Back to Journals » OncoTargets and Therapy » Volume 10
Comparison of autogeneic and allogeneic natural killer cells immunotherapy on the clinical outcome of recurrent breast cancer
Authors Liang S, Xu K, Niu L, Wang X, Liang Y, Zhang M, Chen J, Lin M
Received 19 April 2017
Accepted for publication 5 July 2017
Published 28 August 2017 Volume 2017:10 Pages 4273—4281
DOI https://doi.org/10.2147/OTT.S139986
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Shuzhen Liang,1,2 Kecheng Xu,1,2 Lizhi Niu,1,2 Xiaohua Wang,1 Yingqing Liang,1 Mingjie Zhang,3 Jibing Chen,1,2 Mao Lin1,2
1Department of Central Laboratory, Fuda Cancer Hospital, Jinan University School of Medicine, Guangzhou, Guangdong, China; 2Fuda Cancer Institute, Guangzhou, Guangdong, China; 3Hank Bioengineering Co., Ltd, Shenzhen, China
Abstract: In the present study, we aimed to compare the clinical outcome of autogeneic and allogeneic natural killer (NK) cells immunotherapy for the treatment of recurrent breast cancer. Between July 2016 and February 2017, 36 patients who met the enrollment criteria were randomly assigned to two groups: autogeneic NK cells immunotherapy group (group I, n=18) and allogeneic NK cells immunotherapy group (group II, n=18). The clinical efficacy, quality of life, immune function, circulating tumor cell (CTC) level, and other related indicators were evaluated. We found that allogeneic NK cells immunotherapy has better clinical efficacy than autogeneic therapy. Moreover, allogeneic NK cells therapy improves the quality of life, reduces the number of CTCs, reduces carcinoembryonic antigen and cancer antigen 15-3 (CA15-3) expression, and significantly enhances immune function. To our knowledge, this is the first clinical trial to compare the clinical outcome of autogeneic and allogeneic NK cells immunotherapy for recurrent breast cancer.
Keywords: clinical outcome, autogeneic, allogeneic, natural killer cells, recurrent breast cancer
Introduction
Breast cancer currently represents the most common malignancy and the most common cancer-related cause of death among women in both the developing and developed world.1,2 Almost 48,000 new cases of breast cancer are diagnosed annually in the UK and the annual number of cases has almost doubled during the past three decades.3 To date, the majority of small invasive and noninvasive breast cancers are treated with breast conservation therapy (BCT), but the recurrence rate after BCT in stage 0, I, and II patients ranges between 5% and 22%.4 As recurrent breast cancer cannot be cured, the treatment goal in such cases is to control the disease symptoms, relieve pain, and prolong survival to the greatest extent possible. Chemotherapy also plays an important role in the treatment of recurrent breast cancer;5 however, the related toxicity and side effects may influence the health and quality of life (QOL) of patients.6,7 Hence, more effective and safer treatments need to be developed to improve the survival and QOL of patients with recurrent breast cancer.
The manipulation of the immune system for therapeutic benefit in breast cancer patients has been studied for several decades.8–10 The immune system plays a dual role in breast cancer-promoting tumorigenesis through inflammatory pathways and suppressing adaptive immunity and preventing tumor formation through active immune surveillance. Natural killer (NK) cells are important components of the innate immune system and play a critical role in the early host defense against cancer.11,12 They exert their effector function via direct killing of virally infected cells and tumor cells and via production of immunoregulatory cytokines and chemokines, thereby affecting adaptive immune responses.13,14 With advancements in the NK cell biology field and enhancements in our understanding of NK cell function, NK cell transfer has become a powerful cancer immunotherapy tool in cancer treatment. The animal experiments in mice model show that NK cells are responsible for inhibiting the formation of progressively growing rapid large tumors of breast cells.15–17
There are two types of adoptive NK cells treatment: autogeneic and allogeneic; however, not all cancer patients exhibit clinical effects after autogeneic NK cells treatment.18,19 The killer cell immunoglobulin-like receptors (KIRs) present on NK cells prevent them from killing tumor cells that express similar major histocompatibility complex class I (MHC-I) molecules. Hence, in recent years, several studies have assessed the feasibility of NK cells allograft (rather than autogeneic cells) as an adoptive treatment for cancer. The clinical trial assessing the use of unrelated donor allogeneic NK cells treatment has indicated that there are no side effects in the recipients.20,21
Comparative evaluation of autogeneic and allogeneic NK cells immunotherapy in patients with recurrent breast cancer is not well documented. Therefore, the purpose of this study was to compare the therapeutic efficacy of autogeneic and allogeneic NK cells immunotherapy in patients with recurrent breast cancer.
Materials and methods
Ethics
This clinical trial was registered with the US National Institutes of Health (ID: NCT02853903; Ph1/Ph2) and was approved by the Ethics Committee of Guangzhou Fuda Cancer Hospital. Written informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
Patient eligibility
Patients with recurrent breast cancer, diagnosed via histopathological examination, at Fuda Cancer Hospital (Guangdong, China) between July 2016 and February 2017 were eligible for inclusion in this study. The ideal candidates for this clinical trial included those with lifespan >6 months; Karnofsky performance status (KPS) score ≥70; platelet count ≥80×109/L; white blood cell count ≥3×109/L; neutrophil count ≥2×109/L; hemoglobin ≥90 g/L; prothrombin time international normalized ratio ≥1.5; absence of level 3 hypertension, severe coronary disease, myelosuppression, respiratory disease, and acute or chronic infection; and adequate hepatic function (bilirubin <30 μmol/L, aminotransferase <60 U/L) and renal function (serum creatinine <130 μmol/L, serum urea <10 mmol/L). The patients were randomly divided into two groups: group I was treated with autogeneic NK cells immunotherapy and group II was treated with allogeneic NK cells immunotherapy (four courses of adoptive transfer of NK cells were performed continuously).
NK cells therapy
NK cells were generated according to previously published protocols under good manufacturing practice conditions.22 In brief, peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood samples (80 mL) obtained from patients (group I) and allogeneic donors (group II). To ensure appropriate donor selection, the KIR genotype should not match with the human leukocyte antigen (HLA) class I molecules of the patient.22–26 The peripheral blood samples from allogeneic donors and recipients were tested with the TIANamp Blood DNA Kit (Tiangen Biotech Co., Ltd, Beijing, China) and KIR/HLA-Cw Genotyping Low Resolution Kit (PCR-SSP) (Super Biotechnology Developing Co., Ltd, Tianjin, China).
For NK cells culture, we used the Human HANK Cell In Vitro Preparation Kit (Hank Bioengineering Co., Ltd, Shenzhen, China), along with lethally radiated K562-mb15–41BBL (K562D2) stimulatory cells,27 plasma treatment fluid, lymphocyte culture fluid additives, serum-free medium additives, and cell infusion additives. This kit is specially used for the expansion and activation of NK cells in peripheral blood or umbilical cord blood mononuclear cells in vitro, as well as for the preparation of NK cells in greater quantities and with higher purity and activity (namely, HANK cells).22 According to the instruction, NK cells were cultured in two plastic flasks (T75; Corning Costa, Cambridge, MA, USA) at 37°C with 5% CO2. After culture, 8–10 billion HANK cells were harvested using the NK cell serum-free medium and a culture bag (Haoyang Biological Manufacture Co., Ltd, Tianjin, China). Final cell counting and quality control inspection were performed on day 9 of culture, and the qualified indicators included the proportion of living cells ≥90%; proportion of CD56+ CD3- cells ≥85% (detection via flowcytometry, as previously described22); endotoxin content ≤1 EU/mL; cell viability ≥80% (K562 cells were used as target cells in the cytotoxicity assay described previously22); and absence of bacteria, fungi, and mycoplasma cultures. All the cell preparation processes were performed by the same technician and were assessed by another technician. After 12 whole days of culture, the NK cells were counted and washed with saline. The cell concentration was adjusted to 20×106/mL with a saline solution containing an immune cell infusion additive (HK-005), and then divided into three parts and infused intravenously over 30 min on days 13–15. Right after the infusion, new peripheral blood samples were obtained to begin the new course.
Detection of immune function
Peripheral blood sample (2 mL) was drawn 1 day before treatment and 1 month after the final transfusion for the detection of immune function by flow cytometry (FACSanto™ II; BD Biosciences, San Jose, CA, USA). The number and function of lymphocytes in the peripheral blood of patients were tested based on the protocols described in the instruction manuals. The BD Multitest 6-color TBNK Reagent (BD Biosciences) was used to detect the number of CD3+ CD4+ cells (95% CI, 441–2,156 cells/μL), CD3+ CD8+ cells (95% CI, 125–1,312 cells/μL), total CD3+ cells (95% CI, 603–2,990 cells/μL), CD3-CD19+ cells (95% CI, 107–698 cells/μL), and CD3-CD16+ CD56+ cells (95% CI, 95–640 cells/μL). The BD Cytometric Bead Array Human Th1/Th2 Cytokine Kit II (BD Biosciences) was used to detect the expression levels of interleukin 2 (IL-2) (95% CI, 8–12.5 pg/mL), IL-4 (95% CI, 3.5–6 pg/mL), IL-6 (95% CI, 2.7–8.5 pg/mL), IL-10 (95% CI, 1.8–4 pg/mL), tumor necrosis factor (95% CI, 1.7–2.5 pg/mL), and interferon gamma (95% CI, 1.5–4 pg/mL). The tests were performed according to the protocols given in the instruction manuals. Results above or within the reference range were considered to indicate normal immune function. Patients with one or more than one parameter exhibiting below normal values were considered to have immune dysfunction.
Analysis of circulating tumor cell levels
Peripheral blood sample (7 mL) was drawn 1 day before treatment and 1 month after the final transfusion for the detection of circulating tumor cell (CTC) levels. PBMC from the peripheral blood sample were separated using the human peripheral blood lymphocyte separation liquid (Haoyang Biological Manufacture Co., Ltd.) and were washed twice with sterile Hank’s balanced salt solution (Thermo Fisher Scientific, Waltham, MA, USA). Isolated cells were enriched via binding to magnetic CD326 (EpCAM) MicroBeads (Miltenyi Biotech Ltd., Bergisch Gladbach, Germany) using magnetic activated cell sorting. Enriched isolated cells were then labeled with monoclonal antibodies targeting epithelial cell antigens CD45, CD326, and cytokeratins 8, 18, and 19 (Miltenyi Biotech Ltd.) and incubated in the dark at room temperature for 12 min. Antibodies specific for leukocytes (CD45) labeled with phycoerythrin (PE) (10 μL), specific for epithelial cells (cytokeratins 8, 18, and 19) labeled with fluorescein isothiocyanate (FITC) (10 μL), and specific for epithelial cells (CD326/Ep-CAM) labeled with allophycocyan (10 μL) were added to 7.5 mL of blood. Cell pellets were resuspended in 500 μL PBS and counted by flow cytometry using a BD FACSCanto™ II apparatus (BD Biosciences). Cells that were CD45-negative, and CK- and CD326-positive were defined as CTCs.
Follow-up
Adverse effects
The adverse effects were classified in accordance with the Common Terminology Criteria of Adverse Events, v4.0 (CTCAE v4.0) and were recorded by grade (level of severity) on a scale of 1–5.
Response to treatment
Breast-enhanced spiral computed tomography (CT) was performed at 1 week before treatment, and at 1 and 2 months after the final transfusion to observe the treatment effect. All the CT images were assessed by the same two diagnostic radiologists with specific expertise in breast imaging. The treatment effect was evaluated by analyzing the average variation of maximum transverse diameter and CT value of tumors. According to the Response Evaluation Criteria in Solid Tumors (RECIST),28 the therapeutic effect was classified as complete response (CR; arterial enhancement imaging indicated disappearance of all target lesions), partial response (PR; reduction in the sum of the diameter of target lesions by >30%), stable disease (SD; tumor regression failed to reach PR status or tumor progression failed to reach progressive disease [PD] status), and PD (the sum of the diameter of the tumors increased by >20%). The response rate considered both CR and PR.
Quality of life
After the final transfusion, the patients were divided based on the KPS score into the improved QOL group (KPS increased by ≥10), stable QOL group (KPS increased by <10), and reduced QOL group (KPS reduced by ≥10).
Changes in biochemical indicators
The expressions of CA15-3 and CEA were examined before treatment, and at 1 and 2 weeks and 1 month after the final transfusion.
Statistical analysis
Radiographic local tumor control was assessed using image-guided tumor ablation criteria.29 SPSS version 13.0 (IBM Corporation, Armonk, NY, USA) was used for the statistical analyses, and the results were expressed as the mean ± standard deviation values. GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA) was used to plot graphs. All statistical tests were two-sided, and the differences were considered significant at P<0.05.
Results
Patients
A total of 36 patients with recurrent breast cancer were included in the study and were randomly assigned to two groups (18 in each group). The demographic and clinical characteristics of the two groups were compared, and no significant between-group differences were observed (P>0.05, Table 1).
  | Table 1 Patient clinical information |
Adverse effects
All the adverse effects experienced by the patients, including local (mainly nausea, emesis, chest distress, and hypoglycemia) and systemic (mainly chills, fatigue, and fever) effects, during the study were recorded. The occurrence of adverse events was compared using the chi-square test, and no significant between-group difference was noted (P>0.05, Figure 1). No other side effects, such as blood or bone marrow changes, were detected. All the adverse events were classified as Grade 1; after symptomatic treatment, all the symptoms were relieved within a day and did not reappear.
  | Figure 1 Adverse effects of the two treatment. |
Immune function detection
Lymphocyte count and function were compared before treatment and after the final transfusion. The test data of all patients before treatment were merged and compared with the test results obtained after the final transfusion (Table 2). With regard to the lymphocyte count, the absolute number of total T cells, NK cells, and B cells exhibited significant increases in group II after treatment (P<0.01). In terms of lymphocyte function, the Th1-type cytokine levels exhibited significant increases in group II after treatment (P<0.01), whereas the changes in the levels of Th2-type cytokines were not significant (P>0.05).
Analysis of CTC levels
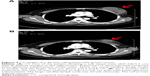
The CTC levels were compared before treatment and after the final transfusion (Figure 2). The number of CTC in group II decreased significantly (P<0.01), from 13.13±5.83 before treatment to 6.88±4.95 at 1 month after the final transfusion. In contrast, the changes in CTC levels in group I were not significant (P>0.05). The representative results from one of the study patients are shown in Figure 3.
Response to treatment
The maximum transverse diameter and CT value are shown in Table 3. The tumor volume and CT value of the two groups decreased at 1 and 2 months after the final transfusion. Moreover, the maximum tumor diameter and CT value were both significantly lower in group II than in group I (P<0.05). The representative results from one of the study patients are shown in Figure 4.
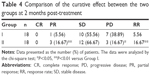
Clinical efficacy was evaluated at 2 months after the final transfusion (Table 4). According to the RECIST evaluation, 3 (16.67%) patients in group II achieved PR and 12 (66.67%) patients exhibited SD. Moreover, in group II, only 3 (16.67%) patients exhibited PD. The clinical efficacy in group II was superior to that in group I, which indicated PR in 1 (5.56%) patient (P<0.05), SD in 10 (55.56%) patients (P>0.05), and PD in 7 (38.89%) patients (P<0.05).
Quality of life
The KPS score in groups I and II was 66.7±5.0 and 71.1±5.9, respectively, before treatment, and it was 78.8±5.6 and 84.3±5.4, respectively, at 2 months after the final transfusion. The KPS score of the two groups markedly improved after treatment (Figure 5A, P<0.05); moreover, the KPS score in group II was significantly higher than that in group I at 2 months after treatment (Figure 5B, P<0.05).
Changes in biochemical indicators
As indicated by the biochemical examination results shown in Figure 6, the expressions of CEA and CA15-3 in the two groups were higher than normal at 1 day before treatment, and it decreased gradually at 1 and 2 weeks and 1 month after the final transfusion. There was no significant difference between the two groups at 1 and 2 weeks after treatment (P>0.05), but CEA and CA15-3 expression was significantly lower in group II than in group I at 1 month after treatment (P<0.05).
Discussion
Breast cancer is the most frequently diagnosed type of cancer in women worldwide. Although surgery, chemotherapy, radiotherapy, and endocrine therapy have greatly enhanced the clinical outcomes, the recurrence rate of breast cancer remains high.30 As there is no difference in survival rate between BCT and mastectomy, there has been a marked shift in surgical therapy toward less radical and disfiguring treatments.31
Cancer development and progression in patients with recurrent breast cancer are known to be influenced by immune responses to the tumor.32,33 The recent success of immunotherapy has highlighted the potential of immune-based therapy approaches for breast cancer treatment.9,34,35 As a part of the innate immune response, NK cells serve as the body’s first line of defense against infected or transformed cells. NK cells recognize and eliminate their target cells that lack self-MHC-I molecules by activating receptors such as NKG2D, NKp30, NKp40, and NKp46. Tumor cells are more susceptible to NK cells because of their lack of MHC class I molecules.36,37 There are two types of NK cells treatment, autogeneic and allogeneic. It is generally accepted that allogeneic NK cells may be more potent in the treatment of malignancies.21,38,39
In the present study, we prospectively compared the clinical outcomes of autogeneic and allogeneic NK cells immunotherapy in patients with recurrent breast cancer in order to obtain information regarding which type of NK cells immunotherapy can improve patients’ clinical outcomes. We found that allogeneic NK cells therapy showed better outcomes than autogeneic NK cells therapy with regard to improving the antitumor effect and enhancing the immune function of patients. The increase in the total number of T cells and NK cells observed after allogeneic NK cells therapy may be related to the improvement of cellular immunity and prevention of apoptosis of T cells.40 Immunocytokines can induce tumor-specific T cells selectively and activate NK cells to sites of tumor. The increase in the expression of Th1 cytokines may be related to the activation of NK cells.41 Therefore, allogeneic NK cells therapy can improve the body’s immunosuppression status by promoting the immunocytokines functions. Moreover, allogeneic NK cells can markedly decrease the levels of CTCs, CEA, and CA15A. Our previous studies have shown that the CTC level is a robust biomarker of the effects of immunotherapy and its decrease may be related to tumor shrinkage.42,43 Therefore, the decrease in CTC level observed in the present study may reflect the efficacy of treatment. Furthermore, the clinical efficacy and QOL of patients treated with allogeneic NK cells therapy were markedly improved compared to those treated with autogeneic NK cells therapy. On the other hand, the postoperative adverse effects were minimal. Thus, observations indicate that allogeneic NK cells therapy is more beneficial for recurrent breast cancer.
In conclusion, allogeneic NK cells immunotherapy yields better outcomes in recurrent breast cancer patients. Furthermore, to our knowledge, this is the first clinical trial (ID: NCT02853903; Ph1/Ph2) to compare the clinical outcome of autogeneic and allogeneic NK cells immunotherapy for recurrent breast cancer. However, further research is needed to evaluate the progression-free survival and overall survival associated with allogeneic NK cells immunotherapy in recurrent breast cancer.
Acknowledgments
We would like to thank the native English-speaking scientists of Elixigen Company for editing our manuscript. This work was supported by the Science and Technology Program of Tianhe District, Guangdong Province, China (grant no 201504KW008).
Disclosure
The authors report no conflicts of interest in this work.
References
DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. | ||
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Benson JR, Jatoi I. The global breast cancer burden. Future Oncol. 2012;8(6):697–702. | ||
Huston TL, Simmons RM. Locally recurrent breast cancer after conservation therapy. Am J Surg. 2005;189(2):229–235. | ||
Smith IC, Heys SD, Hutcheon AW, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002;20(6):1456–1466. | ||
Broeckel JA, Jacobsen PB, Horton J, Balducci L, Lyman GH. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol. 1998;16(5):1689–1696. | ||
Chia SK, Speers CH, D’Yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110(5):973–979. | ||
Spellman A, Tang SC. Immunotherapy for breast cancer: past, present, and future. Cancer Metastasis Rev. 2016;35(4):525–546. | ||
Hadden JW. The immunology and immunotherapy of breast cancer: an update. Int J Immunopharmacol. 1999;21(2):79–101. | ||
Kubo M, Morisaki T, Kuroki H, et al. Combination of adoptive immunotherapy with Herceptin for patients with HER2-expressing breast cancer. Anticancer Res. 2003;23(6a):4443–4449. | ||
Zhao Y, Hu J, Li R, et al. Enhanced NK cell adoptive antitumor effects against breast cancer in vitro via blockade of the transforming growth factor-beta signaling pathway. Onco Targets Ther. 2015;8:1553–1559. | ||
Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10(3):230–252. | ||
Papamichail M, Perez SA, Gritzapis AD, Baxevanis CN. Natural killer lymphocytes: biology, development, and function. Cancer Immunol Immunother. 2004;53(3):176–186. | ||
Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13(4):458–464. | ||
Dewan MZ, Terunuma H, Takada M, et al. Role of natural killer cells in hormone-independent rapid tumor formation and spontaneous metastasis of breast cancer cells in vivo. Breast Cancer Res Treat. 2007;104(3):267–275. | ||
Hwang MH, Li XJ, Kim JE, et al. Potential therapeutic effect of natural killer cells on doxorubicin-resistant breast cancer cells in vitro. PLoS One. 2015;10(8):e0136209. | ||
Roberti MP, Mordoh J, Levy EM. Biological role of NK cells and immunotherapeutic approaches in breast cancer. Front Immunol. 2012;3:375. | ||
Mikelsaar AV, Sunter A, Mikelsaar R, et al. Epitope of titin A-band-specific monoclonal antibody Tit1 5 H1.1 is highly conserved in several Fn3 domains of the titin molecule. Centriole staining in human, mouse and zebrafish cells. Cell Div. 2012;7(1):21. | ||
Burns LJ, Weisdorf DJ, DeFor TE, et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32(2):177–186. | ||
Re F, Staudacher C, Zamai L, Vecchio V, Bregni M. Killer cell Ig-like receptors ligand-mismatched, alloreactive natural killer cells lyse primary solid tumors. Cancer. 2006;107(3):640–648. | ||
Geller MA, Cooley S, Judson PL, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13(1):98–107. | ||
Zhang M, Daniel S, Huang Y, et al. Anti-west nile virus activity of in vitro expanded human primary natural killer cells. BMC Immunol. 2010;11:3. | ||
Witt CS, Christiansen FT. The relevance of natural killer cell human leucocyte antigen epitopes and killer cell immunoglobulin-like receptors in bone marrow transplantation. Vox Sang. 2006;90(1):10–20. | ||
Forte P, Baumann BC, Schneider MK, Seebach JD. HLA-Cw4 expression on porcine endothelial cells reduces cytotoxicity and adhesion mediated by CD158a+ human NK cells. Xenotransplantation. 2009;16(1):19–26. | ||
Kunert K, Seiler M, Mashreghi MF, et al. KIR/HLA ligand incompatibility in kidney transplantation. Transplantation. 2007;84(11):1527–1533. | ||
Moretta L, Moretta A. Killer immunoglobulin-like receptors. Curr Opin Immunol. 2004;16(5):626–633. | ||
Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106(1):376–383. | ||
Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): new guidelines. Med Pediatr Oncol. 2001;37(1):1–3. | ||
Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005;16(6):765–778. | ||
Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–2746. | ||
Fisher B, Redmond C, Poisson R, et al. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989;320(13):822–828. | ||
Nagalla S, Chou JW, Willingham MC, et al. Interactions between immunity, proliferation and molecular subtype in breast cancer prognosis. Genome Biol. 2013;14(4):R34. | ||
Schmidt M, Bohm D, von Torne C, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68(13):5405–5413. | ||
Page DB, Naidoo J, McArthur HL. Emerging immunotherapy strategies in breast cancer. Immunotherapy. 2014;6(2):195–209. | ||
Ernst B, Anderson KS. Immunotherapy for the treatment of breast cancer. Curr Oncol Rep. 2015;17(2):5. | ||
Cho D, Kim SK, Carson WE 3rd. NK cell-based immunotherapy for treating cancer: will it be promising? Korean J Hematol. 2011;46(1):3–5. | ||
Lanier LL, Phillips JH. Inhibitory MHC class I receptors on NK cells and T cells. Immunol Today. 1996;17(2):86–91. | ||
Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. | ||
Iliopoulou EG, Kountourakis P, Karamouzis MV, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010;59(12):1781–1789. | ||
Zhang B, Sun T, Xue L, et al. Functional polymorphisms in FAS and FASL contribute to increased apoptosis of tumor infiltration lymphocytes and risk of breast cancer. Carcinogenesis. 2007;28(5):1067–1073. | ||
Osenga KL, Hank JA, Albertini MR, et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children’s Oncology Group. Clin Cancer Res. 2006;12(6):1750–1759. | ||
Shi J, Li Y, Liang S, et al. Analysis of circulating tumor cells in colorectal cancer liver metastasis patients before and after cryosurgery. Cancer Biol Ther. 2016;17(9):935–942. | ||
Shi J, Li Y, Liang S, et al. Circulating tumour cells as biomarkers for evaluating cryosurgery on unresectable hepatocellular carcinoma. Oncol Rep. 2016;36(4):1845–1851. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.