Back to Journals » Patient Preference and Adherence » Volume 12
Comparison of claims vs patient-reported adherence measures and associated outcomes among patients with nonvalvular atrial fibrillation using oral anticoagulant therapy
Authors Stephenson JJ , Shinde MU, Kwong WJ, Fu A, Tan H, Weintraub WS
Received 10 August 2017
Accepted for publication 22 November 2017
Published 12 January 2018 Volume 2018:12 Pages 105—117
DOI https://doi.org/10.2147/PPA.S148697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Judith J Stephenson,1 Mayura U Shinde,1 Winghan Jacqueline Kwong,2 An-Chen Fu,1 Hiangkiat Tan,1 William S Weintraub3
1HealthCore, Inc., Wilmington, DE, 2Daiichi Sankyo, Inc., Basking Ridge, NJ, 3Christiana Care Health System, Newark, DE, USA
Objective: To compare oral anticoagulant (OAC) adherence among patients with nonvalvular atrial fibrillation (NVAF) using patient-reported and claims-based measures, and to evaluate the effect of OAC adherence on health care costs and patient satisfaction with OAC therapy.
Methods: This was a hybrid US observational study consisting of a longitudinal cohort survey followed by linkage and analysis of respondents’ administrative claims data. Patients with NVAF receiving warfarin, dabigatran, rivaroxaban, or apixaban completed an initial survey and follow-up surveys at 4, 8, and 12 months. Patient-reported adherence was measured at each survey by Morisky Medication Adherence Scale (MMAS-8) and pharmacy claims-determined adherence by the proportion of days covered (PDC) for the 12-month period following the initial survey date; adherence was defined as MMAS-8 score =8 and PDC ≥80%. Patient satisfaction with OAC therapy was assessed by the Duke Anticoagulation Satisfaction Scale (DASS).
Results: Overall, 675 patients completed at least the initial survey (warfarin, n=271; dabigatran, n=266; rivaroxaban, n=128; apixaban, n=10). Fewer than half (47.9%) were PDC adherent, 37.2% were MMAS-8 adherent, and 19.4% were adherent by both measures. Total medical costs of PDC-adherent patients were significantly lower vs PDC-nonadherent patients (US$640 vs $993 per-patient per-month, respectively, p<0.05). MMAS-8-adherent patients reported higher treatment satisfaction; total DASS score was significantly lower among MMAS-8-adherent than MMAS-8-nonadherent patients (37.3 vs 42.9, respectively, p<0.001).
Conclusion: Using claims-based or patient-reported methods to measure OAC adherence may lead to different results when assessing impact on health care costs and satisfaction with anticoagulation medication. These results underscore the importance of considering both claims-based and patient-reported measures when evaluating treatment adherence in real-world settings.
Keywords: adherence, atrial fibrillation, OAC, health care costs
Introduction
Oral anticoagulants (OACs) have long been recommended for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF) in the absence of elevated bleeding risk or contraindications.1 The introduction of non-vitamin K antagonist OACs (NOACs), which include dabigatran, rivaroxaban, apixaban, and edoxaban, enabled effective anticoagulation without the inconvenience associated with warfarin.2–6 Patients with NVAF require sustained OAC therapy, but maintaining adherence on a long-term basis can be challenging.3,7–12 Nonadherence to OACs may result in increased health care costs, more inpatient admissions and emergency department (ED) visits, and higher risk of stroke,7,9–11,13–15 compared with patients who take their OACs as prescribed.
Studies aimed at evaluating OAC adherence in everyday clinical practice have commonly measured adherence using either prescription fill data or patient self-reports, each of which is subject to limitations. Patient self-reports are subjective and may be influenced by errors in recall,16–18 whereas objective claims-based prescription fill data may contain coding errors, and documentation of a prescription fill is not proof that the patient took the medication as prescribed.3,10,11,19,20 To our knowledge, no studies have assessed OAC adherence using both prescription claims and patient-reported data. To fill this research gap, the current study compared OAC adherence in patients with NVAF using both prescription fill data obtained from administrative claims and patient self-reports collected in a series of surveys. Additionally, the effect of OAC adherence on health care costs and patient satisfaction with OAC therapy was evaluated.
Methods
Study design and patients
This was a hybrid US observational study consisting of a longitudinal cohort survey of adults with NVAF who were treated with warfarin, dabigatran, rivaroxaban, or apixaban followed by linkage and analysis of respondents’ survey and administrative claims data.
The patient survey sample was identified from medical and pharmacy claims in the HealthCore Integrated Research Database (HIRD). The HIRD contains a broad, clinically rich and geographically diverse spectrum of longitudinal administrative claims from 14 geographically dispersed health plans in the US. The HIRD is generally representative of the US Census population, although patients aged 65 years and older are underrepresented.21 Patients were selected for the survey based on claims submitted between October 2011 and June 2014 (the patient sample list identification period). Currently active, survey-eligible patients (18 years of age or older) had at least one medical claim for atrial fibrillation (International Classification of Diseases, Version 9, Clinical Modification [ICD-9-CM] code 427.31) and at least one pharmacy claim for warfarin, dabigatran, rivaroxaban, or apixaban during the patient sample identification period.
Patients’ initial OAC cohort assignment was based on the OAC drug (ie, index drug) associated with the date of the last prescription fill for warfarin, dabigatran, rivaroxaban, or apixaban (ie, index fill date) in the patient sample identification period and confirmed at the time of the initial survey. Patients’ final OAC cohort assignment was based on patients’ self-report of the OAC they were currently taking. Patients remained classified according to OAC treatment use at the time of initial survey throughout the 12-month follow-up period. However, current OAC treatment was assessed at each follow-up survey (ie, 4-, 8-, and 12-month surveys) in order to determine if and when switching or discontinuation occurred.
All patients were active members of a commercial (employer-provided) or Medicare Advantage health insurance plan at the time of the initial survey. Patients were required to have at least 6 months continuous enrollment prior to the index fill date to allow for identification of the survey-eligible population. Patients were excluded if they had one or more medical claims for valvular heart disease, valve replacement, cardiovascular surgery, or hyperthyroidism in the 6 months prior to the index fill date.
Eligible patients received a pre-notification letter with opt-out/opt-in telephone numbers ~2 weeks prior to the initial survey. After the 2-week window had elapsed, patients who had neither opted-in nor opted-out were contacted by telephone by professional interviewers, presented with a brief description of the study, and invited to participate in the study. Consenting patients who qualified for the study were surveyed regarding their experiences with anticoagulant treatment over a 12-month period. Survey participation consisted of completing a maximum of four surveys either with an interviewer over the phone or via the internet. The follow-up surveys were conducted at 4, 8, and 12 months after the initial survey (Figure 1). While completion of the initial survey was necessary for study participation, patients were not required to complete all three follow-up surveys. That is, if patients could not be contacted within the time window for a specific follow-up survey, an attempt was made to contact them at the next follow-up time point rather than exclude them from further surveys. Thus, it was possible for patients to complete 1 to 4 surveys.
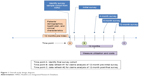  | Figure 1 Overall study design diagram. |
The protocol and all survey-related materials were approved by the New England Institutional Review Board and all patient data were handled in compliance with the regulations of the US Insurance Portability and Accountability Act of 1996.
Using the date of the initial survey as the survey index date, a study database consisting of the medical and pharmacy claims of survey respondents was extracted from the HIRD for the 12-month periods prior to and after the initial survey from which a patient-level analytic file was developed and linked with respondents’ survey data. The combined survey and claims analytic file was used to examine medication adherence, patient-reported outcomes, health care resource utilization (HCRU), and costs of care. Patients were grouped into one of four OAC cohorts (ie, warfarin, dabigatran, rivaroxaban, or apixaban) based on their OAC treatment at the time of the initial survey.
Adherence measures
Adherence for the 12-month period following the initial survey was assessed in two ways: patient self-report and pharmacy claims data. Patient-reported adherence was determined using the eight-item Morisky Medication Adherence Scale (MMAS-8), a validated patient-reported questionnaire that measures medication-taking behavior and explores the circumstances influencing adherence, such as difficulty in remembering to take medications. The MMAS-8 is composed of eight questions based on four themes: forgetfulness, negligence, interruption of drug intake after clinical improvement, and restart of drug intake when symptoms worsen.22 The total MMAS-8 score ranges from 0 to 8, with higher scores indicating better medication adherence. The MMAS-8 was administered at all four survey time points (initial survey and 4-, 8-, and 12-month follow-up surveys). For each patient, an average MMAS-8 score was calculated from the patient’s MMAS-8 scores for each time point, depending on the number of surveys the patient had completed.
The proportion of days covered (PDC) used pharmacy claims data to determine the 12-month NOAC PDC over the 12-month period following the initial survey. PDC was calculated by dividing the number of days covered by the index OAC prescription fill by 365 (ie, the number of days in the follow-up period), then multiplying the result by 100%.
Warfarin adherence was determined using a modified Go et al algorithm involving the combination of prescription fills from pharmacy claims and a proxy measure for international normalized ratio (INR) testing.23 The date of INR testing recorded in medical claims (current procedural code 85610) was used instead of INR laboratory results because INR test results are only available for a subset of HIRD patients.
Other measures
HCRU and costs were estimated during the 12-month follow-up period. HCRU was calculated for inpatient hospitalizations, ED visits, outpatient physician office visits, and prescription fills and was reported as proportions. Costs were presented as per-member per-month because although costs were analyzed for the 12-month period after the initial survey, not all patients remained enrolled for the entire period. Bleeding/stroke-related utilization was calculated for services with an ICD-9-CM diagnosis code for bleeding/stroke event.
All-cause, stroke-related, and bleeding-related total health care costs were calculated for overall total medical costs, inpatient hospitalizations, ED visits, and physician office visits and other outpatient services. Pharmacy costs were not included in total costs because of substantial price differentials between the generic warfarin and the proprietary NOACs. All costs were adjusted using the 2015 annual medical care component of the Consumer Price Index. All HCRU costs were presented on a per-member per-month basis.
Patient demographic and clinical characteristics were compared among OAC treatment cohorts during the 12-month baseline period prior to the initial survey. Baseline comorbid conditions were identified by ICD-9-CM codes associated with medical claims at any position during the pre-index period. Overall pre-index comorbidity burden was assessed using the Deyo-Charlson Comorbidity Index24 (DCI). The CHADS2 stroke risk score, based on the presence of congestive heart failure, hypertension, diabetes, prior stroke or transient ischemic attack (TIA), and age 75 years or older, was calculated during the pre-index period.25,26 Bleeding risk was assessed using two alternative bleeding score schemes: the HEMORR2AGES score27 (based on the presence of prior bleed, hepatic or renal disease, alcohol abuse, malignancy, reduced platelet count or function, hypertension, anemia, excessive falls, prior stroke, and age older than 75 years) and the ATRIA bleeding score7 (based on the presence of anemia, severe renal disease [estimated glomerular filtration rate less than 30 mL/min or dialysis dependent], age 75 years or older, any prior hemorrhage diagnosis, and hypertension). The CHADS2 and bleeding risk scores were determined from claims data. Other clinical outcomes included stroke or TIA requiring inpatient hospitalizations or ED visits and bleeding events requiring inpatient hospitalizations or ED or outpatient visits during the follow-up period.
OAC treatment satisfaction was assessed using the Duke Anticoagulation Satisfaction Scale28 (DASS), which was administered at all four survey time points. The DASS was developed to measure the quality of life (QOL) and satisfaction among patients receiving OAC treatment. It consists of 25 items that address both the negative (ie, limitations, hassles, and burdens) and positive (ie, confidence, reassurance, and satisfaction) impacts of anticoagulation. Each item has seven responses: 1) not at all, 2) a little, 3) somewhat, 4) moderately, 5) quite a bit, 6) a lot, and 7) very much. Three domain scores and a total score can be calculated. The limitations domain consists of nine items pertaining to limitations on such things as physical activities due to fear of bleeding and dietary restrictions; item responses are summed with scores ranging from 9 to 63. The hassles/burdens domain consists of eight items dealing with both daily hassles such as remembering to take the medication and occasional hassles such as having to wait while visiting a provider for blood testing with scores ranging from 8 to 56. The psychological impact domain also consists of eight items dealing with such things as reassurance because of OAC treatment with scores ranging from 8 to 56; the total score is calculated by summing the responses to the 25 items with scores ranging from 25 to 175. Six items were reverse-coded before scores were calculated. Lower scores indicate greater satisfaction with the use of OAC medication, fewer hassles and burdens, and less psychological impact. Individual domain and total DASS scores were determined at each survey and an average total DASS score was calculated depending on the number of follow-up surveys completed by the survey respondent.
Statistical analysis
Descriptive analyses were performed and the descriptive results were analyzed separately using PDC- and MMAS-8-adherent and nonadherent groups. A two-sample t-test was used to test differences for continuous variables and the χ2 test was used for categorical variables. The Wilcoxon rank-sum test was used for non-normal continuous variables (eg, cost variables). Comparisons were made within each OAC cohort as changes from the initial survey to each survey time point rather than across OAC cohorts. Results were reported as mean percentages (SD, median) for continuous variables and as relative frequencies for categorical variables.
A generalized linear model with log-link and gamma distribution was used to compare total all-cause and stroke- or bleeding-related medical costs between adherent and nonadherent groups. The model was adjusted for patient age, gender, commercial vs Medicare Advantage health plan type, region of residence, self-reported dosing schedule, DCI score, and pre-index CHADS2 stroke and HEMORR2AGES and ATRIA bleeding risk scores. A conventional alpha of 0.05 and two-tailed level of significance was used. All data analyses were conducted with SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) and Stata version 12 (StataCorp LP, College Station, TX, USA).
Results
Patient characteristics
Overall, 795 patients completed the initial survey: 321 (40.4%) were in the warfarin cohort, 319 (40.1%) in the dabigatran cohort, 141 (17.7%) in the rivaroxaban cohort, and 14 (1.8%) in the apixaban cohort. After merging the survey data of the 795 respondents with their claims data for the 12-month periods prior to and following the initial survey date, 676 respondents had complete claims data for at least 12 months prior to and following the initial survey date. Of these, 675 respondents had an overall mean MMAS score and they comprised the study population: 271 (40.1%) patients in the warfarin cohort, 266 (39.4%) in the dabigatran cohort, 128 (19.0%) in the rivaroxaban cohort, and 10 (1.5%) in the apixaban cohort (Table 1). While commercial health insurance was most common in all treatment groups, a higher proportion of patients in the NOAC groups had Medicare Advantage health plans than in the warfarin group.
The majority of patients in the warfarin (99.6%) and dabigatran cohorts (63.2%) completed the initial survey in 2012, whereas the majority in the rivaroxaban group (51.6%) completed the initial survey in 2013. All of the patients in the apixaban group completed the initial survey in 2014.
Patients in the warfarin cohort were slightly younger (mean 56.0 years) when diagnosed with atrial fibrillation compared with the NOAC cohorts (mean 62.3 years) and had a longer mean duration of atrial fibrillation (8 years warfarin vs 4 years NOAC; Table 2). As expected, self-reported duration of current therapy reflected the order in which each medication entered the market: 53.9% of the warfarin group were on current therapy for longer than 5 years; 52.6% of the dabigatran group were on current therapy for 1 to 5 years; and 76.6% of the rivaroxaban and 100% of the apixaban groups were on current therapy for less than 1 year. A higher proportion of patients in the NOAC groups (81.6% dabigatran, 79.7% rivaroxaban, 90.0% apixaban) reported using at least one OAC other than their current medication, compared with 42.8% in the warfarin group. Across all treatment groups, cardiologists were most likely the initial and current prescribers of OAC treatment, although the proportion of primary care providers currently prescribing OACs was greater than initial prescribers.
  | Table 2 Clinical characteristics overall and by OAC cohort at initial survey |
Overall, DCI, stroke risk, and bleeding risk scores were similar among all four treatment groups (Table 3). During the baseline period, a higher proportion of patients in the rivaroxaban and apixaban treatment groups had medical claims for coronary artery disease, cardiomyopathy, stroke or TIA event, and bleeding events compared with the warfarin and dabigatran treatment groups.
  | Table 3 Claims-determined comorbidities, stroke, and bleeding risk scores overall and by OAC cohort for the 12-month period before the initial survey |
Adherence
Patients had the opportunity to report their adherence in an initial survey plus up to three follow-up surveys 4, 8, and 12 months later. Overall, 156 patients (23.1%) completed all four surveys (ie, the initial plus three follow-up surveys), 143 patients (21.2%) completed three surveys, 126 (18.7%) completed two surveys, and 250 (37.0%) completed only the initial survey (Table 4). Across the treatment cohorts, the proportions of patients completing the surveys roughly followed the overall pattern.
The mean MMAS-8 scores changed little among the initial and follow-up surveys, both for the overall study population as well as for each treatment cohort (Table 4). The mean overall MMAS-8 score was 7.2, beginning at a high of 7.3 at the initial survey before dipping slightly to 7.2 at the 4- and 8-month surveys and 7.1 at the 12-month survey. The overall mean MMAS-8 score by treatment cohort was 7.3 for all cohorts except dabigatran, which was 7.1. The apixaban cohort reported both the highest (7.5) and lowest (6.8) mean MMAS-8 scores, likely due to the small sample size of that cohort.
Slightly less than half of all patients were adherent according to the claims-based PDC measure (Table 5). Using the self-reported MMAS-8 scores, slightly more than a third of patients were considered adherent. Fewer than one in five patients were considered adherent by both PDC and MMAS.
Health care utilization
Among PDC-adherent patients, a significantly lower proportion had inpatient hospitalizations and ED visits compared with patients considered PDC-nonadherent (Figure 2). However, a greater proportion of PDC-adherent patients had outpatient office visits compared with the PDC-nonadherent patients. These differences were largely eliminated when adherence was measured using MMAS-8 scores (Figure 3). No significant differences were found in terms of inpatient hospitalizations or outpatient visits, but a significant difference was maintained in terms of ED visits.
Medical costs
Total medical costs were significantly lower in the PDC-adherent than the PDC-nonadherent cohort (Figure 4). Unadjusted stroke-related medical costs were similar between the two groups but significantly lower for unadjusted bleeding-related medical costs in the PDC-adherent vs PDC-nonadherent cohort. The adjusted stroke-related total medical costs were 86% lower for PDC-adherent vs PDC-nonadherent cohort, and bleeding-related total medical costs were 75% lower for PDC-adherent vs PDC-nonadherent cohort.
When adherence was measured using the MMAS-8, between-group differences in total medical, stroke-related, and bleeding-related medical costs did not reach statistical significance (Figure 5).
Outpatient medical costs made up the largest proportion of total costs in both PDC- and MMAS-8-adherent cohorts (Figure 6). While they were significantly lower for adherent patients than nonadherent patients defined by PDC, they were not significant between MMAS-8 cohorts. Inpatient hospitalization costs were significantly lower for PDC-adherent than PDC-nonadherent cohorts, but were similar between the MMAS-8-adherent and -nonadherent cohorts. ED costs made up the smallest proportion of total costs and were similar between the adherent and nonadherent cohorts for both PDC and MMAS-8 scores.
Treatment satisfaction
The mean DASS total scores were lower for the dabigatran and rivaroxaban cohorts, indicating greater treatment satisfaction, than for the warfarin and apixaban cohorts, as well as for the overall mean DASS total score (Table 4). This pattern was also seen for the DASS domains of limitations, treatment inconvenience, and psychological impact.
Treatment satisfaction, as measured by the DASS, was slightly higher in the PDC-adherent compared with the PDC-nonadherent cohort (Figure 7); however, the difference was not statistically significant. In contrast, the total DASS score was significantly lower in the MMAS-8-adherent than the MMAS-8-nonadherent cohort, indicating higher treatment satisfaction among MMAS-8-adherent patients. The individual domain scores for limitations, treatment inconvenience, and psychological impact also trended lower among the MMAS-8-adherent cohort compared with the MMAS-8-nonadherent cohort, indicating greater satisfaction, but were statistically similar among the PDC-adherent and PDC-nonadherent cohort patients.
Discussion
This real-world study assessed OAC adherence among patients with NVAF using two alternative methods: the claims-based PDC and the patient-reported MMAS-8. Each measure represents a different perspective: while the PDC provides a broad view of adherence over a 12-month period, the MMAS-8 gives a snapshot of the specific time point at which the questionnaire is completed. Regardless of the measure used, fewer than half of patients with NVAF adhered to their OAC regimen. Adherence rates were higher when measured using PDC rather than MMAS-8 (47.9% PDC vs 37.2% MMAS-8), but only one in five patients (19.4%) were adherent according to both measures.
Our findings in general are consistent with previous results that showed adherence with OAC therapy was associated with reduced health care utilization and lower total health care costs.16 However, the type of HCRU significantly reduced by adherence varied by the criteria used to define adherent behavior. Research in a variety of therapeutic areas, including hypertension and depression as well as atrial fibrillation, has supported the positive relationship between treatment satisfaction and adherence.29–33 While the relationship between treatment satisfaction and adherence may be evident, a literature review highlighted the lack of consistent definitions and measures of adherence and satisfaction.33 The differences between claims-based and patient-reported adherence in the current study further underscores the need for a “gold standard” for measuring adherence and satisfaction.
Overall, patients who were nonadherent according to PDC had significantly higher utilization of inpatient and ED services than patients who were adherent, but only ED use was significantly different between the adherent and nonadherent cohorts according to MMAS-8 scores. On the other hand, MMAS-8-defined adherent patients had greater satisfaction with anticoagulant treatment than MMAS-8-nonadherent patients, but DASS scores were not different between PDC-adherent and PDC-nonadherent cohorts. Although our results support PDC as a more sensitive measure in predicting economic outcomes, it may be less predictive of long-term adherence behavior than the MMAS-8, considering treatment satisfaction is an important factor for maintaining adherence of chronic medications.34 Further research is warranted.
To our knowledge, this is the first study comparing both claims-based and patient-reported adherence measures and their impact on economic burden in patients with NVAF receiving OAC therapy. Strengths of this study included the use of a large administrative claims database as a sampling frame to identify eligible patients to survey and the unique ability to link patients’ medical and pharmacy claims data with their survey data. Our findings suggested that prescription refill patterns and MMAS-8 assess different aspects of adherence that are complementary to each other. While the claims-based PDC may be a more sensitive measure than self-reported adherence in predicting economic outcomes, the MMAS-8 provided supplemental insights in terms of how patients took their OAC on a day-to-day basis. Potential factors that may contribute to adherence – such as lack of patients’ knowledge about OAC use and treating physicians’ compliance with guidelines35 and their role in individualizing treatment management36 – remain subjects for future research.
Our findings should be viewed in light of several limitations. First, the study analyzed data between October 2011 and June 2014. Given that apixaban was approved for stroke prevention in December 2012, the number of patients using apixaban in the study was much smaller than those using other NOACs and particularly those using warfarin. Patients using warfarin tended to have lived with an atrial fibrillation diagnosis longer and had a considerably longer duration of therapy than those using NOACs, which may have affected adherence and the generalizability of the study findings. Second, the study population was identified from the HIRD administrative claims database, which primarily consists of patients with commercial insurance. Therefore, the results may not be generalizable to other populations, such as individuals without insurance, those with government-sponsored insurance (eg, Medicaid), or those living outside the US. Third, PDC was estimated using prescription claims records. We were unable to assess whether patients took the medication as prescribed, and adherence may have been confounded by unobserved patient characteristics that could not be controlled for in our analysis. Lastly, the self-reported patient survey data collected by the MMAS-8 and DASS could not be validated and may have been subject to recall biases. Also, the CHADS2 and bleeding risk scores were determined from claims data. Claims data may have contained undetected coding errors that may have affected the stroke and bleeding risk scores.
Conclusion
Findings from this study showed that using a claims-based or patient self-reported method to measure OAC adherence in patients with NVAF may yield different results when assessing treatment impact on HCRU and costs. Patients who self-reported higher adherence to treatment reported greater treatment satisfaction, which may influence daily prescription-taking behavior. These results underscore the importance of considering both claims-based and patient-reported measures when evaluating treatment adherence in a real-world setting.
Acknowledgment
This study was sponsored by Daiichi Sankyo, Inc. Cheryl Jones, Senior Medical Writer, HealthCore, Inc., wrote the first draft of the manuscript based on input from authors.
Disclosure
WJ Kwong is an employee of Daiichi Sankyo, Inc. JJ Stephenson and H Tan are employees of HealthCore, Inc., a wholly owned subsidiary of Anthem, Inc., which received funding from Daiichi Sankyo, Inc. for the conduct of the study. MU Shinde and A-C Fu were employees of HealthCore, Inc. at the time of the study. WS Weintraub received consulting fees from Daiichi Sankyo, Inc. The authors report no other conflicts of interest in this work.
References
Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clnical practice guidelines. Chest. 2012;141:7S–47S. | ||
AbuDagga A, Stephenson JJ, Fu AC, Kwong WJ, Tan H, Weintraub WS. Characteristics affecting oral anticoagulant therapy choice among patients with non-valvular atrial fibrillation: a retrospective claims analysis. BMC Health Serv Res. 2014;14:310. | ||
Brown JD, Shewale AR, Talbert JC. Adherence to rivaroxaban, dabigatran, and apixaban for stroke prevention in incident, treatment-naive nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2016;22:1319–1329. | ||
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. | ||
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. | ||
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. | ||
Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395–401. | ||
Gallagher AM, Rietbrock S, Plumb J, van Staa TP. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thromb Haemost. 2008;6:1500–1506. | ||
Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Bimingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. | ||
McHorney CA, Crivera C, Laliberte F, et al. Adherence to non-vitamin-K-antagonist oral anticoagulant medications based on the Pharmacy Quality Alliance measure. Curr Med Res Opin. 2015;31:2167–2173. | ||
Zalesak M, Siu K, Francis K, et al. Higher persistence in newly diagnosed nonvalvular atrial fibrillation patients treated with dabigatran versus warfarin. Circ Cardiovasc Qual Outcomes. 2013;6:567–574. | ||
Martinez C, Katholing A, Wallenhorst C, Freedman SB. Therapy persistence in newly diagnosed non-valvular atrial fibrillation treated with warfarin or NOAC. Thromb Haemost. 2015;115:31–39. | ||
Davis NJ, Billet HH, Cohen HW, Amsten JH. Impact of adherence, knowledge, and quality of life on anticoagulation control. Ann Pharmacother. 2005;39:632–636. | ||
Deitzelweig SB, Buysman E, Pinsky B, et al. Warfarin use and stroke risk among patients with nonvalvular atrial fibrillation in a large managed care population. Clin Ther. 2013;35:1201–1210. | ||
Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–2696. | ||
Castellucci LA, Shaw J, van der Salm K, et al. Self-reported adherence to anticoagulation and its determinants using the Morisky medication adherence scale. Thromb Res. 2015;136:727–731. | ||
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. | ||
Patel SI, Cherington C, Scherber R, et al. Assessment of patient adherence to direct oral anticoagulant vs warfarin therapy. J Am Osteopath Assoc. 2017;117:7–15. | ||
Obamiro KO, Chalmers L, Bereznicki LR. A summary of the literature evaluating adherence and persistence with oral anticoagulants in atrial fibrillation. Am J Cardiovasc Drugs. 2016;16:349–363. | ||
Yao X, Abraham NS, Alexander GC, et al. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc. 2016;5(2). pii:e003074. | ||
Wasser TWB, Ycas J, Tunceli O. Applying weighting methodologies to a commercial database to project US Census demographic data. Am J Accountable Care. 2015 September:33–38. | ||
Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10:348–354. | ||
Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–2692. | ||
Deyo RA, Cherkin DC, Cox MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. | ||
Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. | ||
Joundi RA, Cipriano LE, Sposato LA, Saposnik G. Ischemic stroke risk in ptients with atrial fibrillation and CHA2DS2-VASc score of 1: systematic review and meta-analysis. Stroke. 2016;47:1364–1367. | ||
Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713–719. | ||
Samsa G, Matchar DB, Dolor RJ, et al. A new instrument for measuring anticoagulation-related quality of life: development and preliminary validation. Health Qual Life Outcomes. 2004;2:22. | ||
Aljumah K, Hassali AA, AlQhatani S. Examining the relationship between adherence and satisfaction with antidepressant treatment. Neuropsychiatr Dis Treatment. 2014;10:1433–1438. | ||
Hanon O, Chaussade E, Gueranger P, Gruson E, Bonan S, Gay A. Patient-reported treatment satisfaction with rivaroxaban for stroke prevention in atrial fibrillation. A French observational study, the SAFARI Study. PLoS One. 2016;11:e0166218. | ||
Mueller S, Meinecke A-K, Buchwald S, Eiriksson D, Wilke T. Does treatment satisfaction influence adherence to treatment? Impact of AF patients’ treatment satisfaction on adherence to oral anticoagulation treatment. ASH 59th Annual Meeting & Exposition; December 9–12, 2017; Atlanta, GA; 2017. | ||
Zyoud S, Al-Jabi SW, Sweileh WM, Morisky DE. Relationship of treatment satisfaction to medication adherence: findings from a cross-sectional survey among hypertensive patients in Palestine. Health Qual Life Outcomes. 2013;11:191. | ||
Barbosa CD, Balp M-M, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Pat Pref Adherence. 2012;6:39–48. | ||
Almeida Gde Q, Noblat Lde A, Passos LC, do Nascimento HF. Quality of life analysis of patients in chronic use of oral anticoagulant: an observational study. Health Qual Life Outcomes. 2011;9:91. | ||
Potpara TS, Lane DA, Lip GY. Optimizing stroke prevention in atrial fibrillation: better adherence and compliance from patients and physicians leads to better outcomes. Europace. 2015;17:507–508. | ||
Mas Dalmau G, Sant Arderiu E, Enfedaque Montes MB, Sola I, Pequeno Saco S, Alonso Coello P. Patients’ and physicians’ perceptions and attitudes about oral anticoagulation and atrial fibrillation: a qualitative systematic review. BMC Fam Pract. 2017;18:3. | ||
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.









