Back to Journals » Infection and Drug Resistance » Volume 13
Comparison of Clinical, Laboratory and Radiological Characteristics Between COVID-19 and Adenovirus Pneumonia: A Retrospective Study
Authors Jiang J, Wan R, Pan P, Hu C, Zhou R, Yin Y, Zhou T, Huang H, Li Y
Received 23 May 2020
Accepted for publication 20 August 2020
Published 2 October 2020 Volume 2020:13 Pages 3401—3408
DOI https://doi.org/10.2147/IDR.S264132
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Juan Jiang,1 Rongjun Wan,1 Pinhua Pan,1 Chengping Hu,1 Rihua Zhou,2 Yiping Yin,2 Ting Zhou,2 Hua Huang,2 Yuanyuan Li1
1Department of Respiratory Medicine, Xiangya Hospital, Central South University, Changsha, Hunan Province, People’s Republic of China; 2Medical Center of Tuberculosis, The Second People’s Hospital of Chenzhou, Chenzhou, Hunan Province, People’s Republic of China
Correspondence: Yuanyuan Li
Department of Respiratory Medicine, Xiangya Hospital, Central South University, Changsha 410008, People’s Republic of China
Tel +86 13975806790
Email [email protected]
Hua Huang
Medical Center of Tuberculosis, The Second People’s Hospital of Chenzhou, Chenzhou 423000, People’s Republic of China
Tel +86 18673561888
Email [email protected]
Background: The pandemic of coronavirus disease 2019 (COVID-19) has become a global public health problem. It is important for clinical physicians to differentiate COVID-19 from other respiratory infectious diseases caused by viruses, such as human adenovirus.
Subjects and Methods: This was a retrospective observational study. We analyzed and compared the clinical manifestations, laboratory findings and radiological features of two independent cohorts of patients diagnosed with either COVID-19 (n=36) or adenovirus pneumonia (n=18).
Results: COVID-19 did not show a preference in males or females, whereas 94.4% of patients with adenovirus pneumonia were males. Fever and cough were common in both COVID-19 and adenovirus pneumonia. But the median maximal body temperature of the adenovirus pneumonia cohort was significantly higher than in COVID-19 (P< 0.001). Furthermore, 77.8% of patients with adenovirus pneumonia had a productive cough versus only 13.9% of COVID-19 patients (P< 0.001). Compared with adenovirus pneumonia, constitutional symptoms were less common in COVID-19, including headache (16.7% vs 38.9%, P=0.072), sore throat (8.3% vs 27.8%, P=0.058), myalgia (8.3% vs 61.1%, P< 0.001) and diarrhea (8.3% vs 44.4%, P=0.002). Furthermore, patients with COVID-19 were less likely to develop respiratory failure (8.3% vs 83.3%, P< 0.001) and showed less prominent laboratory abnormalities, including lymphocytopenia (61.1% vs 88.9%, P=0.035), thrombocytopenia (2.8% vs 61.1%, P< 0.001), elevated procalcitonin (2.8% vs 77.8%, P< 0.001) and elevated C-reactive protein (36.1% vs 100%, P< 0.001). Besides, a higher percentage of patients with adenovirus pneumonia showed elevated transaminase, myocardial enzymes, creatinine and D-dimer compared with COVID-19 patients. On chest CT, the COVID-19 cohort was characterized by peripherally distributed ground-glass opacity and patchy shadowing, while the adenovirus pneumonia cohort frequently presented with consolidation and pleural effusion.
Conclusion: There were many differences between patients diagnosed with COVID-19 and those with adenovirus pneumonia in their clinical, laboratory and radiological characteristics. Compared with adenovirus pneumonia, COVID-19 patients tended to show a lower severity of illness.
Keywords: coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2, human adenovirus, adenovirus pneumonia, differential diagnosis
Introduction
Since early December 2019, a newly emerged coronavirus, which was officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been spreading worldwide.1 Caused by SARS-CoV-2 infection and first identified in Wuhan, China, coronavirus disease 2019 (COVID-19) has become a serious global public health problem. So far, confirmed cases of COVID-19 have been increasing rapidly, to over 34 million in 213 countries and areas, and have caused more than 230,000 confirmed deaths.2
Clinical manifestations of COVID-19 patients are quite non-specific, such as fever, dry cough and fatigue.3 While peripherally distributed ground-glass opacity (GGO) in the lungs has been considered as the most important radiological feature of COVID-19, it is also frequently seen in other pulmonary viral infections.4 This non-specificity makes it difficult to distinguish COVID-19 from other respiratory tract infections at first sight, especially from viral infections, such as human adenovirus. Human adenovirus is a group of double-stranded non-enveloped DNA viruses. Based on previous studies, it is responsible for at least 5–10% of pediatric and 1–7% of adult respiratory tract infections.5 In recent years, several outbreaks of adenovirus pneumonia have been reported in different countries, which have caused high respiratory mortality.6–8 Similarly to COVID-19, adenovirus pneumonia outbreaks usually occur in the winter or early spring. Both SARS-CoV-2 and human adenovirus can be transmitted through respiratory droplets and direct contact, cause severe respiratory tract infections and rapidly progress to respiratory failure. Radiological features of adenovirus pneumonia also partially overlap those of COVID-19. Thus, in the latest official clinical guideline released by the National Health Commission of China,9 differential diagnosis between COVID-19 and adenovirus pneumonia was deemed necessary for suspected COVID-19 cases on admission.
Therefore, in the present study, we retrospectively analyzed and compared the clinical, laboratory and radiological features of COVID-19 and adenovirus pneumonia. It is hoped that our findings will help more clinical physicians to characterize these two diseases in the early stage of the clinical course.
Subjects and Methods
Study Design and Participants
This was a retrospective study among patients diagnosed with COVID-19 or adenovirus pneumonia. A total of 36 consecutive patients with laboratory-confirmed SARS-CoV-2 infection who were admitted to the Department of Respiratory Medicine of Central South University Xiangya Hospital and the Second People’s Hospital of Chenzhou from January 15 to March 20, 2020 were enrolled in this study. COVID-19 was diagnosed according to the WHO interim guidance.10 Diagnosis of COVID-19 in patients included in this study was made strictly based on the positive detection of virus nucleic acids, by either reversetranscriptase polymerasechainreaction (PCR) or next-generation sequencing. Meanwhile, the medical records were reviewed of 18 consecutive patients with adenovirus pneumonia, which was diagnosed based on the positive detection of virus nucleic acids in bronchoalveolar lavage fluid or blood samples by next-generation sequencing, at the Department of Respiratory Medicine of these two hospitals from November 1, 2018 to March 20, 2020. Routine microbiological detection tests were performed in all patients on admission, including culture of bacteria and fungi, serological tests (specific to human adenovirus, respiratory syncytial virus, influenza B virus, influenza A virus, human parainfluenza viruses, Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella pneumophila and Coxiella burnetii) and PCR of influenza viruses. Patients who met any of the following criteria were excluded: 1) patients younger than 14 years old; 2) patients with confirmed co-infection with other pathogens by routine microbiological detection on admission, including culture of bacteria and fungi, serological tests and PCR of influenza viruses; 3) patients with confirmed or suspected active tuberculosis; 4) pregnant or breast-feeding women; and 5) patients whose medical records were incomplete.
Data Collection
Baseline clinical characteristics, and laboratory and radiological findings of each patient on hospital admission were obtained from electronic medical records. Clinical characteristics included the age, gender, smoking history, symptoms and underlying diseases of patients. Laboratory indicators included blood cell count, blood chemical analysis, coagulation testing, liver and renal function, procalcitonin (PCT), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH) and creatine kinase (CK). Radiological assessments were made based on chest computed tomography (CT). The presence of a radiological abnormality was determined according to the documentation or description in the medical records, which was double-checked by two senior radiologists.
Statistical Analysis
Statistical analysis was performed using SPSS 25.0 software (IBM Corp., Armonk, NY, USA). Continuous variables are presented as medians and interquartile ranges, and categorical variables as counts and percentages. The Mann–Whitney U-test was used to compare continuous variables between COVID-19 and adenovirus pneumonia groups. For categorical variables, differences between these two groups were assessed with the chi-squared test or Fisher’s exact test. Significance was fixed at P<0.05.
Ethics Statement
This study was approved by the Institutional Review Board and Ethics Committee of Central South University. The ethics committee approved the waiver of informed consent owing to the retrospective nature of the review. All research data accessed were de-identified and anonymously analyzed. The study was conducted according to the Declaration of Helsinki.
Results
Clinical Characteristics of Patients with COVID-19 or Adenovirus Pneumonia
Clinical characteristics of patients with COVID-19 or adenovirus pneumonia are summarized in Table 1. In total, there were 36 patients with COVID-19 and 18 patients with adenovirus pneumonia enrolled in the present study. The median ages of these two groups were similar (41 vs 34 years old, P=0.135). COVID-19 did not show a preference in male or female populations, whereas patients with adenovirus pneumonia were almost exclusively male (17/18). Fever, cough and fatigue were the most common symptoms in COVID-19 patients, and were observed in 77.8%, 69.4% and 44.4% of cases, respectively. Fever was observed in all patients with adenovirus pneumonia, and their median maximal body temperature was significantly higher than in COVID-19 patients (40.0 vs 38.0°C, P<0.001). Furthermore, 94.4% of patients with adenovirus pneumonia had a cough, and 77.8% showed expectoration, instead of a dry cough as in COVID-19. Compared with COVID-19, hemoptysis (27.8% vs 0.0%, P=0.001), dyspnea (50.0% vs 11.1%, P=0.002), myalgia (61.1% vs 8.3%, P<0.001) and diarrhea (44.4% vs 8.3%, P=0.002) were significantly more common in adenovirus pneumonia. In terms of underlying diseases, there was no significant difference in the history of chronic obstructive pulmonary disease, diabetes, hypertension, coronary heart disease, chronic liver disease or chronic renal disease between the COVID-19 and adenovirus pneumonia cohorts.
 |
Table 1 Clinical Characteristics of Patients with COVID-19 or Adenovirus Pneumonia |
Laboratory Findings of Patients with COVID-19 or Adenovirus Pneumonia
Table 2 presents the laboratory findings of patients with COVID-19 or adenovirus pneumonia. The median white blood cell counts were comparable in the two groups (4.5 vs 4.6×109 cells/L, P=0.707). Leukocytopenia (defined as white blood cell count <4×109 cells/L) was seen in the same percentage (38.9%) of COVID-19 patients and adenovirus pneumonia patients. Median neutrophil counts of COVID-19 and adenovirus pneumonia cohorts were not statistically different (4.1 vs 2.8×109 cells/L, P=0.137). However, compared with COVID-19, patients with adenovirus pneumonia had a significantly lower median lymphocyte count (0.50 vs 0.98×109 cells/L, P<0.001) and median platelet count (88 vs 169×109 cells/L, P=0.002). Lymphocytopenia, which was defined as a lymphocyte count of less than 1.1×109 cells/L, occurred in 61.1% of COVID-19 and 88.9% of adenovirus pneumonia patients (P=0.035). An elevated level of PCT was present in 2.8% of COVID-19 patients, CRP in 36.1% and ESR in 41.7%. In comparison, 77.8% of patients with adenovirus pneumonia showed elevated PCT (P<0.001), 100% showed elevated CRP (P<0.001) and 44.4% showed elevated ESR (P=0.846). Elevated levels of aspartate aminotransferase (11.1% vs 94.4%), alanine aminotransferase (13.9% vs 72.2%), lactic dehydrogenase (50.0% vs 100%), creatine kinase (8.3% vs 100%) and D-dimer (13.9% vs 83.3%) were significantly less common in COVID-19 patients (P<0.001). In addition, patients with adenovirus pneumonia showed a significantly higher median creatinine level than COVID-19 patients (90.1 vs 63.0 µmol/L, P<0.001).
 |
Table 2 Laboratory Findings of Patients with COVID-19 or Adenovirus Pneumonia on Admission |
Radiological Features of Patients with COVID-19 or Adenovirus Pneumonia
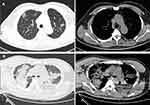
As shown in Table 3, both the COVID-19 and adenovirus pneumonia cohorts mainly presented with bilateral (72.2% vs 88.9%, P=0.165) and multifocal (91.7% vs 100%, P=0.208) pulmonary lesions on chest CT. However, 91.7% of patients with COVID-19 presented with a peripheral distribution of pulmonary lesions, while patients with adenovirus pneumonia did not show this radiological pattern (P<0.001). Patchy shadowing was common in both COVID-19 and adenovirus pneumonia (77.8% vs 100%, P=0.030). GGO (88.9% vs 22.2%, P<0.001) and interstitial abnormalities (36.1% vs 5.6%, P=0.016) were much more frequent in COVID-19 compared with adenovirus pneumonia. Only 2.8% of patients with COVID-19 showed consolidation or pleural effusion. In comparison, 77.8% of patients with adenovirus pneumonia showed consolidation and 72.2% showed pleural effusion. Pericardial effusion and lymphadenopathy were rarely seen in COVID-19, while in the adenovirus pneumonia cohort, pericardial effusion was observed in 16.7% and lymphadenopathy in 33.3% of patients. Figure 1 shows representative chest CT images of patients with COVID-19 and adenovirus pneumonia. COVID-19 was characterized by multiple GGO distributed in the peripheral zone of multiple lobes. However, the most typical abnormalities of adenovirus pneumonia on chest CT were bilateral consolidation, patchy shadowing and pleural effusion.
 |
Table 3 Radiological Features of Patients with COVID-19 or Adenovirus Pneumonia on Chest CT |
Discussion
In this study, we compared the clinical, laboratory and radiological features of patients with COVID-19 and adenovirus pneumonia. To the best of our knowledge, this is the first study focusing on the differential diagnosis between COVID-19 and adenovirus pneumonia. Our data showed that COVID-19 patients were more likely to exhibit mild-to-moderate fever and dry cough, while patients with adenovirus pneumonia usually had high fever and productive cough with more common constitutional symptoms. In addition, patients with adenovirus pneumonia were more likely to develop severely or critically ill subtypes and present more laboratory abnormalities, including lymphocytopenia, thrombocytopenia, and elevated serum levels of PCT, CRP, transaminase, myocardial enzymes, creatinine and D-dimer. On chest CT, COVID-19 was characterized by peripherally distributed GGO and patchy shadowing, while adenovirus pneumonia mainly presented as patchy shadowing, consolidation and pleural effusion.
SARS-CoV-2 is a highly infectious single-stranded enveloped RNA virus, and COVID-19 constitutes a threat to global public health. Early identification and diagnosis are crucial for interrupting the transmission of COVID-19. Because of the non-specific clinical symptoms and radiological changes, confirmed diagnosis of COVID-19 relies on the detection of SARS-CoV-2 nucleic acid.11 However, the problem is that false-negative results are frequently seen in COVID-19. A retrospective study including 1014 cases suspected of having COVID-19 revealed that only 59% of them had positive RT-PCR results. Among 413 patients with negative RT-PCR results, 36% had typical chest CT findings of COVID-19 and were considered as highly likely cases.12 Fang et al13 found that, in a cohort of 51 COVID-19 patients who had throat swabs (45 patients) or sputum samples (six patients) taken, followed by one or more RT-PCR assays, the sensitivity of RT-PCR of the throat swab or sputum sample was only 71%. Xiao et al14 reported that 21.6% of patients with COVID-19 received false-negative results in the first and second consecutive tests for SARS-CoV-2 nucleic acid with nasopharyngeal or oropharyngeal swabs. Therefore, analysis of the similarities and differences in clinical, laboratory and radiological characteristics between COVID-19 and other viral pneumonias, including adenovirus pneumonia, is of significance in clinical practice.
Our data showed that there were similarities and differences between COVID-19 and adenovirus pneumonia. Consistent with previous studies, both COVID-19 and adenovirus pneumonia were characterized by fever and cough.15 However, patients with COVID-19 mainly had mild-to-moderate fever and dry cough, while patients with adenovirus pneumonia exclusively had high fever (Tmax >39°C) and productive cough. Besides, constitutional symptoms such as headache, sore throat, myalgia and gastrointestinal symptoms (including nausea, vomiting and diarrhea) were more common in the adenovirus pneumonia cohort than in COVID-19. In terms of laboratory findings, we found that, compared with COVID-19, adenovirus pneumonia had more prominent laboratory abnormalities, including lymphocytopenia, thrombocytopenia, and elevated levels of inflammatory markers such as PCT and CRP. Target organ injuries were also more common in adenovirus pneumonia, including liver, heart and kidney, which were indicated by the higher percentage of patients revealing elevated levels of transaminases, muscle enzymes and creatinine. These differences in clinical features and laboratory findings could provide valuable clues for clinical physicians to differentiate COVID-19 from adenovirus pneumonia.
According to the latest official guidance issued by the Health Commission of China, chest CT scan, which shows a higher sensitivity (86–98%) and improved false-negative rates compared to RT-PCR,16 is considered the main diagnostic basis of COVID-19 when SARS-CoV-2 nucleic acid testing is negative. In our study, both COVID-19 and adenovirus pneumonia were characterized by bilateral multifocal pulmonary lesions. Specifically, GGO and interstitial abnormalities were much more frequent in COVID-19, whereas consolidation and pleural effusion were more common in adenovirus pneumonia, which is consistent with previous studies.4,17 The peripheral distribution of pulmonary lesions was unique in COVID-19. Therefore, this radiological feature strongly suggests the diagnosis of COVID-19, instead of adenovirus pneumonia.
It is worth noting that, overall, patients with adenovirus pneumonia had more critical conditions than those with COVID-19 in our study. The incidence rate of respiratory failure, which was defined as PaO2/FiO2 ≤300 mmHg, was markedly lower in COVID-19 compared to adenovirus pneumonia. Laboratory abnormalities indicating target organ injuries, such as heart, liver and kidney, were also less common in COVID-19. The high virulence of human adenovirus could be one reason for this. Adenovirus pneumonia requiring hospitalization is uncommon in adults, and current knowledge is mainly derived from small case series. Even though the pathogenic mechanism of human adenovirus remains unclear, it has been reported to be an important viral cause of acute respiratory distress syndrome (ARDS) and respiratory mortality.18 Adenovirus genotypes 7 and 55 can infect both immunocompetent and immunocompromised individuals, and have been implicated in outbreaks of severe pneumonia and ARDS, even in immunocompetent young men.19–23 In the present study, all patients with adenovirus pneumonia were young or middle-aged immunocompetent individuals, which also supported the high virulence of human adenovirus. Among patients with adenovirus-associated ARDS, the mortality could be as high as 80%.23 La Rosa et al24 reported that human adenovirus had a mortality rate of 26% in 76 adult recipients of hematopoietic stem cell transplantation. Furthermore, the national health policy also increased the percentage of hospitalized patients with mild-to-moderate COVID-19. Since the outbreak of COVID-19, the Chinese government has been making every effort to control the sources of infection and cut the chain of transmission. SARS-CoV-2 testing kits and medical care services are freely provided to all confirmed COVID-19 cases, so that many patients with mild-to-moderate COVID-19 are admitted to hospital for isolation and treatment. However, patients with mild adenovirus pneumonia only present non-specific and self-limiting respiratory symptoms. Thus, most of these patients may not be admitted to hospitals.
There are several limitations to the present study. First of all, this was a retrospective study that included data from two centers, which may have caused unavoidable selection bias. Second, the total patient number was small; in particular, there were only 18 patients with adenovirus pneumonia enrolled in this study owing to its low incidence. The small sample size could have reduced the representativeness of these patients. Finally, the data for the adenovirus pneumonia cohort originated from a 2-year span, while the data for the COVID-19 cohort originated from a 2-month span, which may also affect the present results.
Conclusion
There were many differences between COVID-19 and adenovirus pneumonia in their clinical, laboratory and radiological characteristics. Compared with adenovirus pneumonia, COVID-19 patients tended to show a lower severity of illness. Large-scale studies are needed in the future to provide more experience in differentiating COVID-19 from not only adenovirus pneumonia but also other viral infections in the respiratory tract.
Funding
This work was supported in part by grants from the National Natural Science Foundation of China (8160025, 81873406), the Natural Science Foundation of Hunan Province (S2019JJQNJJ1970), the National Key Technology Research and Development Program of China (2015BAI12B00) and the National Key Research and Development Program of China (2018YFC1311900).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
2. World Health Organization. Coronavirus disease (COVID-19) outbreak situation. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
4. Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275–1280. doi:10.1007/s00259-020-04735-9
5. Lynch JP
6. Cao B, Huang GH, Pu ZH, et al. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest. 2014;145(1):79–86. doi:10.1378/chest.13-1186
7. Tan D, Zhu H, Fu Y, et al. Severe community-acquired pneumonia caused by human adenovirus in immunocompetent adults: a multicenter case series. PLoS One. 2016;11(3):e0151199. doi:10.1371/journal.pone.0151199
8. Yoon H, Jhun BW, Kim H, Yoo H, Park SB. Characteristics of adenovirus pneumonia in Korean Military Personnel, 2012-2016. J Korean Med Sci. 2017;32(2):287–295. doi:10.3346/jkms.2017.32.2.287
9. National Health Commission of the People’s Republic of China. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7). Available from:https://covid19.21wecan.com/covid19en/c100036/202004/7983d6f5725c42dabc761c010589cd21.shtml.
10. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. January 28, 2020. Available from:https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf.
11. Wang H, Wei R, Rao G, Zhu J, Song B. Characteristic CT findings distinguishing 2019 novel coronavirus disease (COVID-19) from influenza pneumonia. Eur Radiol. 2020;30(9):4910–4917. doi:10.1007/s00330-020-06880-z
12. Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;200642.
13. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;200432.
14. Xiao AT, Tong YX, Zhang S. False-negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J Med Virol. 2020. doi:10.1002/jmv.25855
15. Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med. 2020;35(5):1545–1549. doi:10.1007/s11606-020-05762-w
16. Udugama B, Kadhiresan P, Kozlowski HN, et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14(4):3822–3835. doi:10.1021/acsnano.0c02624
17. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi:10.1016/S1473-3099(20)30086-4
18. Radke JR, Cook JL. Human adenovirus infections: update and consideration of mechanisms of viral persistence. Curr Opin Infect Dis. 2018;31(3):251–256. doi:10.1097/QCO.0000000000000451
19. Lu QB, Tong YG, Wo Y, et al. Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009-2012. Influenza Other Respir Viruses. 2014;8(3):302–308. doi:10.1111/irv.12232
20. Zhao S, Wan C, Ke C, et al. Re-emergent human adenovirus genome type 7d caused an acute respiratory disease outbreak in Southern China after a twenty-one year absence. Sci Rep. 2014;4:7365. doi:10.1038/srep07365
21. Cui X, Wen L, Wu Z, et al. Human adenovirus type 7 infection associated with severe and fatal acute lower respiratory illness and nosocomial transmission. J Clin Microbiol. 2015;53(2):746–749. doi:10.1128/JCM.02517-14
22. Tang L, Wang L, Tan X, Xu W. Adenovirus serotype 7 associated with a severe lower respiratory tract disease outbreak in infants in Shaanxi Province, China. Virol J. 2011;8:23. doi:10.1186/1743-422X-8-23
23. Sun B, He H, Wang Z, et al. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: a prospective observational study. Crit Care. 2014;18(4):456. doi:10.1186/s13054-014-0456-6
24. La Rosa AM, Champlin RE, Mirza N, et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32(6):871–876. doi:10.1086/319352
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.