Back to Journals » Cancer Management and Research » Volume 12
Comparison of chemoembolization with CalliSpheres® microspheres and conventional chemoembolization in the treatment of hepatocellular carcinoma: a multicenter retrospective study
Authors Liang B , Xiang H, Ma C, Xiong B, Ma Y, Zhao C, Yao Y, Zhang Z, Chen C , Li H, Long Q, Zhou J, Luo C, Qiu H, Hu H, Zhao H , Zhou G , Zheng C
Received 13 September 2018
Accepted for publication 27 June 2019
Published 10 February 2020 Volume 2020:12 Pages 941—956
DOI https://doi.org/10.2147/CMAR.S187203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Bin Liang, 1,* Hua Xiang, 2,* Cong Ma, 3 Bin Xiong, 1 Yilong Ma, 4 Chang Zhao, 4 Yuanhui Yao, 2 Zishu Zhang, 3 Changyong Chen, 5 Haiping Li, 5 Qingyun Long, 6 Jun Zhou, 6 Chao Luo, 7 Huaiming Qiu, 7 Hongyao Hu, 8 Hui Zhao, 8 Guofeng Zhou, 1 Chuansheng Zheng 1
1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Department of Interventional Radiology, Hunan Provincial People’s Hospital, Changsha, People’s Republic of China; 3Department of Radiology, The Second Xiangya Hospital of Central South University, Changsha, People’s Republic of China; 4Department of Interventional Radiology, Cancer Hospital Affiliated to Guangxi Medical University, Nanning, People’s Republic of China; 5Department of Radiology, Xiangya Hospital General South University, Changsha, People’s Republic of China; 6Department of Interventional Radiology, Zhongnan Hospital of Wuhan University, Wuhan, People’s Republic of China; 7Department of Radiology, Wuhan General Hospital of Guangzhou Military Region, Wuhan, People’s Republic of China; 8Department of Interventional Radiology, Renmin Hospital of Wuhan University, Hubei General Hospital, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chuansheng Zheng
Department of Radiology, Hubei Key Laboratory of Molecular Imaging, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Road, Wuhan 430022, People’s Republic of China
Email [email protected]
Guofeng Zhou
Department of Radiology, Hubei Key Laboratory of Molecular Imaging, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, People’s Republic of China
Email [email protected]
Purpose: This study aimed to compare the efficacy and safety between transarterial chemoembolization (TACE) with CalliSpheres® microspheres (CSM-TACE) and conventional TACE (cTACE) in patients with hepatocellular carcinoma (HCC).
Patients and Methods: Three hundred and thirty-five HCC patients receiving CSM-TACE or cTACE were consecutively enrolled in this multi-center, retrospective cohort study, and then divided into CSM-TACE group and cTACE group accordingly. Complete response (CR), objective response (ORR) and disease control response (DCR) was assessed according to mRECIST criteria at 1 month (M1), 3 months(M3) and 6 months(M6) after treatment. Progression-free survival (PFS) and overall survival (OS) were assessed. Liver function indexes and adverse events (AEs) were also evaluated.
Results: CR at M3 (P=0.020) and ORR at M1 (P< 0.001), M3 (P< 0.001) and M6 (P=0.017) after treatment were significantly higher in the CSM-TACE compared with cTACE group. DCRs, PFS (25.3 months vs 24.2 months, P=0.503) and OS (27.8 months vs 25.3 months, P=0.203) were similar between the two groups. CSM-TACE was independently correlated with higher ORR at M1 (P=0.002) and longer OS (P=0.023). Abnormal alkaline phosphatase (ALP) (P=0.049) was independently associated with lower ORR at M3, and history of alcohol intake (P=0.019) and largest nodule size ≥ 7 cm (P=0.015) independently correlated with lower ORR at M6 (P=0.015). Largest nodule size ≥ 7 cm (P=0.029) and abnormal albumin (ALB) (P=0.046) were independently associated with shorter PFS. Child-Pugh stage B/C (P=0.023), abnormal ALB (P=0.001), ALP (P=0.008) and alpha-fetoprotein (AFP) (P=0.005) were independently associated with shorter OS. Most liver function indexes and AEs were similar between the two groups (P> 0.05), except that ALP (P=0.005), total bilirubin (P=0.031), pain during procedure (P=0.034) and occurrence of fever post(treatment (P=0.017) were significantly elevated in the CSM-TACE compared with cTACE group.
Conclusion: CSM-TACE presents with a better treatment response and similar survival profile compared with cTACE in HCC patients.
Keywords: chemoembolization, CalliSpheres microspheres, hepatocellular carcinoma, treatment response, survival, predictive factor
Introduction
Hepatocellular carcinoma (HCC), the most prevalent liver cancer, is the sixth most common cancer and the third leading etiology of cancer-related deaths worldwide. HCC demands a large amount of medical resources and is expected to be even more prevalent in the ensuing decades.1,2 Apart from the relatively high prevalence of HCC, poor prognosis is another critical issue in its management, with the median survival of advanced stage patients being 6-8 months and the median survival of terminal stage patients being <3-4 months according to the updated Barcelona Clinic Liver Cancer (BCLC) staging system.2 Although there are potential curative therapies for HCC patients, which includes surgical resection, liver transplantation and ablation, these treatment options are only restricted to patients in very early and early stages.
Transarterial chemoembolization (TACE) is a well-established procedure for patients with unresectable HCC. Conventional TACE (cTACE) has presented a survival advantage in selected HCC patients,3,4 but the procedure has several limitations, which include the relatively high incidence of systemic toxicity and varied procedure standards.5,6 Drug-eluting bead TACE (DEB-TACE), using an advanced technology that has a more controlled release and sustained concentration of chemotherapeutics in patients, has been developed and it has become an increasingly popular TACE technique in unresectable HCC patients.7 There are growing evidence revealing that DEB-TACE is at least equal to cTACE in regard to treatment response and survival. However, the comparison of their efficacy in a multicenter study including a larger sample size is rare.8,9 Moreover, CalliSpheres® microspheres (CSM), the first microsphere produced in the People’s Republic of China that is used in DEB-TACE procedure, has not been sufficiently investigated in view of its efficacy compared with cTACE in treating HCC patients.
Therefore, the aim of this study was to compare the treatment response and survival profiles between TACE with CalliSpheres® microspheres (CSM-TACE) and cTACE in HCC patients.
Materials and methods
Study design
DECTH study (DEB-TACE versus cTACE for HCC) was a multi-center, retrospective cohort study with the purpose of comparing the efficacy and safety between CSM-TACE treatment and cTACE treatment in Chinese HCC patients. It included eight medical centers in People’s Republic of China (Table S1) and was approved by the Institutional Review Board of each participating center. This study was part of DECTH study and compared the treatment response and survival profiles between CSM-TACE and cTACE. The study was conducted in accordance with the Declaration of Helsinki.
Participants
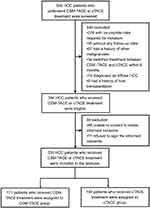
Three hundred and thirty-five HCC patients who received CSM-TACE or cTACE treatment between September 2014 and August 2017 were consecutively enrolled in this multi-center, retrospective cohort study. The inclusion criteria included: 1) patients diagnosed as primary HCC confirmed by clinical or pathological findings; 2) patients aged at least 18 years old; 3) patients who underwent CSM-TACE or cTACE treatment; 4) with complete demographic data, history, diagnosis, clinical detail, pathology results, treatment, measurement and assessment. The exclusion criteria were: 1) patients who were diagnosed with diffuse HCC, hepatobiliary cell carcinoma, mixed cell carcinoma or lamellar cell carcinoma; 2) patients with history of liver transplantation or other malignancies; 3) patients who were lost to follow-up without any follow-up data; 4) patients who switched treatment between CSM-TACE and cTACE within 6 months. Figure 1 outlines eligibility criteria for study inclusion and pation allocation.
Data collection
After obtaining written informed consents, patients’ data was extracted from electronic medical records and the medical records department, which included the demographic information, medical history, clinical findings, laboratory results of blood investigations, liver and kidney function tests, tumor marker indexes, previous treatments, the records of equipment and drugs used in CSM-TACE and cTACE procedures, assessment of treatment response, documentation of adverse events (AEs) and follow-up of patients’ survival. Patients’ baseline information was collected, including: 1) demographic characteristics: age and gender; 2) medical history: alcohol intake, hepatitis B, hepatitis C and cirrhosis; 3) clinical features: tumor location (unilobar or bilobar), tumor distribution (multifocal disease or unifocal disease), largest nodule size, portal vein invasion, hepatic vein invasion, Eastern Cooperative Oncology Group (ECOG) performance status, Child-Pugh stage and BCLC stage; 4) laboratory indexes of blood routine investigations, liver and kidney function: white blood cell, red blood cell, absolute neutrophil count, haemoglobin (Hb), platelet, albumin (ALB), total protein (TP), total bilirubin (TB), total bile acid (TBA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), blood creatinine (BCr) and blood urea nitrogen (BUN); 5) tumor marker indexes: alpha-fetoprotein (AFP), carcino-embryonic antigen (CEA) and carbohydrate antigen199 (CA199); 6) previous treatments: cTACE, surgery, systematic chemotherapy, radiofrequency ablation and targeted therapy.
Grouping
Depending on the treatment options, patients who received CSM-TACE treatment were assigned to CSM-TACE group (N=171), and the others who received cTACE treatment were assigned to the cTACE group (N=164) accordingly.
TACE procedures
In the CSM-TACE group, the CSMs of 100–300 μm or 300–500 μm in size (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Province, People’s Republic of China) were used as both drug carrier and embolic agent. The loading and preparation of CSM was carried out using an aseptic technique. Fifty or 80 mg of epirubicin powder was first diluted with saline and then added into one vial of CSM solution to obtain an initial loading volume of 8 cc. Thirty minutes later, the doxorubicin-loaded CSMs solution was mixed with 8 mL of nonionic isotonic contrast medium to obtain a final injectable volume of 16 cc. All CSM-TACE procedures were performed under the guidance of digital subtraction angiography. Briefly, after accessing the common femoral artery through the Seldinger technique and subsequent insertion of an arterial introducer sheath, a 5F visceral catheter was introduced to catheterize the common hepatic artery. Selective arteriography was performed to detect hypervascular tumor and its supplying arteries. A 2.7F coaxial microcatheter system (Progreat, Terumo, Tokyo, Japan) was advanced into the tumor-feeding arteries, and then the epirubicin-loaded CSMs were administered manually through the microcatheter under fluoroscopic guidance. The embolization endpoint was complete disappearance or remarkable decrease of tumor stain. For massive HCC lesion, if the embolization endpoint was not obtained after injection of one vial of CSM, another vial of CSM was injected or a repeat course of CSM-TACE was scheduled.
In the cTACE group, after superselective catheterization of the tumor-feeding arteries, a solution of single (epirubicin 50–80 mg) or multiple chemotherapeutic agents (epirubicin 50–80 mg, cisplatin, oxaliplatin or lobaplatin 50–100 mg, and 5-Fu or floxuridine 1.0 g) with ethiodized oil was subsequently injected. This was followed by injection of embolic particles, such as gelatin sponge, polyvinyl alcohol or calibrated microspheres. The embolization endpoint was complete stasis or near stasis of the blood flow. For massive HCC lesion, multiple cTACE procedures were performed.
Pre-procedure and post-procedure treatments
Pre-procedure and post-procedure treatments were provided in all patients. Antiemetic and antibiotic prophylaxis were conventionally given according to standard institutional protocols before the CSM-TACE or cTACE procedure. Pain medication, antibiotic prophylaxis, antiemetic therapy, and gastric protection were provided after TACE procedure.
Response assessments and definitions
Treatment response was assessed by triple-phase CT or MR imaging, which was conducted at 1 month(M1), 3 months (M3) or 6 months (M6) after TACE treatment. The evaluation criteria of treatment response were in accordance with the modified Response Evaluation Criteria in Solid Tumors, which were defined as follows: 1) complete response (CR): disappearance of any intratumoral arterial enhancement in all target lesions; 2) partial response (PR): at least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions; 3) stable disease (SD): any cases that did not qualify to be either PR or progressive disease (PD); 4) PD: an increase of at least 20% in the sum of the diameters of the viable (enhancing) target lesions. In addition, objective response rate (ORR) was defined as CR+PR, and disease control rate (DCR) was defined as CR+PR+SD.
Safety assessment
Liver function tests including ALT, AST, ALP, TB, ALB, TP and TBA at 1-month post treatment were assessed. Common AEs, which consisted of pain and hypertension during treatment and pain, fever, nausea and vomiting post treatment, were evaluated as well.
Survival assessments
Patients were followed up for survival assessment. Progression-free survival (PFS) was defined as the duration from the time of treatment to the time of disease progression or death. Overall survival (OS) was defined as the duration from the time of treatment to the time of death.
Statistical analysis
SPSS 22.0 statistical software (SPSS Inc., Chicago, USA) was used for statistical analysis, and GraphPad Prism 6.01 software (GraphPad Software Inc., San Diego, USA) was used to make figures. Count data were expressed as count (percentage), and the comparison between the two groups was determined by Chi-square test; normally distributed continuous data were presented as mean±standard deviation, and the comparison between the two groups was determined by t-test. Skewed distributed continuous data were described as median (25th–75th quantiles), and the comparison between two groups was determined by Wilcoxon rank sum test. Factors affecting ORR were determined by multivariate logistic regression analysis with Forward Stepwise (Conditional) method. Survival analysis was performed using Kaplan–Meier method and log-rank test. Moreover, prognostic factors of PFS and OS were determined by multivariate Cox’s proportional hazards regression analysis with Forward Stepwise (Conditional LR) method. P-value <0.05 was considered significant, and the significant results were shown in boldface.
Results
Baseline characteristics
The overall patient characteristics are listed in Table 1. The median largest tumor size (P=0.004), median TP value (P=0.024), median TB value (P=0.001) and percentage of patients receiving previous cTACE treatment (P=0.018) were significantly higher in the CSM-TACE group than those in the cTACE group. In the other baseline characteristics, no significant difference was found between the two groups (P>0.05).
 |
Table 1 Baseline characteristics of HCC patients |
Comparison of tumor response between the CSM-TACE and cTACE group
The responses were compared between the CSM-TACE and cTACE group using Chi-square test. As for overall response, CR rate in the CSM-TACE group was significantly higher than that in the cTACE group at M3 (P=0.020), whereas no significant difference was found between the two groups at M1 (P=0.301) and M6 (P=0.129) (Table 2). ORR in the CSM-TACE group was also significantly higher than that in the cTACE group at M1 (P<0.001), M3 (P<0.001) and M6 (P=0.017). In contrast, no significant difference was noted in DCRs between the two groups at M1 (P=0.536), M3 (P=0.913) and M6 (P=0.219).
 |
Table 2 Comparison of treatment response between CSM-TACE group and cTACE group |
As for lesion response, the CSM-TACE group showed significant increase in CR rate compared with the cTACE group at M3 (P=0.039), whereas there was no significant difference between the two groups at M1 (P=0.213) or M6 (P=0.274). Similarly, the ORR was significantly higher in the CSM-TACE group than that in the cTACE group at M1 (P=0.018), M3 (P=0.023) and M6 (P=0.043). In DCR, the CSM-TACE group showed significant elevation at M1 (P=0.004) and M3 (P=0.001) compared with the cTACE group.
Factors affecting ORR
Multivariate logistic regression model analysis was performed to evaluate the predicting factors for ORR, which showed that CSM-TACE (P=0.002) was independently associated with higher ORR at M1 (Table 3). Additionally, abnormal ALP (P=0.049) was independently associated with lower ORR at M3; history of drinking alcohol (P=0.019) and largest nodule size ≥7 cm (P=0.015) were independently predicting factors for lower ORR at M6.
 |
Table 3 Factors affecting ORR by multivariate logistic regression model analysis with Forward Stepwise (Conditional) method |
Subgroup analysis of ORR
Comparisons of ORR in subgroups were also conducted using Chi-square test, which disclosed that at M1, the CSM-TACE group presented with elevated ORR in patients with age ≥60 years (P=0.003), age <60 years (P=0.007), male gender (P=0.001), largest nodule size ≥7cm (P=0.007), largest nodule size <7cm (P=0.004), portal vein invasion (P=0.005), no portal vein invasion (P=0.007), no hepatic vein invasion (P<0.001), Child-pugh stage A (P<0.001), BCLC stage A/B (P=0.013), BCLC stage C/D (P=0.002), AFP ≥120.5 μg/L (P=0.006) and AFP <120.5 μg/L (P=0.049) compared with cTACE, however, CSM-TACE group showed lower ORR in patients without largest nodule size ≥7cm (P=0.004) than that in cTACE group. At M3, the ORR was increased in the CSM-TACE group than that in the cTACE group in patients with age <60 years (P<0.001), male gender (P=0.001), no largest nodule size ≥7 cm (P<0.001), portal vein invasion (P=0.024), no portal vein invasion (P=0.002), no hepatic vein invasion (P<0.001), Child-Pugh stage A (P<0.001), BCLC stage A/B (P=0.001), AFP ≥120.5 μg/L (P=0.002) and AFP <120.5 μg/L (P=0.014). At M6, patients with age ≥60 years (P=0.050), male gender (P=0.004), portal vein invasion (P=0.011), no hepatic vein invasion (P=0.003), Child-Pugh stage A (P=0.024), AFP ≥120.5 μg/L (P=0.041) presented with elevated ORR rate in CSM-TACE group compared with cTACE group (Table 4).
 |
Table 4 Comparison of ORR in subgroup analysis |
 |
Table 5 Factors affecting PFS and OS by multivariate Cox’s proportional hazards regression model analysis with Forward Stepwise (Conditional LR) method |
Comparisons of PFS and OS between the CSM-TACE and cTACE group
The patients were followed up until March 2018 and the median follow-up duration was 11.0 months (range: 1.0–37.0 months). The PFS and OS of patients was recorded and compared using the Kaplan–Meier method and log-rank test between the CSM-TACE and cTACE group, which showed that the difference in PFS (P=0.503) (Figure 2A) and OS (P=0.203) (Figure 2B) was not significant between the two groups.
Factors affecting PFS and OS
Multivariate Cox’s proportional hazards regression model analysis was performed to evaluate the independent factors affecting PFS and OS in HCC patients, which revealed that the largest nodule size ≥7 cm (P=0.029) and abnormal ALB (P=0.046) independently predicted shorter PFS (Table 5). As for OS, CSM-TACE (P=0.023) was an independent predictive factor for longer OS, whereas Child-Pugh stage B/C (P=0.036), abnormal ALB (P=0.001), ALP (P=0.008) and AFP (P=0.005) also independently predicted worse OS.
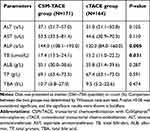 |
Table 6 Liver function tests at 1 month after treatment |
Subgroup analysis of PFS and OS
In addition, the PFS was compared between the CSM-TACE and cTACE group in subgroups by Kaplan–Meier method and log-rank test, which showed that no difference was found in all subgroups (P>0.05) (Figure 3A–P). As for OS, patients with Child-Pugh stage A in CSM-TACE group presented better OS than that in the cTACE group (P=0.032) (Figure 4K) (Figure 4A–J and L–P).
Comparisons of liver function and AEs between the CSM-TACE and cTACE group
The liver function indexes at M1 post treatment were assessed and compared between the CSM-TACE and cTACE group, which demonstrated that the median levels of ALP (P=0.005) and TB (P=0.031) were significantly higher in the CSM-TACE group than those in the cTACE group, whereas the differences in ALT (P=0.105), AST (P=0.110), ALB (P=0.287), TP (P=0.591) and TBA (P=0.474) level did not reach statistical significance between the two groups (Table 6).
In addition, the AEs were also evaluated and compared between the two groups. During treatment, the pain incidence (P=0.034) and pain grade (P=0.040) in the CSM-TACE group were significantly higher than those in the cTACE group (Table 7). The other AEs, including hypertension (P=0.192), nausea and/or vomiting (P=0.766), were similar between the two groups. Five to 7 days after treatment, the CSM-TACE group showed significant increase only in fever incidence compared with the cTACE group (P=0.017).
 |
Table 7 Adverse events which occurred during and after treatment |
Discussion
In this study, we demonstrated that: 1) CSM-TACE group achieved better treatment response compared with cTACE group, and further multivariate logistic regression model analysis revealed that CSM-TACE independently correlated with better ORR; 2) although PFS and OS displayed no difference between the CSM-TACE and cTACE group, CSM-TACE was identified as an independent predictive factor for more favorable OS in multivariate Cox’s proportional hazards regression model analysis; 3) abnormal ALP, history of alcohol intake and largest nodule size ≥7 cm were independently predicting factors for worse treatment response, and largest nodule size ≥7 cm, Child-Pugh stage B/C, abnormal ALB, ALP and AFP were independently associated with an unfavorable survival; 4) the majority of liver function indexes and AEs were similar between the two groups, except that ALP, TB, pain incidence during operation and occurrence of fever post treatment were elevated in the CSM-TACE compared with the cTACE group.
Although DEB-TACE has been widely used for treatment of HCC, comparison of tumor response after DEB-TACE with cTACE remains controversial. A retrospective cohort study demonstrated that there was no difference of DCR between DEB-TACE with DC Bead® and cTACE in HCC patients.11 However, another study with a large sample size validated a better ORR in HCC patients receiving DEB-TACEcompared with cTACE.12 Moreover, a randomized phase Ⅱ study indicated that the DEB-TACE had higher rates of CR, ORR and DCR compared with cTACE.13 In our study, we found that CR at M3 and ORR at all visits were higher in HCC patients treated by CSM-TACE compared with those in HCC patients treated by cTACE, which confirm DEB-TACE has better local tumor control. The advantage of DEB-TACE in tumor response may result from the fact that CSMs have the ability to sequester chemotherapeutic agents and release them in a controlled pattern, and thus maintain a sustained high concentration of chemotherapeutics drugs in tumor tissue.10,14,15
It is also debatable whether DEB-TACE presents with superior survival benefits compared with cTACE in HCC patients. A previous study displayed comparable OS between DEB-TACE with DC Bead® and cTACE in HCC patients.11 Another retrospective cohort study also revealed that there was no difference regarding the median OS in advanced HCC patients with portal vein thrombosis treated by DEB-TACE with LC bead® and patients treated by cTACE.16 However, a retrospective cohort study conducted by Rahman et al elucidateed that in unresectable HCC patients, DEB-TACE presented with a more prolonged median survival time compared with cTACE.17 Furthermore, a meta-analysis demonstrated that the 1 year, 2 year and 3 year survival rates were elevated in HCC patients treated by DEB-TACE compared with cTACE, and the 1 year- as well as 2 year- relapse-free survival rates were also increased in patients treated with DEB-TACE.18 In this study, no significant difference was found in both PFS and OS between the CSM-TACE and cTACE group, which probably be due to the short follow-up period. However, the multivariate Cox’s proportional hazards regression model analysis revealed that CSM-TACE was independently correlated with more prolonged OS. Additional research is necessary to asses patient survival after CSM-TACE
Risk stratification of HCC patients before TACE is of considerable value in prediciting treatment response and survival, which has been investigated in recent studies. Vesselle et al19 revealed that tumor size was a negative predicitive factor for CR in HCC patients receiving DEB-TACE. Brown et al20 and Sellers et al21 showed that Child-Pugh stage was a negative predicitive factor for survival in HCC patients treated by TACE. Other factors, such as albumin-bilirubin, platelet-albumin-bilirubin grades, ECOG stage, BCLC stage, portal vein invasion, CA-199, Hb, ALP, ALB and AFP level, have also been proved to predict tumor response and survival.22–24 In this study, abnormal ALP, history ofalcohol intake and largest nodule size ≥7 cm independently predicted worse tumor response, and largest nodule size ≥7 cm, Child-Pugh stage B/C, abnormal ALB, ALP and AFP were independently associated with shorter survival in HCC patients treated by CSM-TACE or cTACE. Our results revealed similar predictive factors except the alcohol intake history. A study showed that continuous and limited alcohol consumption promoted progression and metastasis of HCC by activating NF-κB pathway, which might explain why the history of alcohol intake was independently associated with worse treatment response in our study.25
Moreover, we also evaluated the safety of CSM-TACE and cTACE treatments in HCC patients. In this study, the two groups were similar in most of the liver function indexes at 1 month post treatment, except that the levels of ALP and TB were higher in the CSM-TACE compared with the cTACE group. This finding was different from previous studies in that DEB-TACE demonstrated a safety advantage.13 One possible explanation for this could be that both ALP and TB level at baseline in the CSM-TACE group were higher than those in the cTACE group. In addition, we also compared the postembolization syndrome during and after treatment between the two groups, which showed that the CSM-TACE group had higher pain incidence, pain grades and fever incidence compared with the cTACE group. This finding was also distinct from previous results where DEB-TACE had an improved tolerability profile.13 This discrepancy may be due to the difference in the tumor size between the two groups. Lager tumor size in the CSM-TACE group probably mean that the tumor need more aggressive treatment and accordingly achieve more substantial tumor necrosis, which may result in more serious postembolization syndrome.
This study had several limitations. Firstly, as a retrospective study, several baseline characteristics were disparate between the two groups, which may influence the comparison of therapeutic efficacy and safety. A randomized controlled trial should be done in the future. Secondly, we included BCLC stage A, B, C and D patients in this study. Although TACE is the first-line therapy for intermediate-stage HCC, in real clinical practice TACE is also indicated for the HCC patients with portal vein invasion or end-stage HCC patients within the Milan criteria.26 Thirdly, the follow-up period was relatively short in this study. Further follow-up is required to assess the long-term efficacy.
Conclusion
In conclusion, CSM-TACE presents with more favorable treatment response and survival profile compared with cTACE in HCC patients. Future randomized clinical trials with larger sample sizes and longer follow-up periods are required to determine the efficacy and safety of CSM-TACE in selected HCC patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.21294
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. doi:10.1016/j.jhep.2018.03.019
3. Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi:10.1016/S0140-6736(02)07571-2
4. Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi:10.1053/jhep.2002.33156
5. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773. doi:10.1002/hep.23892
6. Lee JK, Chung YH, Song BC, et al. Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2002;17:52–58. doi: 10.1046/j.1440-1746.2002.02664.x
7. Kim YW, Kwon JH, Nam SW, et al. Sustained multiple organ ischaemia after transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. Exp Ther Med. 2018;15:1479–1483.
8. Song JE, Kim DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol. 2017;9:808–814. doi:10.4254/wjh.v9.i18.808
9. Zou JH, Zhang L, Ren ZG, et al. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis. 2016;17:510–517. doi:10.1111/1751-2980.12380
10. Zhang S, Huang C, Li Z, et al. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Deliv. 2017;24:1011–1017. doi:10.1080/10717544.2016.1267822
11. Lee YK, Jung KS, Kim DY, et al. Conventional versus drug-eluting beads chemoembolization for hepatocellular carcinoma: emphasis on the impact of tumor size. J Gastroenterol Hepatol. 2017;32:487–496. doi:10.1111/jgh.13501
12. Cheung AH, Lam CS, Tam HS, et al. Nine-year experience of doxorubicin-eluting beads chemoembolization for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2016;15:493–498. doi:10.1016/S1499-3872(16)60133-9
13. Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. doi:10.1007/s00270-009-9711-7.
14. Poon RT, Tso WK, Pang RW, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100–1108. doi:10.1016/j.cgh.2007.04.021
15. Guan YS, He Q, Jin Y, et al. Development of CalliSpheres(R) embolic microspheres. Zhonghua Gan Zang Bing Za Zhi. 2016;24:549–551.
16. Gorodetski B, Chapiro J, Schernthaner R, et al. Advanced-stage hepatocellular carcinoma with portal vein thrombosis: conventional versus drug-eluting beads transcatheter arterial chemoembolization. Eur Radiol. 2017;27:526–535. doi:10.1007/s00330-016-4445-9
17. Rahman FA, Naidu J, Ngiu CS, et al. Conventional versus doxorubicin-eluting beads transarterial chemoembolization for unresectable hepatocellular carcinoma: a tertiary medical centre experience in Malaysia. Asian Pac J Cancer Prev. 2016;17:4037–4041. doi:10.7314/apjcp.2016.17.4.2119
18. Chen P, Yuan P, Chen B, et al. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41:75–85. doi:10.1016/j.clinre.2016.05.013
19. Vesselle G, Quirier-Leleu C, Velasco S, et al. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol. 2016;26:1640–1648. doi:10.1007/s00330-015-3982-y
20. Brown DB, Fundakowski CE, Lisker-Melman M, et al. Comparison of MELD and Child-Pugh scores to predict survival after chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2004;15:1209–1218. doi:10.1097/01.RVI.0000128123.04554.C1
21. Sellers MT, Huggins S, Kegley K, et al. Multivariate analysis of prognostic factors for survival following doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2013;24:647–654. doi: 10.1016/j.jvir.2012.12.003
22. Hansmann J, Evers MJ, Bui JT, et al. Albumin-bilirubin and platelet-albumin-bilirubin grades accurately predict overall survival in high-risk patients undergoing conventional transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2017;28:1224–1231 e1222. doi:10.1016/j.jvir.2017.05.020
23. Wu X, Chen R, Zheng W, et al. Comprehensive analysis of factors affecting clinical response and short-term survival to drug-eluting bead transarterial chemoembolization for treatment in patients with liver cancer. Technol Cancer Res Treat. 2018;17:1533033818759878. doi:10.1177/1533033818759878
24. Gomes AS, Monteleone PA, Sayre JW, et al. Comparison of triple-drug transcatheter arterial chemoembolization (TACE) with single-drug TACE using doxorubicin-eluting beads: long-term survival in 313 patients. AJR Am J Roentgenol. 2017;209:722–732. doi:10.2214/AJR.17.18219
25. Wang F, Yang JL, Yu KK, et al. Activation of the NF-kappaB pathway as a mechanism of alcohol enhanced progression and metastasis of human hepatocellular carcinoma. Mol Cancer. 2015;14:10. doi:10.1186/s12943-014-0278-9
26. Horikawa M, Miyayama S, Irie T, et al. Development of conventional transarterial chemoembolization for hepatocellular carcinomas in japan: historical, strategic, and technical review. Am J Roentgenol. 2015;205:764–773. doi:10.2214/AJR.15.14825
Supplementary material
 |
Table S1 Number of patients included in this study by medical center |
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.