A slit angled 2.65 mm arched knife (Mani, Inc.) was used to make a main incision and insert an UltraSert AcrySof IQ Single Piece intraocular lens (Alcon Vision LLC). During surgery, balanced salt solution PLUS Irrigating Solution 0.0184% (Alcon Vision LLC) was used. Ophthalmic viscoelastic device (OVD) was PROVISC (Alcon Vision LLC); other OVDs could be used if medically necessary.
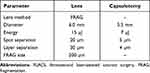 |
Table 1 FLACS Settings |
Assessments
Subjects were examined at the preoperative visit (within 60 days before surgery), the surgery day visit, and 5 postoperative visits (day 1, day 4–10, day 20–40, day 60–120, and day 150–210).
The primary endpoint was CDE, recorded after the ultrasound portion of the surgery. Total CDE = (longitudinal time × average longitudinal power) + (torsional time × 0.4 × average torsional amplitude), where 0.4 represents the approximate reduction of heat dissipated at the incision compared with conventional phacoemulsification.21
Secondary endpoints included percent change of ECD at day 150–210 compared with the preoperative visit and average torsional amplitude on surgery day. Specular microscopy (EM-3000 Specular Microscope, Tomey) was performed and central corneal ECD were assessed by the same masked observer both at the preoperative visit and the day 150–210 visit. To reduce variability of ECD assessments, the same instruments were used for all measurements at all site visits. Cell counting was done automatically by the instrument.
Slit lamp examinations were conducted at the preoperative visit and all postoperative visits. At the preoperative visit, lens condition was graded 1–5 by 2 independent specialists (other than the surgeon) according to the Emery-Little classification. Exploratory endpoints included central corneal thickness and CDVA, evaluated at the preoperative visit and all postoperative visits. CDVA was assessed under photopic lighting conditions using the decimal visual acuity chart.
Statistics
The full analysis set included eyes with successful cataract surgery, where anterior capsulotomy and lens fragmentation were completed using the assigned surgical technique. The primary endpoint (CDE) and secondary endpoints (percent change in ECD and torsional amplitude) were assessed using descriptive statistics for each surgical technique. Means were estimated using 95% confidence intervals based on a mixed model for repeated measures (MMRM), considering correlation between both eyes within a patient. Superiority of FLACS to the conventional technique was to be demonstrated using t-tests based on MMRM. The multiplicity of statistical tests for primary and secondary endpoints was adjusted in a sequential manner as follows. The primary endpoint (CDE) was tested first. Percent ECD change was tested only if there was a significant difference in CDE. Per planned analysis method, average torsional amplitude was to be tested for superiority only if there was a significant difference in percent ECD change. Because the percent change of ECD did not show a statistically significant difference between surgical techniques, the superiority testing was not performed. The sample size was determined according to this statistical analysis strategy, and overall type 1 error was controlled at one-sided 2.5%. Demographic data and exploratory effectiveness endpoints, including estimated aspiration fluid use, central corneal thickness, and CDVA, were summarized descriptively.
Safety
Adverse events (AEs) and device deficiencies were assessed at the surgery visit and all postoperative visits. Serious adverse events (SAEs), defined as an AE that led to death or serious deterioration in the health resulting in a life-threatening illness or injury, any potentially sight-threatening event, in-patient hospitalization, a congenital anomaly, or a medically important event, were evaluated by the investigator. Number and percentage of eyes with AEs after cataract surgery were presented by each surgical technique.
Results
Subject Characteristics
Of the 57 subjects enrolled in this study, 2 discontinued before surgery and 55 completed the study and were included in the safety analysis set. Two additional subjects were excluded from the full analysis set because surgery on both the first and second eyes was not performed according to planned randomization (Figure 1). In the full analysis set, 53 eyes were in the FLACS group and 53 eyes were in the conventional group. The mean cataract grade was 2.92±0.58 in the FLACS group and 2.94±0.57 in the conventional group. Grade 3 cataract was the most common type in both the FLACS (66%; 35/53) and conventional (68%; 36/53) groups. In the safety analysis set, 64% of subjects were female (35/55; Table 2), and the mean ± SD age was 73.2±6.6 years.
 |
Table 2 Subject Demographics and Baseline Characteristics |
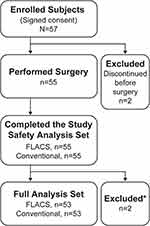 |
Figure 1 Subject disposition. *Surgery was not performed according to the randomization table. Abbreviation: FLACS, femtosecond laser-assisted cataract surgery. |
Cumulative Dissipated Energy
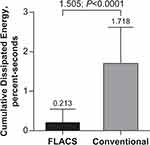
On the day of the surgery, mean ± SD CDE was 0.213±0.334%-seconds for FLACS (n=53) and 1.718±0.898%-seconds for conventional methods (n=53; Figure 2). The CDE least squares mean difference of 1.505%-seconds between the techniques was significant (P<0.0001), and superiority of FLACS versus conventional method for CDE was demonstrated.
Endothelial Cell Density
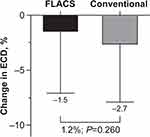
At the preoperative visit, mean ECD was 2629±236 cells/mm2 for FLACS (n=53) and 2634±229 cells/mm2 for conventional technique (n=53), and the least squares mean difference between the techniques was 4.2 cells/mm2 (P=0.848). At the day 150–210 visit, mean ECD was 2583±215 cells/mm2 for FLACS (n=53) and 2561±260 cells/mm2 for conventional technique (n=53), and the least squares mean difference between the techniques was 22 cells/mm2 (P=0.395). The percent ECL at the day 150–210 visit compared with the preoperative visit was 1.5±5.6% for FLACS (n=53) and 2.7±5.2% for conventional (n=53; Figure 3). Although ECL was numerically greater for conventional technique compared with FLACS, the 1.2% difference in ECL between the techniques was not significant (P=0.260).
Torsional Amplitude
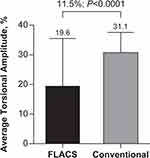
On the day of the surgery, mean torsional amplitude was 19.6±16.0% for FLACS (n=53) and 31.1±6.6% for conventional (n=53; Figure 4). The torsional amplitude was significantly smaller for FLACS versus conventional technique (least squares mean difference of 11.5%; P<0.0001). However, superiority was not demonstrated according to the stated analysis plan.
Exploratory Endpoints
Aspiration fluid usage on the day of the surgery was similar for both FLACS and conventional techniques. In the full analysis set, mean estimated aspiration fluid usage was 25.0±8.0 mL in the FLACS group versus 23.9±5.3 mL in the conventional group after the ultrasound and was 46.7±8.5 mL for FLACS versus 44.1±6.5 mL for conventional after the entire procedure.
Central corneal thickness in the full analysis set increased at the day 1 visit compared with the preoperative visit for both FLACS and conventional techniques. There was a significantly greater increase in mean central corneal thickness for subjects in conventional group versus those in the FLACS group (6.1 µm; P=0.0048; Figure 5). For both FLACS and conventional groups, central corneal thickness at 20–40 days, 60–120 days, and 150–210 days was similar to the preoperative visit; no significant differences were observed between FLACS and conventional techniques.
At the preoperative visit, CDVA ≥0.7 was reported for 59% of subjects in the FLACS group and 57% of subjects in the conventional group. Mean CDVA was 0.161±0.125 logMAR for the FLACS group (n=53) and 0.179±0.127 logMAR for the conventional group (n=53; Figure 6). At the 4–10-day visit and all later visits, all subjects achieved CDVA ≥0.7. Subjects in both the FLACS and conventional groups achieved comparable CDVA. At day 150–210 visit, mean CDVA was −0.029±0.040 logMAR for the FLACS group (n=53) and −0.024±0.045 logMAR for the conventional group (n=53).
Safety
In the FLACS group, ocular AEs were reported in 6/55 subjects (11%), including conjunctival haemorrhage, posterior capsule opacification, and cystoid macular edema (n=1 each; Table 3). In the conventional group, ocular AEs were reported in 2/55 subjects (4%), including posterior capsule opacification, vitreous prolapse, and ciliary zonular dehiscence (vitreous prolapse and ciliary zonular dehiscence occurred in the same subject; Table 3). Posterior capsular opacification was reported in the same subject, using either FLACS or conventional techniques. None of the ocular AEs were assessed as related to the device and none led to discontinuation from the study. No ocular SAEs were reported.
 |
Table 3 Subjects with Ocular AEs (Safety Analysis Set) |
Non-ocular AEs were reported in 3/55 subjects (6%; Table 4). Non-ocular SAEs included chondrocalcinosis pyrophosphate (1/55; 2%) and epilepsy (1/55; 2%). None of the non-ocular AEs were assessed as related to the study device or to the conduct of the study by the investigator and none led to discontinuation from the study. Device deficiency (patient interface docking failure) was reported in 1 subject in the FLACS group. This was resolved and there were no related health hazards or AEs.
 |
Table 4 Subjects with Non-Ocular AEs and SAEs |
Discussion
In this study, significantly lower CDE levels and torsional amplitude were achieved by subjects receiving phacoemulsification by the FLACS (with LenSx) surgical technique compared with the conventional surgical technique (P<0.0001 for both), and superiority of FLACS was demonstrated for CDE. Low ECL was observed with both techniques (1.5% for FLACS and 2.7% for conventional) at 150 to 210 days after surgery. Although central corneal thickness was significantly smaller for FLACS versus conventional technique at the day 1 visit, it was comparable for both techniques at all other visits. Additionally, CDVA was comparable for both techniques at all postoperative visits.
The lower levels of CDE achieved with FLACS compared with conventional techniques were consistent with previous reports. A recent observational retrospective study in 735 eyes found that mean CDE during phacoemulsification was significantly lower for FLACS versus conventional techniques in subjects with grade 4 cataracts (P=0.05).10 A prospective comparative nonrandomized study in 570 eyes reported that mean CDE was significantly lower in FLACS versus conventional group when using either a Centurion active-fluidics phacoemulsification platform or INFINITI (Alcon Vision LLC) gravity-fluidics platform (P=0.0008 and P=0.0003, respectively).12 Recent meta-analyses addressing efficacy and safety of FLACS similarly reported lower CDE for subjects in the FLACS group compared with those in the conventional technique group.22,23
The ECL rates of 1.5% to 2.7% observed in the current study were substantially lower than rates of 6.4% to 18.4% reported in other studies.24 There are a number of parameters that can contribute to the ECL risk after cataract surgery. A regression analysis of ECL in 50 patients after routine cataract surgery found that phacoemulsification time was a significant factor for ECL (P<0.01).25 Shallow anterior chamber depth (ACD) and short axial length also contributed to the risk of ECL (P=0.02 and P=0.001, respectively).25 In patients with grade NO3 and NO4 cataracts, ACD ≤2.5 mm resulted in significantly greater ECL compared with ACD between 3.5 and 4.5 mm (P<0.05 for both).26 The OVD type can also affect ECL. In a study that assessed OVDs, the use of dispersive versus cohesive viscoelastic resulted in 1.2% versus 9.6% ECL, respectively (P<0.0001).27 A study that assessed FLACS versus conventional technique in 400 patients using the previous generation Infiniti system reported greater mean ECL using FLACS (10.2±13.7%) compared with the conventional technique (9.7±13.7%).28 The mean ECL was substantially greater using the Infinity compared with the Centurion in the current study, suggesting better fluidics efficiency with the latest generation system. The Centurion system, which can be operated at higher vacuum settings, can use significantly less aspiration fluid and provide better surgical efficiency compared with the Infinity system.15,29,30
Previous studies showed mixed results for effects of torsional phacoemulsification on ECL. The use of torsional versus conventional ultrasound resulted in 3.2% versus 7.9% ECL, respectively, 1 month after surgery in patients with moderate cataract and 23.5% versus 13.5% ECL, respectively, in patients with hard cataract.31 Another study reported ECL of 4.2% in torsional versus 6.7% in conventional groups 2 months after surgery.32 Torsional phacoemulsification can result in shorter cumulative tip travel and shorter procedure time, indicating increased nuclear follow ability. Collectively, these surgical technique improvements may lead to increased phacoemulsification efficiency and safety.33
Although ECL can be affected by multiple factors, it may be feasible to evaluate how technique, equipment, accessories, and other components contribute to favourable ECL outcomes with both FLACS and manual phacoemulsification methods. The surgical parameters used in the current study likely contributed to low CDE that resulted in ECL that was considerably below previously reported levels, regardless of surgical method.
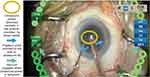
In this study, the eye was stabilized to maintain the correct biomechanical orientation when creating the incision and to avoid trauma to the adjacent Descemet’s membrane. The VERION Image Guided System (Alcon Vision LLC) contributed as a visual guiding tool, allowing the incision to be at the correct axis. The use of a balanced tip designed specifically for torsional phacoemulsification allowed low movement at the incision site and efficient amplification of movement at the distal end, contributing to low CDE. Linear control of power with a delineated foot pedal movement control made it possible to use the minimum amount of energy for the cataract removal. At the torsional maximum ultrasound power setting of 50%, CDE at the incision site was low for an average grade cataractous material. Delivering ultrasound energy centrally to the anterior chamber, just below the iris plane, may decrease the risks of potential complications when emulsification and aspiration of cortical materials occur near the posterior capsule or too close to the cornea. The ultrasound tip was positioned with the bevel facing down to reduce effects of cavitation and minimize turbulence, which may help protect the endothelium and contribute to low ECL.34 The irrigation ports were oriented to the side of the ultrasound tip and vacuum was engaged when ultrasound power was delivered (Figure 7), improving efficiency of the phacoemulsification procedure and optimizing the use of ultrasound energy. The combination of FLACS and Centurion that resulted in small fragmentation size (200 µm) facilitated easier aspiration of the lens and could have also contributed to the reduction of CDE and ECL.
Limitations of this study include a relatively small number of subjects evaluated and a single-site study design. Superiority of ECL was not demonstrated in this study because the difference between surgical techniques (1.2%) was smaller than initially forecast (5.5%). A larger sample size would be needed to statistically identify a 1.2% difference. Additional studies assessing effects of FLACS on CDE and ECL in larger populations in general practice settings are needed, as well as further evaluation of the phacoemulsification parameters and their effects on surgical outcomes. Finally, evaluation of cost-effectiveness of FLACS versus the conventional surgical approach was beyond the scope of this study. In addition to further evaluation of clinical benefits of FLACS in general practice settings, evaluation of the cost-effectiveness of FLACS would be of value.
In conclusion, phacoemulsification using the FLACS (with LenSx) surgical technique achieved significantly lower cumulative dissipated energy levels (P<0.0001) and torsional amplitude (P<0.0001) compared with the conventional method. Low ECL was achieved with both FLACS and conventional phacoemulsification (1.5% and 2.7%, respectively) when using low phacoemulsification energy and selecting an efficient ultrasound tip and modality; proficient surgical technique and efficient use of fluidics and OVD can also contribute to low ECL.
Data Sharing Statement
Study data not reported in this publication are confidential.
Acknowledgments
This study was funded by Alcon Vision LLC. Medical writing assistance was provided by Natalia Zhukovskaya, PhD, of ICON (North Wales, PA), and was funded by Alcon.
Funding
Research funding was provided by Alcon Japan Ltd.
Disclosure
VP Injev and N Sasaki are Alcon employees. Y Oka received grant support from Alcon Japan Ltd. The authors report no other conflicts of interest in this work.
References
1. Khairallah M, Kahloun R, Bourne R, et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis Sci. 2015;56(11):6762–6769. doi:10.1167/iovs.15-17201
2. Wang W, Yan W, Fotis K, et al. Cataract surgical rate and socioeconomics: a global study. Invest Ophthalmol Vis Sci. 2016;57(14):5872–5881.
3. Nagy ZZ, Kranitz K, Takacs AI, et al. Comparison of intraocular lens decentration parameters after femtosecond and manual capsulotomies. J Refract Surg. 2011;27(8):564–569.
4. Reddy KP, Kandulla J, Auffarth GU. Effectiveness and safety of femtosecond laser-assisted lens fragmentation and anterior capsulotomy versus the manual technique in cataract surgery. J Cataract Refract Surg. 2013;39(9):1297–1306.
5. Titiyal JS, Kaur M, Singh A, Arora T, Sharma N. Comparative evaluation of femtosecond laser-assisted cataract surgery and conventional phacoemulsification in white cataract. Clin Ophthalmol. 2016;10:1357–1364.
6. Conrad-Hengerer I, Hengerer FH, Schultz T, Dick HB. Effect of femtosecond laser fragmentation on effective phacoemulsification time in cataract surgery. J Refract Surg. 2012;28(12):879–883.
7. Day AC, Burr JM, Bennett K, et al. Femtosecond laser-assisted cataract surgery versus phacoemulsification cataract surgery (FACT): a randomized noninferiority trial. Ophthalmology. 2020;127(8):1012–1019.
8. Filkorn T, Kovacs I, Takacs A, et al. Comparison of IOL power calculation and refractive outcome after laser refractive cataract surgery with a femtosecond laser versus conventional phacoemulsification. J Refract Surg. 2012;28(8):540–544.
9. Kranitz K, Takacs A, Mihaltz K, et al. Femtosecond laser capsulotomy and manual continuous curvilinear capsulorrhexis parameters and their effects on intraocular lens centration. J Refract Surg. 2011;27(8):558–563.
10. Ang RET, Quinto MMS, Cruz EM, Rivera MCR, Martinez GHA. Comparison of clinical outcomes between femtosecond laser-assisted versus conventional phacoemulsification. Eye Vis. 2018;5(1):8. doi:10.1186/s40662-018-0102-5
11. Chen X, Xiao W, Ye S, Chen W, Liu Y. Efficacy and safety of femtosecond laser-assisted cataract surgery versus conventional phacoemulsification for cataract: a meta-analysis of randomized controlled trials. Sci Rep. 2015;5(1):13123. doi:10.1038/srep13123
12. Yesilirmak N, Diakonis VF, Sise A, et al. Differences in energy expenditure for conventional and femtosecond-assisted cataract surgery using 2 different phacoemulsification systems. J Cataract Refract Surg. 2017;43(1):16–21.
13. Nagy ZZ, Mastropasqua L, Knorz MC. The use of femtosecond lasers in cataract surgery: review of the published results with the LenSx system. J Refract Surg. 2014;30(11):730–740.
14. Christakis PG, Braga-Mele RM. Intraoperative performance and postoperative outcome comparison of longitudinal, torsional, and transversal phacoemulsification machines. J Cataract Refract Surg. 2012;38(2):234–241.
15. Solomon KD, Lorente R, Fanney D, Cionni RJ. Clinical study using a new phacoemulsification system with surgical intraocular pressure control. J Cataract Refract Surg. 2016;42(4):542–549.
16. Mahdy MA, Eid MZ, Mohammed MA, Hafez A, Bhatia J. Relationship between endothelial cell loss and microcoaxial phacoemulsification parameters in noncomplicated cataract surgery. Clin Ophthalmol. 2012;6:503–510.
17. Rosado-Adames N, Afshari NA. The changing fate of the corneal endothelium in cataract surgery. Curr Opin Ophthalmol. 2012;23(1):3–6.
18. Ho JW, Afshari NA. Advances in cataract surgery: preserving the corneal endothelium. Curr Opin Ophthalmol. 2015;26(1):22–27.
19. Abell RG, Kerr NM, Howie AR, et al. Effect of femtosecond laser-assisted cataract surgery on the corneal endothelium. J Cataract Refract Surg. 2014;40(11):1777–1783.
20. Saeedi OJ, Chang LY, Ong SR, et al. Comparison of cumulative dispersed energy (CDE) in femtosecond laser-assisted cataract surgery (FLACS) and conventional phacoemulsification. Int Ophthalmol. 2019;39(8):1761–1766.
21. Chen M, Anderson E, Hill G, Chen JJ, Patrianakos T. Comparison of cumulative dissipated energy between the Infiniti and Centurion phacoemulsification systems. Clin Ophthalmol. 2015;9:1367-1372.
22. Kolb CM, Shajari M, Mathys L, et al. Comparison of femtosecond laser-assisted cataract surgery and conventional cataract surgery: meta-analysis and systematic review. J Cataract Refract Surg. 2020;46(8):1075–1085.
23. Popovic M, Campos-Moller X, Schlenker MB, Ahmed IIK. Efficacy and safety of femtosecond laser-assisted cataract surgery compared with manual cataract surgery: a meta-analysis of 14 567 eyes. Ophthalmology. 2016;123(10):2113–2126.
24. Fea AM, Consolandi G, Pignata G, et al. A comparison of endothelial cell loss in combined cataract and MIGS (Hydrus) procedure to phacoemulsification alone: 6-month results. J Ophthalmol. 2015;2015:769289.
25. Walkow T, Anders N, Klebe S. Endothelial cell loss after phacoemulsification: relation to preoperative and intraoperative parameters. J Cataract Refract Surg. 2000;26(5):727–732.
26. Hwang HB, Lyu B, Yim HB, Lee NY. Endothelial cell loss after phacoemulsification according to different anterior chamber depths. J Ophthalmol. 2015;2015:210716.
27. Moschos MM, Chatziralli IP, Sergentanis TN. Viscoat versus Visthesia during phacoemulsification cataract surgery: corneal and foveal changes. BMC Ophthalmol. 2011;11:9.
28. Roberts HW, Wagh VK, Sullivan DL, et al. A randomized controlled trial comparing femtosecond laser-assisted cataract surgery versus conventional phacoemulsification surgery. J Cataract Refract Surg. 2019;45(1):11–20.
29. Ting DSJ, Rees J, Ng JY, Allen D, Steel DHW. Effect of high-vacuum setting on phacoemulsification efficiency. J Cataract Refract Surg. 2017;43(9):1135–1139.
30. Sharif-Kashani P, Fanney D, Injev V. Comparison of occlusion break responses and vacuum rise times of phacoemulsification systems. BMC Ophthalmol. 2014;14:96.
31. Kim DH, Wee WR, Lee JH, Kim MK. The comparison between torsional and conventional mode phacoemulsification in moderate and hard cataracts. Korean J Ophthalmol. 2010;24(6):336–340.
32. Bozkurt E, Bayraktar S, Yazgan S, et al. Comparison of conventional and torsional mode (OZil) phacoemulsification: randomized prospective clinical study. Eur J Ophthalmol. 2009;19(6):984–989.
33. Davison JA. Cumulative tip travel and implied followability of longitudinal and torsional phacoemulsification. J Cataract Refract Surg. 2008;34(6):986–990.
34. Coelho RP, Raskin E, Paula JS, Cruz AA. Tip position during phaco. Ophthalmology. 2008;115(12):2315–2315 e2311.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.