Back to Journals » Clinical Interventions in Aging » Volume 17
Comparison of Clinical Outcomes Between Ticagrelor and Clopidogrel in Elderly Patients Undergoing Percutaneous Coronary Intervention: A Cohort Study
Authors Meng S , Guo L , Ye Z, Wang J, Ding H, Wu S, Huang R
Received 4 January 2022
Accepted for publication 10 March 2022
Published 2 April 2022 Volume 2022:17 Pages 331—341
DOI https://doi.org/10.2147/CIA.S355210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Shaoke Meng,1,* Lei Guo,1,* Zhishuai Ye,2 Junjie Wang,1 Huaiyu Ding,1 Shanshan Wu,3 Rongchong Huang2
1Department of Cardiology, The First Affiliated Hospital of Dalian Medical University, Dalian City, People’s Republic of China; 2Cardiac Center/Division of Cardiovascular Diseases, Beijing Friendship Hospital, Capital Medical University, Beijing City, People’s Republic of China; 3Department of Clinical Epidemiology and EBM, Beijing Friendship Hospital, Capital Medical University, Beijing City, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Rongchong Huang, Department of Cardiology, Beijing Friendship Hospital, Capital Medical University, 95 Yong’an Road, Beijing City, 100053, People’s Republic of China, Email [email protected]
Background: Age is a strong predictor of adverse outcomes due both to a higher risk of bleeding and ischemia. The purpose of this study was to evaluate the safety and efficacy of ticagrelor in elderly patients.
Methods: Patients ≥ 75 years of age admitted to our center from January, 2015 to December, 2019 who had undergone percutaneous coronary intervention (PCI) and received dual antiplatelet therapy (DAPT) were included in our study. Eligible patients were divided into clopidogrel and ticagrelor groups according to the P2Y12 receptor inhibitor and were followed up for 1 year. The primary safety endpoint was types 2, 3, and 5 bleeding, as defined by Bleeding Academic Research Consortium (BARC), and the primary efficacy endpoint was combined major adverse cardiovascular and cerebrovascular events (MACCEs). A Cox proportional hazard model and propensity score matching were used to correct confounding factors.
Results: Of 1505 patients enrolled in this study, 442 were assigned to ticagrelor group and 1063 were assigned to clopidogrel group. The incidence of BARC 2, 3, and 5 bleeding (HR, 2.304; 95% CI, 1.540– 3.447), and any bleeding (HR, 2.476; 95% CI, 1.802– 3.403) in ticagrelor group was significantly higher than clopidogrel group. There were no significant difference between the two groups with respect to BARC 3 and 5 bleeding (HR, 1.566; 95% CI, 0.767– 3.198) and MACCEs (HR, 0.957; 95% CI, 0.702– 1.305).
Conclusion: Compared with clopidogrel, DAPT with ticagrelor significantly increased the risk of BARC 2, 3, and 5 bleeding without reducing MACCEs in elderly patients who underwent PCI.
Trial Registration: The study was retrospectively registered in clinicaltrials.gov (NCT 04999293).
Keywords: ticagrelor, elderly, percutaneous coronary intervention, dual antiplatelet therapy
Introduction
Platelet activation and aggregation, followed by thrombosis, have important roles in the occurrence of coronary ischemic events. Compared with fibrinolytic therapy and medical treatment, percutaneous coronary intervention (PCI) has significantly improved the prognosis of patients presenting with acute coronary syndrome (ACS); however, PCI may result in iatrogenic plaque rupture and stent implantation, both of which are the potential basis of ischemic events. Therefore, antiplatelet therapy is the cornerstone of drug treatment for patients undergoing PCI.
Since the results of the Percutaneous Coronary Intervention–Clopidogrel in Unstable angina to prevent Recurrent Events (PCI-CURE) study were published, dual antiplatelet therapy (DAPT [aspirin combined with clopidogrel]) has become the standard regimen for antithrombotic therapy within 1 year after PCI.1 Clopidogrel is a prodrug that requires hepatic conversion into an active metabolite to exert its antiplatelet response. After absorption from the intestine, approximately 85% of the prodrug is inactivated by carboxylase, and only 15% is metabolized by hepatic cytochrome (CYP) P450 enzyme in a 2-step process.2 A number of studies have suggested a significant interindividual variation in the clopidogrel response mainly due to a CYP2C19 polymorphism.3,4 As a new type of platelet P2Y12 receptor inhibitor, ticagrelor is an active drug does not require metabolism in the liver, and can trigger a more rapid, powerful, and stable anti-platelet aggregation effect.5 The Platelet Inhibition and Patient Outcomes (PLATO) trial showed that ticagrelor combined with aspirin significantly reduced the risk of ischemia compared to clopidogrel in ACS patients, albeit with a greater risk of non-coronary artery bypass graft (CABG)-related major bleeding but without an increase in the incidence of fatal bleeding.6 At present, all relevant guidelines recommend the use of ticagrelor for patients with high-risk coronary heart disease.7,8
Elderly patients often have multiple chronic co-morbidities, changes in multiple organs, slow drug metabolism, and other factors that significantly increase the risk of bleeding and ischemia. Accordingly, older age is included in a variety of ischemic and bleeding risk scores. At the same time, a number of studies have shown that perioperative bleeding in patients undergoing PCI and long-term use of antithrombotic drugs were associated with increased risks of death, recurrent myocardial infarction (MI), stent thrombosis, and stroke, which makes antithrombotic treatment in the elderly challenging.9
The mean global age is increasing year-after-year. Patients ≥ 75 years of age account for greater than one-third of hospitalizations and two-thirds of deaths due to ACS.10 Although elderly patients represent the fastest-growing patient subgroup undergoing PCI, elderly patients tend to be underrepresented in randomized clinical trials.11 The purpose of this study was to determine the safety and efficacy of ticagrelor in elderly patients with coronary heart disease undergoing PCI in a real-world setting.
Methods
Study Population
Patients ≥ 75 years of age who were treated with PCI (limited to stent implantation) at our center from January 1, 2015 to December 31, 2019 were screened for additional enrollment criteria. Patients were survivors and treated with DAPT (aspirin [100 mg once daily], cilostazol, or indobufen combined with a P2Y12 receptor antagonist [clopidogrel (75 mg once daily) or ticagrelor (90 mg twice daily)]) at the time of hospital discharge. The exclusion criteria were as follows: (1) coronary artery bypass graft (CABG) or only drug conservative treatment during hospitalization; (2) concurrent use of oral anticoagulants; (3) inability to tolerate long-term antiplatelet therapy, such as active bleeding and a bleeding tendency; (4) acute infectious diseases; and (5) cognitive impairment, declined re-examination, and lost to follow-up. Our center has a standardized follow-up system. All patients receiving PCI treatment received detailed secondary prevention education at the time of hospital discharge and were required to keep regular follow-up appointments 1, 3, 6, 9, and 12 months after PCI in the coronary heart disease specialist clinic. The study was approved by institutional review boards of the First Affiliated Hospital of Dalian Medical University (reference number: PJ-KS-KY-2021-146) in accordance with the principles of the Declaration of Helsinki. The data are anonymous, and the requirement for informed consent was therefore waived owing to the retrospective and observational nature of the study.
Group
The clinical data were retrospectively analyzed and divided into clopidogrel and ticagrelor groups according to the P2Y12 receptor inhibitor used in DAPT at the time of hospital discharge. To analyze the effect of ticagrelor on ischemic events and bleeding, an intention-to-treat analysis was performed. Although some patients were permitted to switch from ticagrelor-to-clopidogrel or ticagrelor-to-clopidogrel during follow-up, such patients remained in their original group.
Definition of Clinical Outcomes
All patients were followed for 1 year in the outpatient clinic after hospital discharge. Endpoint events were recorded and collected through the medical data intelligent platform system of Yidu cloud for rehospitalization and outpatient medical records. Telephone follow-up was available to confirm relevant information as needed.
The primary safety endpoint of the study was types 2, 3, and 5 bleeding, as defined by the Bleeding Academic Research Consortium (BARC).12 The primary efficacy endpoint was the combined major adverse cardiovascular and cerebrovascular events (MACCEs), including all-cause mortality, myocardial infarction, ischemic stroke, and any revascularization. Secondary end points were all-cause mortality, myocardial infarction, revascularization, ischemic stroke (ie, the incidence of individual components of the MACCEs), the incidence of BARC 3 and 5 bleeding, major and minor bleeding defined by the PLATO study,13 major and minor bleeding defined by the thrombolysis in myocardial infarction (TIMI) study,14 and the occurrence of any bleeding.
The diagnostic criteria for coronary heart disease subtypes were based on the relevant guidelines.7,8,15 Multivessel disease was defined as ≥ 2 coronary arteries with a lumen stenosis ≥ 70%.
Statistical Analysis
Continuous variables were compared using the independent sample t-test or Mann–Whitney rank-sum test and are presented as the mean ± standard deviation or as the median and interquartile range. Categorical variables were analyzed using Pearson’s chi-square test or Fisher’s exact test and are expressed as counts with percentages. Kaplan Meier survival curves were constructed to describe the cumulative incidence of each end point and assessed by a Log rank test. Cox proportional hazard regression model was used to construct univariate and multivariate survival analyses and identify influencing factors of MACCEs and bleeding. Covariates with p values < 0.10 in univariate analysis or potentially important clinically-relevant factors including age, gender, weight, hypertension, diabetes, old myocardial infarction(OMI), the estimated glomerular filtration rate (eGFR), PCI, CABG, ischemic stroke, atrial fibrillation (AF)/atrial flutter (AFL), left ventricle ejection fraction (LVEF), malignant tumors, gastrointestinal diseases, prior bleeding, multivessel disease, and hemoglobin concentration were considered to be candidate variables to be included in multivariate analysis. Binary logistic regression was used to construct propensity scores with a caliper = 0.05. The two groups were matched with a 1:1 propensity score by the nearest matching method using variables shown in Table 1. Statistical analysis was performed using SPSS (version 25.0; IBM SPSS Inc., Chicago, IL, USA) and R (version 3.3.3; https://cran.r-project.org) software. All analyses were 2-tailed and a P-value <0.05 was considered statistically significant.
 |
Table 1 Baseline Clinical Characteristics of Study Population |
Results
Study Population and Baseline Clinical Characteristics
Of 1505 patients enrolled in this study, 442 were assigned to the ticagrelor group and 1063 were assigned to the clopidogrel group (Figure 1). The clinical baseline data of the enrolled patients are shown in Table 1. Of the patients, 722 (48.0%) were ≥ 80 years of age, 43.6% were women, and most patients (>99%) were diagnosed with ACS and 64.8% present with myocardial infarction. As shown in Table 1, the clopidogrel group had a higher proportion of women, and older age, a lower eGFR, lower hemoglobin concentration, non-high-density lipoprotein-cholesterol (HDL-C), and LVEF evaluated by echocardiography and were more complicated by a history of hypertension, ischemic stroke, AF/AFL, gastrointestinal diseases, and prior bleeding, while in the ticagrelor group, left main lesions were more common.
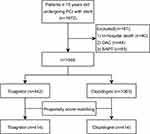 |
Figure 1 Flow chart of study. Abbreviations: OAC, oral anticoagulation; PCI, percutaneous coronary intervention; SAPT, single antiplatelet therapy. |
Clinical Outcomes and Multivariate Cox Proportional Hazard Analysis
As shown in Table 2, during the 12-month follow-up 208 patients (13.8%) developed MACCEs. There was no significant difference in MACCEs (adjusted HR, 0.957; 95% CI, 0.702–1.305; p=0.782) and its independent endpoints (all-cause death, myocardial infarction, revascularization, and ischemic stroke) between the two groups based on multivariate Cox regression analysis.
 |
Table 2 Clinical Outcomes and Hazard Ratio in Ticagrelor as Compared with Clopidogrel Group in Elderly Patients Undergoing Percutaneous Coronary Intervention |
Bleeding occurred in 159 cases (10.6%). The incidence of different types of bleeding is shown in Tables 2 and 3. The incidence of BARC2, 3, and 5 bleeding (10.6% vs 4.9%; adjusted HR, 2.304; 95% CI, 1.540–3.447; p<0.001), PLATO major and minor bleeding (10.2% vs 4.7%; adjusted HR, 2.296; 95% CI, 1.522–3.464; p<0.001), and any bleeding (17.6% vs 7.6%; adjusted HR, 2.476; 95% CI, 1.802–3.403; p < 0.001) in the ticagrelor group were significantly higher than the clopidogrel group. There was no significant difference between the two groups with respect to BARC 3 and 5 bleeding (2.9% vs 1.9%; adjusted HR, 1.566; 95% CI, 0.767–3.198; p=0.218) and TIMI major and minor bleeding (2.9% vs 2.1%; adjusted HR, 1.511; 95% CI, 0.750–3.045; p=0.248).
 |
Table 3 Bleeding Outcomes According to Different BARC Types and Sites in Elderly Patients Receiving Dual Antiplatelet Therapy After Percutaneous Coronary Intervention |
Results from the Propensity Score Matched Approach
Eight hundred twenty-eight patients (414 pairs) were obtained after propensity score matching. A standard deviation histogram (Supplementary Figure 1), univariate SD scatter plot (Supplementary Figure 2), and standardized difference change line (Supplementary Figure 3) showed that the two groups were well-matched.
The cumulative incidence of BARC 2, 3, and 5 bleeding, BARC 3 and 5 bleeding, MACCEs, death, myocardial infarction, ischemic stroke, and revascularization is depicted by Kaplan-Meier survival curves (Figures 2 and 3). The Log rank test showed that there was a significance difference between the two groups for BARC 2, 3, and 5 bleeding (Figure 2). There was no significant difference in the cumulative incidence of BARC 3 and 5 bleeding, MACCEs, and the individual components of MACCES between the two groups (Figures 2 and 3). Analysis by propensity score matching (414 pairs) did not significantly affect the results (Table 2). Compared with clopidogrel, DAPT based on ticagrelor significantly increased the risk of BARC 2, 3, and 5 bleeding (HR, 2.325; 95% CI, 1.565–3.456; p=0.006) without reducing MACCEs (HR, 0.922; 95% CI, 0.645–1.317; p=0.656).
Risk Factors for MACCEs and Bleeding Events
By constructing a Cox proportional regression model, variables with a P < 0.10 on univariate analysis (ticagrelor use, mineralocorticoid receptor antagonist [MRA], ischemic stroke, gastrointestinal diseases, prior bleeding, and malignant tumors) and important clinical factors (age, sex, weight, hemoglobin concentration, eGFR, and proton pump inhibitor [PPI]/Histamine 2 receptor antagonist [H2RA]) were included in the multivariate regression analysis. Use of ticagrelor, a history of bleeding, and malignant tumors were associated with an increased risk of types 2, 3, and 5 bleeding, as defined by BARC (Supplemental Table 1). Similarly, variables with a P<0.10 based on univariate analysis (MRA, type of coronary heart disease, AF/AFL, diabetes, OMI, ischemic stroke, peripheral artery disease [PAD], hemoglobin concentration, eGFR, single- and/or multi-vessel disease, and LVEF) and clinically important factors (age and malignant tumors) were included in multivariate regression analysis for MACCEs. After adjusting for confounding factors, OMI, ischemic stroke, PAD, eGFR, and multivessel disease were independent risk factors for MACCEs (Supplemental Table 2).
Discussion
In the present study 1505 patients with coronary heart disease ≥ 75 years of age who had successfully undergone PCI and received DAPT were divided into ticagrelor and clopidogrel groups according to the P2Y12 inhibitor used in the DAPT regimen. MACCEs and the MACCEs individual endpoints were not significantly different between the two groups during the 12-month follow-up period. The incidence of BARC 2, 3, and 5 bleeding, PLATO major and minor bleeding, and any bleeding was significantly higher in the ticagrelor group than the clopidogrel group. There was no significant difference in BARC 3 and 5 bleeding, and TIMI major and minor bleeding between the two groups.
Antiplatelet therapy has an essential role in drug treatment after PCI. Compared with clopidogrel, the guidelines recommend the use of more effective P2Y12 inhibitors (ticagrelor or prasugrel) in high-risk coronary heart disease patients who have undergone PCI treatment, especially ACS to prevent recurrent atherosclerotic thrombotic events.7,8 Compared with clopidogrel, the use of prasugrel and ticagrelor comes at the cost of increased bleeding, which may offset the ischemic benefits in a more vulnerable patient population. Elderly patients are a representative population in whom the risks of ischemia and bleeding complications after ACS are higher.
The PLATO study showed that despite the increase in non-CABG-related bleeding events in the ticagrelor group, the incidence of fatal bleeding was not increased, and the net adverse clinical events (NACE) was still better than clopidogrel.6 The PLATO subgroup analysis of elderly patients showed that among ACS patients, the clinical benefit and safety of the ticagrelor group did not depend on age compared with clopidogrel.16 However, the external authenticity of randomized control trials (RCTs) is low. For a long time, elderly patients have been underrepresented in clinical studies. Less than 10% of ACS trials recruited patients ≥ 75 years of age and the enrolled patients often had fewer co-morbidities that did not fully reflect the real-world situation.11 The proportion of patients ≥ 75-years-old in the PLATO studies was 15%,6 which was much lower than the 28–38% in the NRMI,17 CRUSADE,18 and GRACE registration studies.19 The efficacy and safety of elderly patients in real-world studies need further evaluation.
The POPular AGE study is the only RCT that compared the safety and clinical net benefits of antiplatelet drugs in elderly ACS patients.20 Due to slow recruitment, the age criterion was adjusted from ≥ 75 years to ≥ 70 years of age 6 months after enrollment commenced. In the end, only 326 patients ≥ 75 years of age were treated with ticagrelor. Studies have shown that compared with ticagrelor or prasugrel, clopidogrel significantly reduced the occurrence of PLATO-defined major and minor bleeding (HR, 0.71; 95% CI, 0.54–0.94; P=0.02) with comparable efficacy in non-ST-elevation acute coronary syndrome (NSTE-ACS) patients ≥ 70 years of age.
A registered study from Germany evaluated the efficacy of ticagrelor in 1087 ST-segment elevation myocardial infarction (STEMI) patients ≥ 75 years of age.21 The study showed that compared with clopidogrel, ticagrelor reduced the combined ischemic endpoint events by 31% (HR, 0.69; 95% CI, 0.49–0.97), and there was no difference in the incidence of bleeding or death.21 In the current study, the incidence of out-of-hospital bleeding was extremely low (0.4% in the clopidogrel group and 1.8% in the ticagrelor group), which was much lower than approximately 4.8% in the real world within 1 year. This finding may indicate that not all bleeding events have been recorded. A registered study by SWEDEHEART from Sweden studied > 14,000 patients with myocardial infarction registered in the Swedish national system from 2010–2017 and found that compared with clopidogrel, patients in the ticagrelor group had increased mortality (HR, 1.17; 95% CI, 1.03–1.32) and re-admission due to bleeding (HR, 1.48; 95% CI, 1.25–1.76).22 A recent paper by Bianco et al reported 1-year clinical outcomes of ticagrelor versus clopidogrel in patients ≥75 years from two real-world registry showing benefit of ticagrelor in the incidence of all-cause death (HR, 0.32; 95% CI, 0.1–0.8; P = 0.012) without a statistical significant increase in the major bleeding (HR, 1.49; 95% CI, 0.70–3.0; P = 0.257) compared to clopidogrel.23 However, only 175 patients in the ticagrelor group were older than 75 years old in the patients analyzed after propensity score matching.
The inconsistency of the above results may be related to the different definitions of clinical endpoint events and sample sizes. What may be more important is the heterogeneity of the risk of ischemia and bleeding among different populations in the study. In addition, it must be acknowledged that biological and chronologic ages may differ. Although multiple bleeding scoring systems include age as an independent factor, age itself is not considered to be a major bleeding risk factor in definition of high-risk bleeding risk documented by the Academic Research Consortium when other co-morbidities and co-existing risk factors are not present.24
The above four studies were all conducted in Europe, and the East Asian population differed with respect to some baseline characteristics. The Asian population subgroup analysis of the PLATO study showed that the benefits and bleeding risk were similar to the non-Asian population.25 Only 548 Asians were included in the ticagrelor group of the PLATO study, and the interquartile range of age (52–70 years) suggested even fewer elderly patients.25 The East Asian population had a lower mean weight and were more complicated by ischemic stroke, both of which may increase the risk of bleeding. Based on the subgroup analysis, the conclusion needs to be further confirmed by a larger cohort study.
The present study also showed that although there was no significant increase in BARC 3 and 5 bleeding in the ticagrelor group; BARC 1 and 2 bleeding (ie, minor and minimal bleeding) increased significantly, which is consistent with previous studies. Even minor bleeding can lead to drug discontinuation in addition to other reasons, which can affect the long-term prognosis. An analysis of data from 4 RCTs involving > 45,000 patients showed that post-discharge bleeding after ACS had an equivalent prognostic impact as post-discharge myocardial infarction on long-term all-cause mortality.26
In addition, this study showed that malignant tumors were also an independent risk factor for bleeding, which is also consistent with the Academic Research Consortium findings, which used active malignant tumors within 12 months as the main criterion for high-risk bleeding.24 A study reported that one of 13 cases of post-discharge bleeding in ACS patients was related to a newly diagnosed malignant tumor.27 Bleeding in related parts of the urogenital system, gastrointestinal tract, and bronchopulmonary system was more likely to be diagnosed with a new tumor. Data from baseline and follow-up of our study showed that approximately 10% of patients ≥ 75 years of age had malignant tumors. Therefore, more attention should be paid to the choice of antithrombotic drugs for this population.
Previous studies have shown that clopidogrel resistance or high on-treatment platelet reactivity was more common in elderly patients;28 however, our study did not show the ischemic benefits of ticagrelor (a more potent antiplatelet agent), which was consistent with the SWEDEHEART study (a sample size of > 14,000 cases).22 Antithrombotic therapy after ACS or PCI in recent years has focused on individualized treatment to achieve a balance between ischemia and bleeding. Escalation or de-escalation antiplatelet therapy under the guidance of genetic or platelet function testing may play a particularly role in the elderly, although the guidelines do not recommend routine use in clinical practice. This study showed that the independent risk factors for long-term MACCEs in patients who have undergone successful PCI, which were more common in the elderly, were as follows: OMI; ischemic stroke; PAD; multivessel disease; and eGFR. Different vascular diseases have different antithrombotic strategies. The different benefits and risks of clopidogrel and ticagrelor in atherosclerotic cardiovascular diseases, other than coronary artery disease, may partially explain the results of this study.
There may be the possibility of switching between drugs during the long-term treatment follow-up. According to previous studies and clinical experience, the incidence of ticagrelor de-escalation to the clopidogrel group may be more common, which would make it possible to narrow the difference in efficacy endpoints between the two groups. This study used an intention-to-treat analysis, which has practical significance in clinical applications. There was a significant difference in the incidence of bleeding between the two groups, which also reflected the low incidence of drug switching between the two groups.
Limitations
There were several limitations to this study. First, it was a single center, retrospective study, which may underestimate the incidence of minimal bleeding, but minimal bleeding was not the main safety endpoint of this study. Our center had a standardized follow-up system after PCI, which can further reduce the bias of a retrospective study. In addition, due to the small effective sample size of endpoint events, stratified analysis was not conducted according to the bleeding risk score.
Conclusions
Compared with clopidogrel, DAPT based on ticagrelor significantly increased the risk of BARC2, 3, and 5 bleeding without reducing MACCEs in elderly patients who received PCI. In clinical practice, accurate risk stratification of elderly patients is required to weigh the risks of bleeding and ischemia. Before the availability of additional strong evidence in support of the ischemic benefit of ticagrelor, clopidogrel remains a rational choice for some elderly patients.
Data Sharing Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank Dr Xuhe Gong, Dr Mingyuan Liu, Dr Jixuan Liu (Beijing Friendship Hospital, Capital Medical University) for their help in the design of this study. Shaoke Meng and Lei Guo share first authorship.
Funding
This study was supported by the Beijing United Heart Foundation, Cardiacare Sponsored Optimizing Antithrombotic Research Fund (grant no. BJUHFCSOARF 201801-02) (Beijing,China).
Disclosure
The authors declare that they have no conflict of interest.
References
1. Mehta SR, Yusuf S, Peters RJG, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527–533. doi:10.1016/s0140-6736(01)05701-4
2. Akhtar T, Bandyopadhyay D, Ghosh RK, Aronow WS, Lavie CJ, Yadav N. Advances in the pharmacogenomics of antiplatelet therapy. Am J Ther. 2020;27(5):e477–e484. doi:10.1097/mjt.0000000000001013
3. Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107(23):2908–2913. doi:10.1161/01.Cir.0000072771.11429.83
4. Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45(2):246–251. doi:10.1016/j.jacc.2004.09.067
5. Husted S, van Giezen JJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009;27(4):259–274. doi:10.1111/j.1755-5922.2009.00096.x
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. doi:10.1056/NEJMoa0904327
7. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177. doi:10.1093/eurheartj/ehx393
8. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi:10.1093/eurheartj/ehaa575
9. Genereux P, Giustino G, Witzenbichler B, et al. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66(9):1036–1045. doi:10.1016/j.jacc.2015.06.1323
10. Aronow WS. Approach to symptomatic coronary disease in the elderly: TIME to change? Lancet. 2001;358(9286):945–956. doi:10.1016/s0140-6736(01)06111-6
11. Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001;286(6):708–713. doi:10.1001/jama.286.6.708
12. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–2747. doi:10.1161/CIRCULATIONAHA.110.009449
13. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Thrombolysis. 2005;3(4):692–694. doi:10.1111/j.1538-7836.2005.01204.x
14. Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96(9):1200–1206. doi:10.1016/j.amjcard.2005.06.056
15. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi:10.1093/eurheartj/ehz425
16. Husted S, James S, Becker RC, et al. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: a substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circ Cardiovasc Qual Outcomes. 2012;5(5):680–688. doi:10.1161/CIRCOUTCOMES.111.964395
17. Alexander KP, Newby LK, Cannon CP, et al. Acute coronary care in the elderly, part I: non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549–2569. doi:10.1161/CIRCULATIONAHA.107.182615
18. Alexander KP, Newby LK, Armstrong PW, et al. Acute coronary care in the elderly, part II: ST-segment-elevation myocardial infarction: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2570–2589. doi:10.1161/CIRCULATIONAHA.107.182616
19. Dai X, Busby-Whitehead J, Alexander KP. Acute coronary syndrome in the older adults. J Geriatr Cardiol. 2016;13(2):101–108. doi:10.11909/j.issn.1671-5411.2016.02.012
20. Gimbel M, Qaderdan K, Willemsen L, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. The Lancet. 2020;395(10233):1374–1381. doi:10.1016/s0140-6736(20)30325-1
21. Schmucker J, Fach A, Mata Marin LA, et al. Efficacy and Safety of Ticagrelor in Comparison to Clopidogrel in Elderly Patients With ST-Segment-Elevation Myocardial Infarctions. J Am Heart Assoc. 2019;8(18):e012530. doi:10.1161/JAHA.119.012530
22. Szummer K, Montez-Rath ME, Alfredsson J, et al. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: insights from the SWEDEHEART registry. Circulation. 2020;142(18):1700–1708. doi:10.1161/circulationaha.120.050645
23. Bianco M, Careggio A, Biole CA, et al. Ticagrelor or clopidogrel after an acute coronary syndrome in the elderly: a propensity score matching analysis from 16,653 patients treated with PCI included in two large multinational registries. Cardiovasc Drugs Ther. 2021;35(6):1171–1182. doi:10.1007/s10557-021-07213-y
24. Urban P, Mehran R, Colleran R, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation. 2019;140(3):240–261. doi:10.1161/CIRCULATIONAHA.119.040167
25. Kang HJ, Clare RM, Gao R, et al. Ticagrelor versus clopidogrel in Asian patients with acute coronary syndrome: a retrospective analysis from the Platelet Inhibition and Patient Outcomes (PLATO) Trial. Am Heart J. 2015;169(6):899–905 e1. doi:10.1016/j.ahj.2015.03.015
26. Marquis-Gravel G, Dalgaard F, Jones AD, et al. Post-discharge bleeding and mortality following acute coronary syndromes with or without PCI. J Am Coll Cardiol. 2020;76(2):162–171. doi:10.1016/j.jacc.2020.05.031
27. Raposeiras-Roubin S, Abu-Assi E, Munoz-Pousa I, et al. Usefulness of bleeding after acute coronary syndromes for unmasking silent cancer. Am J Cardiol. 2020;125(12):1801–1808. doi:10.1016/j.amjcard.2020.03.023
28. Verdoia M, Pergolini P, Rolla R, et al. Advanced age and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. J Thromb Thrombolysis. 2016;14(1):57–64. doi:10.1111/jth.13177
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


