Back to Journals » Drug Design, Development and Therapy » Volume 10
Comparison of cisplatinum/paclitaxel with cisplatinum/5-fluorouracil as first-line therapy for nonsurgical locally advanced esophageal squamous cell carcinoma patients
Authors Hu G, Wang Z, Wang Y, Zhang Q, Tang N, Guo J, Liu L, Han X
Received 30 January 2016
Accepted for publication 19 April 2016
Published 1 July 2016 Volume 2016:10 Pages 2129—2136
DOI https://doi.org/10.2147/DDDT.S105441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Wei Duan
Guofang Hu,1 Zhehai Wang,2 Yuan Wang,1 Qingqing Zhang,1 Ning Tang,1 Jun Guo,2 Liyan Liu,2 Xiao Han2
1School of Medicine and Life Sciences, University of Jinan, Shandong Academy of Medical Sciences, 2Department of Oncology, Shandong Cancer Hospital, Shandong University, Jinan, Shandong, People’s Republic of China
Background: To retrospectively evaluate the efficacy and toxicity of definitive concurrent chemoradiotherapy (dCRT) with cisplatinum/paclitaxel versus cisplatinum/5-fluorouracil in patients with locally advanced esophageal squamous cell carcinoma (ESCC) who received nonsurgical treatment.
Methods: This study retrospectively evaluated 202 patients with locally advanced ESCC treated at Shandong Cancer Hospital between January 2009 and December 2013. All the patients initially received dCRT, including platinum and paclitaxel or 5-fluorouracil, with concurrent 1.8 or 2 Gy/fraction radiation (total dose, 54–60 Gy). The patient population was divided into two treatment groups: 105 patients who received the cisplatinum/paclitaxel regimen were allocated to group A, and 97 patients who received the cisplatinum/5-fluorouracil regimen were allocated to group B. We compared the progression-free survival (PFS) and overall survival (OS) by various clinical variables, including prior treatment characteristics, major toxicities (mainly in grade 3 and 4 hematological), and response to dCRT. We used the receiver operating curve analysis to determine the optimal cutoff value of clinical stage and radiation dose. The Kaplan–Meier method was used for survival comparison and Cox regression for multivariate analysis.
Results: Median PFS and OS in group A were significantly better compared with group B (median PFS, 15.9 versus 13.0 months, P=0.016 and median OS, 33.9 versus 23.1 months, P=0.014, respectively). The 1- and 2-year survival rates of the two groups were 82.9% versus 76.3%, and 61.9% versus 47.6%, respectively. The complete response and response rate were 17.1% versus 7.2% (P=0.032) and 52.4% versus 30.9% (P=0.042) in group A and B, respectively. Meanwhile, group B was associated with a significantly lower rate of grade 3/4 overall toxicity than group A (P=0.039).
Conclusion: Our data showed that patients with locally advanced ESCC in group A had longer PFS and OS compared with group B. Cisplatinum/paclitaxel can be considered a good candidate chemotherapy regimen for patients with locally advanced ESCC who are being treated with nonsurgical therapy.
Keywords: esophageal carcinoma, definitive chemoradiotherapy, complete response, survival, toxicity
Introduction
Esophageal carcinoma (EC) is the sixth leading cause of cancer-related deaths and the eighth most common cancer worldwide.1 Locally advanced esophageal cancer was defined as clinical stage IB–IIIC in the 7th edition of Union for International Cancer Control TNM classification, accounts for more than half of all the esophageal cancers in the world, and esophageal squamous cell carcinoma (ESCC) has been the main target of Japan Clinical Oncology Group studies.2 For locally advanced ESCC, surgery is the optimal treatment method. However, for most patients who are not eligible for curative intended surgery or have refused surgery when diagnosed, chemotherapy, radiotherapy, or chemoradiotherapy have to be considered as alternative options. Also, previous studies have shown that definitive concurrent chemoradiotherapy (dCRT) is superior to chemotherapy and radiotherapy alone.3–6 A randomized clinical trial named RTOG 85-01 compared chemoradiotherapy with cisplatinum plus 5-fluorouracil (5-FU) to radiotherapy alone in patients not receiving surgery and demonstrated the superiority of chemoradiotherapy with improved 5-year survival rates of up to 26%.4 Han et al5 compared concurrent chemoradiotherapy (nedaplatin, 20 mg/m2/day, 5-FU, 500 mg/m2/day for 4 days) with radiotherapy alone and demonstrated that the 3-year survival rate in the dCRT group was higher than the radiotherapy group (40% versus 18.5%, P=0.007). van Hagen et al6 compared chemoradiotherapy followed by surgery and surgery alone in patients with esophageal or esophagogastric junction cancer, the median overall survival (OS) was significantly longer in the chemoradiotherapy–surgery group than surgery alone group (49.4 versus 24.0 months, P=0.003). In Western countries, the cisplatinum/5-FU regimen has been widely used clinically for many years and now is recommended as the standard treatment for EC.7,8 However, many Asian clinicians are reluctant to use these regimens directly in their clinical practice because the majority of the enrolled patients in Western trials had adenocarcinoma, while most of the Asian patients suffer from squamous cell carcinoma. Paclitaxel is one of the most promising drugs for esophageal cancer. Several exploratory trials have investigated paclitaxel plus cisplatinum, paclitaxel plus cisplatinum and 5-FU in preoperative and postoperative chemotherapy and have shown promising outcomes.9–11 Zemanova et al11 reported clinical results using cisplatinum and 5-FU with or without paclitaxel. Unfortunately, no clear survival benefit was observed except significantly increased hematological and nonhematological toxicity as a result of the treatment. Based on these backgrounds, the aim of this study was to evaluate the clinical outcomes of dCRT with cisplatinum/paclitaxel versus cisplatinum/5-FU in patients with locally advanced ESCC without surgery and identify prognostic factors for tumor control and survival.
Patients and methods
Patients
Data from patients who were treated at the Shandong Cancer Hospital from January 2009 to December 2013 were screened and 202 patients with locally advanced ESCC were analyzed retrospectively. These patients satisfied the following selection criteria: 1) 18–75 years of age at diagnosis; 2) cytopathologically confirmed as ESCC; 3) clinical stage IIB–IIIC based on the 7th Union for International Cancer Control TNM classification; 4) Eastern Cooperative Oncology Group performance status of 0 or 1; 5) cisplatinum-based doublet first-line chemotherapy for at least two cycles concurrent with radiotherapy; 6) no prior cancer therapy or concomitant malignancy; 7) no uncontrolled diabetes or other serious disease; 8) complete and retrievable clinical medical records; and 9) no surgical treatment related to EC during the follow-up period. The study was approved by the Medical Ethics Committee of the Shandong Cancer Hospital. As the study data were obtained by reviewing medical records, the Medical Ethics Committee of the Shandong Cancer Hospital did not require that participant consent be obtained, although the authors confirm that all patients had signed a written informed consent before treatment.
Treatment
Chemotherapy regimens
In group A, the patients were treated with cisplatinum (75 mg/m2, days 1 and 2) and paclitaxel (50 mg/m2, days 1 and 8), with concurrent radiotherapy. In group B, the patients were treated with cisplatinum (75 mg/m2, days 1 and 2) and 5-FU (1,000 mg/m2, days 1–4), with concurrent radiotherapy. Each cycle of chemotherapy was repeated every 21 days and each patient received at least two cycles of chemotherapy. Complete blood counts were performed before and after every cycle of chemotherapy. In cases of severe hematologic toxicity, dose adjustment was implemented in the next chemotherapy cycle. If the white blood cell was under 3,000/mm3 after the chemotherapy, the patient was treated with recombinant human granulocyte colony-stimulating factor injection. If the white blood cell count was down to 2,000–2,500/mm3, the dose of paclitaxel, 5-FU, and cisplatinum was temporarily decreased by 50% at the beginning of the next course of chemotherapy; radiotherapy was continued as before. In the event of grade 4 hematological or grade 3/4 gastrointestinal reaction, the doses were reduced by 25% in the subsequent course and radiotherapy was continued. Both chemotherapy and radiation were discontinued if grade 4 hematological or grade 3/4 gastrointestinal reaction, or radiation pneumonia/esophagitis were observed; the CRT was discontinued until recovery from toxicity. Chemotherapy treatment was discontinued if disease progression was observed, toxicity recovery before the next cycle of chemotherapy was not possible, or patients refused to continue.
Radiotherapy scheme
Radiotherapy planning was carried out after direct simulation based on diagnostic or three-dimensional treatment planning computed tomography images. Gross tumor volume (GTV) was defined as macroscopic primary tumor and regional lymph node metastases reconstructed using all available information derived from endoscopy, endoscopic ultrasound, computed tomography, and from fluorodeoxyglucose positron emission tomography if available. During direct simulation, margins from GTV to field margin were 5 cm in the caudal/cranial direction and 2 cm in the transverse plane. To generate the planning target volume, a margin of 4 cm in the caudal/cranial direction and 1.5 cm in the transverse plane was used. The clinical target volume was defined as the GTV plus the volume of a 3 cm margin along the length of the esophagus and 1 cm radical margin, as well as elective nodal regions. A 1 cm margin was used around the pathological lymph nodes. The planning target volume was generated by expanding the clinical target volume by a margin of 1 cm in all directions to account for setup uncertainties and organ motion. A total dose of 54–60 Gy was given in fractions of 1.8–2 Gy five times per week and was generally delivered by three-dimensional conformal radiotherapy with at least 6 MV photons. The treated length was equivalent to the tumor length estimated at endoscopy plus 1–2 cm margins in the cranial and caudal directions. The required dose was specified at 1 cm from the radiation source or 0.5 cm under the mucosal surface.
Evaluation methods
Based on the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 guidelines,12 status of response to treatments was classified into complete response (CR), partial response (PR), stable disease, and progressive disease. Response rate (RR) was defined as CR plus PR, and CR, PR, and stable disease were seen as disease control rate. Toxicity of dCRT was evaluated according to the Common Terminology Criteria for Adverse Events 4.0.
Follow-up
Follow-up was performed on all patients. The last follow-up was in December 2015, and the median duration of follow-up was 44.6 months. Survival data were collected through a positive follow-up. OS was measured from the starting date of first-line (chemo) radiotherapy treatment to death from any cause or last follow-up. Progression-free survival (PFS) was measured from the starting date of first-line (chemo) radiotherapy treatment to disease progression or death.
Statistical analysis
Rates were compared using the χ2 test. Fisher’s exact test was used to analyze categorical variables. Median PFS and median OS were calculated using the Kaplan–Meier method. We used the Kaplan–Meier method to draw survival curves and tested these using the log-rank test. The optimal cutoff value of clinical stage and radiation dose were determined using the receiver operating curve analysis with Youden’s index (J, J =sensitivity+specificity - 1). The Cox regression model was used to identify independent prognostic factors for locally advanced ESCC. Two-sided P-value <0.05 was considered to be statistically significant. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, Chicago, IL, USA) software, version 17.0.
Results
Patient and tumor characteristics
Patient and tumor characteristics are shown in Table 1. For the 202 patients included in this study, the median age was 61 years (range 43–75 years), with 194 (96.0%) being female. All patients had cytopathologically diagnosed ESCC; 23 (11.4%) patients had stage IIB disease, 103 (51.0%) had stage IIIA disease, 35 (17.3%) had stage IIIB disease, and 41 (20.3%) had stage IIIC disease. Baseline characteristics were well balanced between the two groups from this study except that group A had more comorbidities (56.2%) compared with group B (40.2%, P=0.023). All the patients were treated with cisplatinum-based doublet chemotherapy and concurrent radiotherapy as the first-line treatment, of them 105 patients received paclitaxel plus cisplatinum in group A and 97 patients received cisplatinum plus 5-FU in group B. The general course was for four to six cycles.
Efficacy
All 202 patients were able to be evaluated for efficacy. Response to treatment for locally advanced ESCC patients are displayed in Table 2. CR was obtained in 18 (17.1%) versus seven (7.2%) patients in groups A and B (P=0.032), respectively. RR was significantly better in group A (52.4%) than group B (38.1%, P=0.042). No difference in disease-control rate was found between the two groups (96.2% versus 94.8%, P=0.740). The efficacy of the first-line treatment is shown in Table 3.
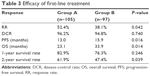  | Table 3 Efficacy of first-line treatment |
Survival
For the whole group, the median PFS was 14.6 months (95% confidence interval [CI], 13.0–16.2 months), median OS was 27.8 months (95% CI, 22.4–33.2 months), and the 1- and 2-year survival rates were 79.7% and 55.0%, respectively. PFS was comparable between the two groups and the difference was significant between groups A and B (15.9 versus 13.0 months, P=0.016). Patients in group A were found to have a longer median OS (33.9 months) and 1- and 2-year survival rates (82.9% and 61.9%, respectively) when compared with group B (23.1 months, P=0.014, and 1- and 2-year survival rates of 76.3% versus 47.4%, respectively). Distant metastasis rate was also significantly lower in group A than in group B (18.1% versus 30.9%, P=0.034). PFS and OS curves of the two groups are shown in Figures 1 and 2, respectively. Univariate analysis of various prognostic factors and the data indicated that the important factors for PFS were radiation dose (P=0.002), different chemotherapy regimens (P=0.018), and response to treatment (CR, P<0.001 and RR, P<0.001). To improve the accuracy of statistical results, if the P-value of a variable was <0.10 in the univariate analysis, it was included in the multivariate analysis. On the contrary, if the P-value of a variable was ≥0.10 in the univariate analysis, it was excluded from the multivariate analysis. Results showed that radiation dose (P<0.001), different chemotherapy regimens (P=0.001), and RR (P<0.001) were independent prognostic factors of PFS. Furthermore, we used the Cox multivariate regression analysis to determine factors influencing OS. The results showed that being earlier clinical stage (P=0.018), treated with cisplatinum/paclitaxel regimen (P=0.043), and CR after first-line dCRT (P=0.010) were independent prognostic factors of OS (Table 4).
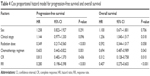  | Table 4 Cox proportional hazard model for progression-free survival and overall survival |
In order to ascertain the specific factors affecting survival, we conducted a further study of the clinical stage and radiation dose with optimal cutoff value. For the entire cohort, the optimal cutoff value of the clinical stage was IIIA, with a sensitivity of 91.43% and a specificity of 14.43%; then we divided all the cases into two groups, namely stage II (stage IIB) and stage III (included stage IIIA–C). We further evaluated the PFS and OS value between the two groups based on different stages. Patients with stage II showed a substantially better PFS and OS than those with stage III (median PFS, 27.7 versus 16.1 months, P=0.001 and median OS 49.9 versus 32.1 months, P=0.001, respectively). The optimal cutoff value of radiation dose to predict survival was 59.5 Gy based on the receiver operating curve analysis (Figure 3) with the area under the curve of 0.566 (95% CI, 0.487–0.645, P=0.105). We divided all the patients into two groups based on the optimal cutoff value of radiation dose and found that there were no significant differences between these two groups both in PFS and OS (median PFS, 18.5 versus 15.5 months, P=0.114 and median OS, 35.7 versus 30.7 months, P=0.131, respectively). To distinguish the influence of clinical stage, we compared the survival of different chemotherapy regimens and radiation doses among all the patients with stage III disease and showed that OS was significantly better in patients treated with the cisplatinum/paclitaxel regimen than cisplatinum/5-FU regimen (median OS, 36.0 versus 26.9 months, P=0.004). However, PFS was not obviously significant (median PFS, 17.4 versus 14.3 months, P=0.055). Moreover, radiation dose had no significant discriminative effect among patients with stage III disease (median PFS, 17.0 versus 14.5 months, P=0.241 and median OS, 33.1 versus 30.2 months, P=0.417, respectively). The final statistical results showed that being earlier clinical stage (stage II), treated with cisplatinum/paclitaxel regimen and CR after first-line dCRT were independent positive prognostic factors of survival.
Toxicity and feasibility of different therapies
Toxicity of dCRT was evaluated according to the Common Terminology Criteria for Adverse Events 4.0. We documented the main grade 3/4 toxicities, and no grade 5 toxicity or side effects-related deaths had occurred among patients in both different groups. The grade 3/4 toxicities of patients in group B were akin to data reported previously.13–15 For all patients, the most common toxicities were hematological toxicity and gastrointestinal reaction. Compared with previous studies, the grade 3/4 adverse effects of dCRT in the two groups were decreased, which was mainly attributed to the administration of antiemetic drugs before chemotherapy to decrease the incidence of gastrointestinal reaction.16,17 Furthermore, the hematologic toxicity was manageable, and prophylactic granulocyte colony-stimulating factor could ameliorate it. In addition, one patient in group A experienced grade 3 radiation pneumonia and one patient in group B experienced grade 3 radiation esophagitis, but they were well tolerated. The occurrence of nonhematological toxicity between the two groups was not significantly different, and patients treated with cisplatinum/paclitaxel regimen showed good tolerance. Detailed toxicities are displayed in Table 5.
  | Table 5 Major toxicities during dCRT |
Discussion
Based on the present clinical evidence, the cisplatinum/paclitaxel regimen with concurrent radiotherapy was associated with improved survival compared with standard cisplatinum/5-FU regimen for patients with locally advanced ESCC. The chemotherapy regimen greatly affected the CR and RR, which has been proven to be independent prognostic factors for survival.18,19 To our knowledge, the cisplatinum/paclitaxel regimen has never been compared with the cisplatinum/5-FU regimen directly, for patients with locally advanced ESCC who are not receiving curative intended surgery. Therefore, the optimal chemotherapy regimen is debatable. Our results indicate that patients who received the cisplatinum/paclitaxel regimen had a significant survival advantage over patients who received the cisplatinum/5-FU regimen.
The neoadjuvant regimen of cisplatinum/5-FU combination has been widely used in clinical practice for EC for many years.20 Its clinical efficacy has been recognized by clinicians. However, its benefit is limited, with RR of 21%–43.2% and 2-year survival rate of 44%–63% being reported for locally advanced EC.21–24 Furthermore, the enrolled patients in these studies had esophageal adenocarcinoma, whilst most of the Asian EC patients had squamous cell carcinoma, therefore, these studies were not suitable for the Asian EC patients. Cisplatinum/paclitaxel as a new chemotherapy regimen used in locally advanced ESCC is gradually being accepted by physicians and certain effects have been achieved. Huang et al10 undertook a comprehensive review of ten articles to quantify the survival benefit of cisplatinum/paclitaxel regimen. Their data demonstrated that patients with locally advanced ESCC who received cisplatinum/paclitaxel regimen had a potentially superior survival benefit over those who received cisplatinum/5-FU regimen in neoadjuvant CRT. Li et al15 investigated 59 patients with ESCC and found that those with cisplatinum/paclitaxel regimen had a better RR and longer survival compared with cisplatinum/5-FU regimen. A study reported by Ruhstaller et al9 found that squamous cell carcinoma was associated with a longer survival compared with adenocarcinoma in cisplatinum/paclitaxel regimen. Our analysis of survival in 202 patients with locally advanced ESCC indicates that those in group A had longer PFS and OS than those in group B. Earlier researches10,18,25,26 had shown that patients achieving CR after treatment had longer survival than patients achieving no CR, suggesting that CR might have a positive effect on survival. Rizvi et al25 found that patients had obviously longer survival (62.73±17.02 versus 41.42 months) with complete pathological response compared to those with residual disease. Scheer et al18 found that median survival times for patients with pathological CR were significantly longer than patients with residual tumor (P=0.011). Our research also indicates that patients with CR had a longer survival time after first-line dCRT than those with no CR (55.5 versus 30.7 months, P<0.001). With respect to the adverse effects of this regimen, there were no grade 5 adverse reactions observed in patients and no toxicity related deaths had occurred. Thus, our finding suggests that the cisplatinum/paclitaxel regimen with concurrent radiotherapy as the first-line treatment had a better curative rate and tolerable side effects for locally advanced ESCC patients who had no surgery. However, this is a nonrandomized comparison. Although the patients in both groups were well matched by confounding the characteristics of patient and tumor before treatment, its retrospective nature still has potential selection bias. Meanwhile, patients in group A had more comorbidities, which could suggest a possible selection bias. Further prospective studies are needed to confirm our current findings.
In this study, we noted differences in PFS, OS, CR, and RR between the two groups. Multivariate analysis revealed that chemotherapy regimen and RR were strongly related to survival, and this is consistent with the results of previous researches.19,27 A study reported by Chang et al19 demonstrated that treatment response is the strongest independent prognostic factor of survival. A previous randomized Phase III clinical trial showed that patients with the higher RR had longer survival than those with lower RR, suggesting that RR might closely be related to longer survival.27 For the whole cohort, multivariate analysis indicated that being earlier clinical stage, radiation dose of 54–60 Gy, chemotherapy with cisplatinum/paclitaxel regimen, and CR after dCRT were independent positive prognostic factors of survival.
Conclusion
Our research, based on a direct comparison of two treatment regimens, indicated a survival benefit from the cisplatinum/paclitaxel regimen over that of the cisplatinum/5-FU regimen in dCRT administered to locally advanced ESCC patients who had not undergone surgery.
Disclosure
The authors report no conflicts of interest in this work.
References
Di Pardo BJ, Bronson NW, Diggs BS, Thomas CR Jr, Hunter JG, Dolan JP. The global burden of esophageal cancer: A disability-adjusted life-year approach. World J Surg. 2016;40(2):395–401. | ||
Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol. 2013;43(7):752–755. | ||
Smit JK, Muijs CT, Burgerhof JG, et al. Survival after definitive (chemo) radiotherapy in esophageal cancer patients: A population-based study in the north-East Netherlands. Ann Surg Oncol. 2013;20(6):1985–1992. | ||
Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281(17):1623–1627. | ||
Han J, Zhu W, Yu C, Zhou X, Li T, Zhang X. Clinical study of concurrent chemoradiotherapy or radiotherapy alone for esophageal cancer patients with positive lymph node metastasis. Tumori. 2012;98(1):60–65. | ||
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. | ||
Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):51–56. | ||
Stahl M, Budach W, Meyer HJ, Cervantes A; ESMO Guidelines Working Group. Esophageal cancer: Clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):46–49. | ||
Ruhstaller T, Widmer L, Schuller JC, et al. Multicenter phase II trial of preoperative induction chemotherapy followed by chemoradiation with docetaxel and cisplatin for locally advanced esophageal carcinoma (SAKK 75/02). Ann Oncol. 2009;20(9):1522–1528. | ||
Huang TC, Hsu CH, Lin CC, Tu YK. Systematic review and network meta-analysis: neoadjuvant chemoradiotherapy for locoregional esophageal cancer. Jpn J Clin Oncol. 2015;45(11):1023–1028. | ||
Zemanova M, Petruzelka L, Pazdro A, et al. Prospective non-randomized study of preoperative concurrent platinum plus 5-fluorouracil-based chemoradiotherapy with or without paclitaxel in esophageal cancer patients: long-term follow-up. Dis Esophagus. 2010;23(2):160–167. | ||
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. | ||
Liu S, Yang H, Zhang P, et al. Neoadjuvant chemoradiotherapy with cisplatin plus vinorelbine versus cisplatin plus fluorouracil for esophageal squamous cell carcinoma: A matched case–control study. Radiother Oncol. 2015;116(2):262–268. | ||
Honing J, Smit JK, Muijs CT, et al. A comparison of carboplatin and paclitaxel with cisplatinum and 5-fluorouracil in definitive chemoradiation in esophageal cancer patients. Ann Oncol. 2014;25(3):638–643. | ||
Li QQ, Liu MZ, Hu YH, Liu H, He ZY, Lin HX. Definitive concomitant chemoradiotherapy with docetaxel and cisplatin in squamous esopheageal carcinoma. Dis Esophagus. 2010;23(3):253–259. | ||
Weinstein C, Jordan K, Green SA, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: results of a randomized, double-blind phase III trial. Ann Oncol. 2016;27(1):172–178. | ||
Abe M, Hirashima Y, Kasamatsu Y, et al. Efficacy and safety of olanzapine combined with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy in gynecological cancer: KCOG-G1301 phase II trial. Support Care Cancer. 2016;24(2):675–682. | ||
Scheer RV, Fakiris AJ, Johnstone PA. Quantifying the benefit of a pathologic complete response after neoadjuvant chemoradiotherapy in the treatment of esophageal cancer. Int J Radiat Oncol Biol Phys. 2011;80(4):996–1001. | ||
Chang WL, Wang WL, Chuang TJ, et al. Response evaluation with endoscopic ultrasound and computed tomography in esophageal squamous cell carcinoma treated by definitive chemoradiotherapy. J Gastroenterol Hepatol. 2015;30(3):463–469. | ||
Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–692. | ||
Courrech Staal EF, Aleman BM, van Velthuysen ML, et al. Chemoradiation for esophageal cancer-Institutional experience with three different regimens. Am J Clin Oncol. 2011;34(4):343–349. | ||
Nomura M, Oze I, Abe T, et al. Impact of docetaxel in addition to cisplatin and fluorouracil as neoadjuvant treatment for resectable stage III or T3 esophageal cancer: a propensity score-matched analysis. Cancer Chemother Pharmacol. 2015;76(2):357–363. | ||
Yang H, Yao J, Wen H, et al. Clinical evaluations of neoadjuvant chemotherapy with DN and FP regimen for patients with middle or lower thoracic locally advanced esophageal squamous cell carcinoma. Zhonghua Yi Xue Za Zhi. 2015;95(19):1530–1533. | ||
Fu C, Li B, Guo L, et al. Phase II study of concurrent selective lymph node late course accelerated hyper-fractionated radiotherapy and pemetrexed and cisplatin for locally advanced oesophageal squamous cell carcinoma. Br J Radiol. 2014;87(1037):20130656. | ||
Rizvi FH, Syed AA, Khattak S, Rizvi SS, Kazmi SA, Khan MQ. Complete pathological response after neoadjuvant treatment in locally advanced esophageal cancer predicts long term survival: a retrospective cohort study. Int J Surg. 2014;12(6):621–625. | ||
Kordes S, van Berge Henegouwen MI, Hulshof MC, et al. Preoperative chemoradiation therapy in combination with panitumumab for patients with resectable esophageal cancer: the PACT study. Int J Radiat Oncol Biol Phys. 2014;90(1):190–196. | ||
Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC – A randomized Phase III trial. J Clin Oncol. 2016;34(5):443–451. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.