Back to Journals » Clinical Ophthalmology » Volume 16
Comparison of Astigmatism Prediction Accuracy for Toric Lens Implantation from Two Swept-Source Optical Coherence Tomography Devices
Authors Melendez RF, Smits G, Nguyen T, Ruffaner-Hanson CD, Ortiz D, Hall B
Received 10 June 2022
Accepted for publication 17 October 2022
Published 17 November 2022 Volume 2022:16 Pages 3795—3802
DOI https://doi.org/10.2147/OPTH.S378019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Robert F Melendez,1,2 Gerard Smits,3 Thao Nguyen,1 Chaselyn D Ruffaner-Hanson,1 Danielle Ortiz,2 Brad Hall4
1Juliette Eye Institute, Albuquerque, NM, USA; 2University of New Mexico School of Medicine, Department of Ophthalmology, Albuquerque, NM, USA; 3CSC, Inc., Santa Barbara, CA, USA; 4Sengi, Penniac, NB, Canada
Correspondence: Robert F Melendez, Juliette Eye Institute, 6401 Holly Ave., NE, Albuquerque, NM, 87113, USA, Tel +1 505-355-2020 ; +1 505-235-4781, Email [email protected]
Purpose: To compare the astigmatism prediction accuracy for toric intraocular lens (IOL) implantation between two swept-source optical coherence tomography (SS-OCT) devices: Argos (Movu, a Santec Company) and the IOLMaster 700 (Carl Zeiss Meditec).
Methods: This was a retrospective chart review of 59 eyes (44 patients) with corneal astigmatism and cataract that underwent cataract surgery or refractive lens exchange surgery with a toric IOL. Biometry was performed on all patients prior to cataract surgery and the Barrett toric IOL calculator was used. Visual acuity was measured postoperatively. Manifest refraction was measured at 1 month and compared to the predicted postoperative residual refraction. Preoperative K measurements between devices were also compared.
Results: Mean cylinder prediction error was − 0.17 ± 0.43 for Argos and 0.12 ± 0.56 for IOLMaster 700. The cylinder prediction error 0.5 D or less was not significantly different between the devices, with 83.1% (49 eyes) for Argos and 76.3% (45 eyes) for IOLMaster 700 (p = 0.206). Spherical equivalent prediction error was 0.13 ± 0.39 for Argos and 0.25 ± 0.50 for IOLMaster 700. The mean spherical equivalent prediction error 0.5 D or less was significantly different between the devices, with 79.7% (47 eyes) for Argos and 61.0% (36 eyes) for IOLMaster 700 (p = 0.016).
Conclusion: The prediction accuracies were similar between the devices, except for spherical equivalent, where a higher percentage of eyes were 0.5 D or less of the prediction with the Argos compared to the IOLMaster.
Keywords: Argos, IOLMaster 700, SS-OCT, prediction error
Plain Language Summary
Cataract surgery is a common procedure performed worldwide. During surgery, the natural opaque lens inside the eye is replaced with an artificial intraocular lens (IOL). It is common for cataract surgery patients to also have astigmatism, which is caused when the cornea (front of the eye) is curved differently in one direction than in the other direction (eg the cornea has the shape of a rugby ball compared to a soccer ball). A specific type of IOL, a toric IOL, can be used to correct astigmatism during cataract surgery. Biometers are devices that allow surgeons to take crucial measurements of the eyes prior to surgery and select the most appropriate toric IOL power. The purpose of this retrospective chart review was to compare the astigmatism prdiction accuracy for toric IOL implantation between two commercially available biometers. The results of this study show that the prediction accuracies were similar between the devices.
Introduction
Cataract surgery patients have increasing demands and desires to achieve near perfect postoperative refractive outcomes. Good refractive outcomes after intraocular lens (IOL) implantation are highly dependent on accurate preoperative biometry measurements. Measurement accuracy is even more crucial in patients with astigmatism requiring a toric lens implant to achieve the absolute best postoperative refractive outcome. It has been reported that for every diopter of postoperative refractive astigmatism, visual acuity at distance degrades by 1.5 lines.1 Corneal astigmatism is common (about 80% of the population has 0.5 D or greater astigmatism),2,3 therefore it is important for surgeons to know the performance of commercially available biometers, especially with regard to refractive accuracy.
The first-generation biometers used partial coherence interferometry (PCI) to measure preoperative parameters such as keratometry (K), anterior chamber depth (ACD), and axial length (AL).4 In addition to PCI technology, biometers based on optical low coherence interferometry (OLCI),5 optical low coherence reflectometry (OLCR),6 and swept-source optical coherence tomography (SS-OCT)7,8 are available. Of the available technologies, SS-OCT has the advantage of better optical penetration. Two popular SS-OCT biometers are the IOLMaster 700 (Carl Zeiss Meditec AG Jena, Germany) and the Argos (Movu, a Santec Company, Santa Clara, USA).
The IOLMaster 700 was the first available SS-OCT and uses a wavelength of 1050 nm to measure AL, ACD, central corneal thickness (CCT), lens thickness (LT), white-to-white (WTW), and K. It uses a refractive index of 1.3375.9 The Argos is a recently available SS-OCT and uses a wavelength of 1060 nm to measure AL, ACD, CCT, LT, WTW, and K. It uses refractive indices of 1.376 for the cornea, 1.410 for the lens, and 1.336 for the aqueous and vitreous.10 Studies of both the IOLMaster 700 and the Argos have shown comparable or even better results than PCI biometers.11,12 However, there are minimal data comparing the astigmatic outcomes when using the K values from the Argos and from the IOLMaster. The purpose of this study was to specifically compare the astigmatism prediction accuracy of the Argos and the IOLMaster with toric IOL implantation.
Patients and Methods
Patients
This was a retrospective, single surgeon, study of patients who underwent cataract or refractive lens exchange surgery at the Juliette Eye Institute between January 2020 and July 2021. Both manual and electronic medical records were used to identify eyes that fit the inclusion and exclusion criteria listed below. This study was submitted for review and approval from an Institutional Review Board (IRB). The IRB (WCG IRB, Puyallup, USA) ruled that approval was not required for this type of retrospective study. Deidentified data from preoperative examinations and 4–6 week postoperative examinations were collected, including age, sex, postoperative refraction, and corrected distance visual acuity. The study was conducted in compliance with the tenets of the Declaration of Helsinki, International Harmonization (ICH) guidelines, and Good Clinical Practice (GCP).
The inclusion criteria were biometry and keratometry measured using the Argos device, biometry and total keratometry measured using the IOLMaster 700 device, either cataract or refractive lens exchange surgery completed without complication, implantation with an AcrySof monofocal toric, PanOptix toric, or Vivity toric IOL (Alcon Vision, LLC), and a final corrected distance visual acuity (CDVA) of 20/32 or better.
To reduce variability related to IOL implanted outcomes, the exclusion criteria were any eyes with a history of corneal refractive surgery (eg, LASIK, SMILE, PRK), clinically significant ocular pathology other than residual refractive error (eg, macular degeneration, advanced glaucoma, keratoconus), suboptimal surgical outcomes that were not related to the treatment plan (eg, cystoid macular edema), eyes that had the toric IOL rotated ≥10 degrees from the implanted axis postoperatively, and clinically significant posterior capsule opacification that could interfere with accurate refraction.
Cataract and Refractive Lens Exchange Surgery
One surgeon performed all cataract surgeries. Phacoemulsification was performed using the Centurion Vision System (Alcon Vision, LLC, Fort Worth, USA). The phacoemulsification tip was introduced and used to emulsify and aspirate the nucleus using a cracking technique. The residual cortex was removed using a manual irrigating/aspirating cannula. The anterior chamber and capsular bag were reformed with viscoelastic agents. The foldable IOL was injected through the main wound and into the capsular bag. The lens was manipulated to achieve good centration, using the ORA System (Alcon Vision, LLC, Fort Worth, USA), the residual viscoelastic was removed, and the anterior chamber was reformed to an appropriate tension using a manual irrigating/aspirating cannula.
Measurements and Refractive Outcomes
Preoperative measurements taken with the Argos included AL, ACD, LT, corneal diameter (also known as the WTW), the flat K and its axis, the steep K and its axis, and the amount of astigmatism with its axis. The Barrett Universal II in the Argos device was used for all IOL power calculations. The Barrett toric calculator was used to determine toric power and toric lens orientation. The surgical treatment data were recorded, including both the planning data and the treatment data. This included the IOL power calculations with expected residual refractive errors, and the toric calculations with the Barrett calculator using the Argos K measurements and the predicted posterior corneal astigmatism. The VERION image guidance (Alcon Vision, LLC, Fort Worth, USA) from the Argos was used during surgery to align the toric IOL on axis. The same calculations (back calculations) were performed using IOLMaster 700 Barrett TK Toric calculator with the TK measurement.
Refractive outcomes were measured between 4 and 6 weeks after surgery with a manifest refraction that included sphere, cylinder, axis, and monocular uncorrected distance visual acuity (UDVA) and CDVA.
Statistical Analysis
The statistical analysis was conducted with Statistical Analysis System software (Version 9.4, SAS Institute, Inc., Cary, USA). Differences between each biometer system’s baseline and postoperative month 1 outcome variables were compared using generalized estimating equation (GEE) models. GEE models were used due to two levels of dependence present in the data. First, 15 of the 44 subjects were bilaterally treated; second, each eye provided data for two biometers. Continuous outcomes were analyzed using an identity link function in the GEE model; binary endpoints were analyzed using a logit link function in the GEE model.
Results
The retrospective chart review uncovered 500 cases, of which 59 eyes of 44 patients met the inclusion/exclusion criteria shown above. The preoperative patient demographics are summarized in Table 1. This study included approximately equal numbers of female and male eyes. All eyes were implanted with a toric IOL. No adverse effects, product issues, or other safety events were observed in this study.
 |
Table 1 Preoperative Patient Demographics |
The refractive outcomes are summarized in Figure 1. Overall, 86% of eyes were within ± 0.5 D of the spherical equivalent target. In addition, 76% of eyes had 0.5 D or less of postoperative residual astigmatism. Visual acuities were acceptable, with 69% and 93% of eyes 20/25 or better in their postoperative UDVA and CDVA, respectively.
Table 2 provides the K steep, K flat, spherical equivalent prediction error, and cylinder prediction error for both the Argos and IOLMaster 700 devices. Pearson correlation coefficients for K steep and K flat were very close to 1 (r = 0.986 and r = 0.984 respectively), indicating a near perfect correlation between devices. There were statistically significant differences for the K steep and K flat measurements between devices (p < 0.001 for K steep and p = 0.0021 for K flat), but they appear to be clinically insignificant. The steep K measurement was 0.16 larger for Argos than IOLMaster 700 and the flat K measurement was 0.11 larger for Argos than IOLMaster 700. Likewise, prediction errors were highly correlated between devices (r = 0.698 for spherical equivalent and r = 0.634 for cylinder). Cylinder and spherical equivalent prediction errors were significantly different between devices (p = 0.017 for spherical equivalent and p < 0.001 for cylinder), but do appear to be clinically insignificant. The Argos tended to underestimate the postoperative cylinder while the IOLMaster 700 had a tendency to overestimate the postoperative cylinder, but the magnitudes of misestimation were approximately the same (−0.17 D and 0.12 D respectively).
 |
Table 2 Summary of Comparison Between Devices |
Bias could be introduced when looking at the results when one lens was recommended by one device and implanted. For example, if the Argos device recommended a TFAT40 with a spherical power of 15 D and that was what was implanted, there could be bias towards less prediction error with the Argos device. We conducted a further analysis and categorized the data based on whether the Argos suggested IOL model and power was implanted, the IOLMaster 700 suggested IOL model and power was implanted, both the suggested IOL model and power from both devices were implanted (eg the Argos and IOLMaster 700 suggested the same IOL model and power), or whether neither of the suggested IOL models and powers from the devices were implanted. However, the combined analyses of spherical equivalent and cylinder prediction error at postoperative month 1, presented in Table 2 included lens choice in the model to adjust for any such bias. It should be noted that in neither analysis did lens choice significantly affect outcomes. The analysis by lens choice is presented in Table 3. These results, stratified by lens choice, are not statistically significant and do not appear to be clinically meaningful.
 |
Table 3 Summary of Comparison Between Devices by Lens Choice |
The percentage of eyes with prediction error for spherical equivalent within ± 0.50 D was 79.7% (47 eyes) for Argos and 61.0% (36 eyes) for IOLMaster 700, a difference of 18.7% (11 eyes). A GEE model, adjusting for baseline delta K, AL, ACD, and lens choice found Argos to be significantly better at achieving a spherical equivalent prediction error 0.50 D or less of target compared to the IOLMaster 700 (p = 0.016). Likewise, the percentage of eyes with prediction error for cylinder within ± 0.50 D was 83.1% (49 eyes) for Argos and 76.3% (45 eyes) for IOLMaster 700, which was a difference of 6.8% (4 eyes). A GEE model, adjusting for baseline delta K, AL, and ACD did not find this difference between Argos and IOLMaster 700 for achieving a cylinder prediction error 0.50 D or less of target to be statistically significant (p = 0.206). These results are summarized in Figures 2 and 3.
 |
Figure 2 Prediction error for spherical equivalent. Abbreviation: D, diopters. |
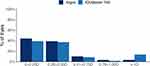 |
Figure 3 Prediction error for cylinder. Abbreviation: D, diopters. |
A sub-analysis was performed categorizing eyes (by preoperative steep K axis) as against-the-rule (ATR; 0° ≤ ATR ≤ 22.5° or 157.5° ≤ ATR ≤ 180°), with-the-rule (WTR; 67.5° ≤ WTR ≤ 112.5°), or oblique (OBL; 22.5° < OBL < 67.5° or 157.5° < OBL < 180°). The categorization resulted in 18 eyes (30.5%) assigned to ATR, 34 eyes (57.6%) assigned to WTR, and 7 eyes (11.9%) assigned to OBL. Adding ATR, WTR, and OBL categorization to the GEE models above indicated no significant differences in prediction accuracies of spherical equivalent and cylinder for ATR, WTR, and OBL eyes between the Argos and the IOLMaster 700 (p > 0.05). Further, the categorization did not interact with biometer (IOLMaster 700 or Argos), indicating that neither biometer was either better or worse for a specific type of astigmatism.
Discussion
Biometers are indispensable diagnostic tools for the cataract and refractive surgeon. They allow for increasingly precise measurements and postoperative refraction prediction accuracy. Understanding the performance of commercially available biometers can allow surgeons to select appropriate devices for their practice. To that end, this study evaluated and compared the prediction accuracy between two SS-OCT biometers, the Argos and IOLMaster 700 devices, with toric IOL implantation.
The primary endpoint of this study was the astigmatism prediction error for the Argos and IOLMaster 700 biometers. We did not find any clinically significant differences between the devices for astigmatism prediction error. Nor did we observe any significant differences in the percentage of eyes that were within 0.5 D or less of cylinder prediction. The IOLMaster 700 is capable of measuring both the anterior corneal astigmatism and the posterior corneal astigmatism.13 Both of these measurements may be crucial for accurate refractive outcomes and happy patients and surgeons. However, we found no evidence of any clinically significant differences in cylinder prediction accuracy between the Argos and IOLMaster 700 devices. This statistical significance, but clinical irrelevance may be caused by the different refractive indices used by the devices. To the best of our knowledge, there are no other studies that have compared the cylinder prediction errors between the Argos and IOLMaster 700 devices.
In addition to cylinder prediction error, we investigated the spherical equivalent prediction error between the Argos and IOLMaster 700 biometers. Although the mean prediction error results revealed statistically significant differences between the devices (0.13 D for Argos and 0.25 D for IOLMaster 700) the results do not appear clinically significant. However, the same is not true when the devices were compared for the percentage of eyes with prediction error less than or equal to 0.5 D. Here, the differences were not only statistically significant (80% for Argos and 61% for IOLMaster 700) but also appear clinically significant. Missing the refractive target during IOL implantation may necessitate an enhancement procedure postoperatively. A difference in spherical equivalent prediction error of 19% could translate, on average, to 19 more eyes per 100 requiring an additional procedure for optimal postoperative refractive outcomes. Our results appear to differ from those of previously published studies.8,14,15 These studies reported no differences in the percentage of eyes with prediction error (spherical equivalent) less than or equal to 0.5 D between the Argos and the IOLMaster 700. Our study focused on toric IOL implantation, and specifically implanted mostly trifocal toric IOLs (86.4% of all IOLs implanted in this study), which may explain these differences. Choi et al16 did compare spherical equivalent prediction error between Argos and IOLMaster 700 with a trifocal IOL. The authors reported no difference in spherical equivalent prediction error less than or equal to 0.5 D between the devices, however this was a study of post-refractive eyes. Many of the previous studies8,14,15 also had a larger sample size, which may also explain the differences to our results.
In this study, there were strong correlations between the Argos and IOLMaster 700 for K flat, K steep, spherical equivalent prediction error, and cylinder prediction error. These results are in line with previous reports, which also indicated strong correlations between the Argos and IOLMaster 700 for K measurements and prediction error.8,12,14,15,17 This is not unexpected, since both of these biometers utilize SS-OCT. From this study and previous reports, it appears that both biometers are clinically useful and can result in acceptable postoperative refractive outcomes.
The limitations of this study include the retrospective design and that the data come from a single site with a single surgeon. A single surgeon study does provide good internal validity; however, a multicenter study should be conducted to confirm our findings. A mixture of AL were included in this study: short (AL < 22 mm), medium (22 ≤ AL ≤ 26 mm), and long (AL > 26 mm); however, no sub-group analysis was performed for each different AL group, since this was not the primary objective of the study. Another limitation is that the data for the IOL Master 700 was obtained with back-calculation. Back-calculations do have an advantage in that each eye acts as its own control, however empirical data would be more robust.
Conclusions
There were strong correlations between the Argos and IOLMaster 700 biometers for K flat, K steep, spherical equivalent prediction error, and cylinder prediction error. The prediction accuracies were similar between the devices, except for spherical equivalent, where a higher percentage of eyes were within 0.5 D of the prediction with the Argos compared to the IOLMaster.
Funding
This study was supported with an investigator-initiated study grant (67187439) from Alcon Vision, LLC, Fort Worth, TX, USA.
Disclosure
Robert F. Melendez reports that he is a consultant for Alcon Laboratories. Gerard Smits reports personal fees from Juliette Eye during the conduct of this study; and that he is a consultant for Zeiss Medical. Brad Hall reports personal fees from Juliette Eye during the conduct of this study; personal fees from Ace Vision Group, outside the submitted work. The authors report no other conflicts of interest for this work.
References
1. Lehmann RP, Houtman DM. Visual performance in cataract patients with low levels of postoperative astigmatism: full correction versus spherical equivalent correction. Clin Ophthalmol. 2012;6:333–338. doi:10.2147/OPTH.S28241
2. Khan MI, Muhtaseb M. Prevalence of corneal astigmatism in patients having routine cataract surgery at a teaching hospital in the United Kingdom. J Cataract Refract Surg. 2011;37:1751–1755. doi:10.1016/j.jcrs.2011.04.026
3. Chen W, Zuo C, Chen C, et al. Prevalence of corneal astigmatism before cataract surgery in Chinese patients. J Cataract Refract Surg. 2013;39:188–192. doi:10.1016/j.jcrs.2012.08.060
4. Vogel A, Dick BH, Krummenauer F. Reproducibility of optical biometry using partial coherence interferometry: intraobserver and interobserver reliability. J Cataract Refract Surg. 2001;27:1961–1968. doi:10.1016/S0886-3350(01)01214-7
5. Hoffer KJ, Shammas HJ, Savini G, Huang J. Multicenter study of optical low-coherence interferometry and partial-coherence interferometry optical biometers with patients from the United States and China. J Cataract Refract Surg. 2016;42:62–67. doi:10.1016/j.jcrs.2015.07.041
6. Hoffer KJ, Shammas HJ, Savini G. Comparison of 2 laser instruments for measuring axial length. J Cataract Refract Surg. 2010;36:644–648. doi:10.1016/j.jcrs.2009.11.007
7. Montes-Mico R, Pastor-Pascual F, Ruiz-Mesa R, Tana-Rivero P. Ocular biometry with swept-source optical coherence tomography. J Cataract Refract Surg. 2021;47:802–814. doi:10.1097/j.jcrs.0000000000000551
8. Yang CM, Lim DH, Kim HJ, Chung TY. Comparison of two swept-source optical coherence tomography biometers and a partial coherence interferometer. PLoS One. 2019;14:e0223114. doi:10.1371/journal.pone.0223114
9. Srivannaboon S, Chirapapaisan C, Chonpimai P, Loket S. Clinical comparison of a new swept-source optical coherence tomography-based optical biometer and a time-domain optical coherence tomography-based optical biometer. J Cataract Refract Surg. 2015;41:2224–2232. doi:10.1016/j.jcrs.2015.03.019
10. Shammas HJ, Ortiz S, Shammas MC, Kim SH, Chong C. Biometry measurements using a new large-coherence-length swept-source optical coherence tomographer. J Cataract Refract Surg. 2016;42:50–61. doi:10.1016/j.jcrs.2015.07.042
11. Higashiyama T, Mori H, Nakajima F, Ohji M. Comparison of a new biometer using swept-source optical coherence tomography and a conventional biometer using partial coherence interferometry. PLoS One. 2018;13:e0196401. doi:10.1371/journal.pone.0196401
12. Montes-Mico R. Evaluation of six biometers based on different optical technologies. J Cataract Refract Surg. 2022;48:16–25. doi:10.1097/j.jcrs.0000000000000690
13. LaHood BR, Goggin M. Measurement of posterior corneal astigmatism by the IOLMaster 700. J Refract Surg. 2018;34:331–336. doi:10.3928/1081597X-20180214-02
14. Omoto MK, Torii H, Masui S, Ayaki M, Tsubota K, Negishi K. Ocular biometry and refractive outcomes using two swept-source optical coherence tomography-based biometers with segmental or equivalent refractive indices. Sci Rep. 2019;9:6557. doi:10.1038/s41598-019-42968-3
15. Tamaoki A, Kojima T, Hasegawa A, et al. Clinical evaluation of a new swept-source optical coherence biometer that uses individual refractive indices to measure axial length in cataract patients. Ophthalmic Res. 2019;62:11–23. doi:10.1159/000496690
16. Choi JY, Choi A, Kwon H, Jeon S. Accuracy of IOL power calculation formulas for quadrifocal AcrySof IQ PanOptix TFNT implantation in patients with previous corneal refractive surgery: comparison of SS-OCT-based biometers. J Refract Surg. 2021;37:836–841. doi:10.3928/1081597X-20210812-02
17. Sabatino F, Matarazzo F, Findl O, Maurino V. Comparative analysis of 2 swept-source optical coherence tomography biometers. J Cataract Refract Surg. 2019;45:1124–1129. doi:10.1016/j.jcrs.2019.03.020
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

