Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Comparison of Adverse Event Profiles of Tumor Necrosis Factor-Alfa Inhibitors: Analysis of a Spontaneous Reporting Database
Authors Wakabayashi T, Hosohata K , Oyama S, Inada A, Ueno S, Kambara H, Iida T, Nakatsuji T, Uchida M , Iwanaga K
Received 16 January 2020
Accepted for publication 4 June 2020
Published 13 August 2020 Volume 2020:16 Pages 741—747
DOI https://doi.org/10.2147/TCRM.S246328
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Tomohito Wakabayashi, Keiko Hosohata, Saki Oyama, Ayaka Inada, Sayaka Ueno, Hiroko Kambara, Tatsuya Iida, Takahiro Nakatsuji, Mayako Uchida, Kazunori Iwanaga
Education and Research Center for Clinical Pharmacy, Osaka University of Pharmaceutical Sciences, Takatsuki, Osaka, Japan
Correspondence: Keiko Hosohata
Education and Research Center for Clinical Pharmacy, Osaka University of Pharmaceutical Sciences, 4-20-1 Nasahara, Takatsuki, Osaka 569-1094, Japan
Tel +81-72-690-1271
Fax +81-72-690-1023
Email [email protected]
Introduction: Concerns over safety profiles of tumor necrosis factor (TNF)-alfa inhibitors have been raised. The purpose of this study was to clarify the adverse events associated with TNF-alfa inhibitors using a spontaneous reporting system database.
Materials and Methods: A retrospective pharmacovigilance disproportionality analysis was conducted using the Japanese Adverse Drug Event Report (JADER) database. Adverse event reports submitted to the Pharmaceuticals and Medical Devices Agency between 2004 and 2017 were analyzed, and the reporting odds ratio (ROR) and 95% confidence interval (CI) for each adverse event were calculated.
Results: Among the 34,031 reports of adverse events associated with TNF-alfa inhibitors, 65.8% were women, who were frequently in their 60s (28.2%). Signals were detected for pneumonia (ROR, 5.36; 95% CI, 5.14– 5.6), interstitial lung disease (ROR, 2.04; 95% CI, 1.95– 2.15), pneumocystis jirovecii pneumonia (ROR, 11.8; 95% CI, 11.1– 12.5), and herpes zoster (ROR, 6.4; 95% CI, 5.92– 6.91) for TNF-alfa inhibitors as a class. There was variability in their signal strength across individual TNF-alfa inhibitors.
Conclusion: The strength of the associations of TNF-alfa inhibitors with adverse events is variable, and further studies are required to evaluate the identified signals.
Keywords: TNF-alfa inhibitors, adverse drug events, spontaneous reporting system, reporting odds ratio, Japanese Adverse Drug Event Report database
Introduction
Tumor necrosis factor (TNF)-alpha is a potent pro-inflammatory cytokine exerting pleiotropic effects on various cell types and plays a central role in the pathogenesis of inflammatory diseases. Antibodies that bind to and neutralize TNF-alfa have been developed in order to inhibit its activity, and have been shown to be effective for patients with rheumatoid arthritis (RA) and other forms of inflammatory disease such as psoriasis, psoriatic arthritis, juvenile rheumatoid arthritis (JRA), ankylosing spondylitis (AS), and inflammatory bowel disease (IBD).1,2 Currently available therapies for them are infliximab, etanercept, adalimumab, golimumab, and certolizumab pegol in Japan. Several Phase III studies showed that TNF-alpha inhibitors had favorable safety profiles.3,4 In clinical practice, however, unexpected adverse events could occur because patients have various backgrounds and etiologies, unlike in clinical trials, where enrollment criteria are strict. Therefore, unexpected adverse drug effects can emerge, and so investigation of their occurrence is important.
In the post-marketing phase, it is important to monitor high-priority adverse events and gain insight into actual drug safety profiles. Spontaneous reporting systems are a primary source of information to detect safety signals, especially for newly marketed drugs.5,6 For the pharmacovigilance approach, the Japanese Adverse Drug Event Report (JADER) database is a large published database managed by the Pharmaceuticals and Medical Devices Agency (PMDA).7,8 In this study, we aimed to clarify the adverse event profiles of five TNF-alfa inhibitors as a class and individual agents in real-world settings using the JADER database.
Methods
The JADER database is freely obtainable from the website of the PMDA, which has been reported.9–12 The data covered the period between April 2004 and January 2017. The JADER consists of 4 tables: patient demographic information (DEMO), drug information (DRUG), adverse events (REAC), and medical history. After we removed duplicate data from each table, the DEMO table was then linked to the REAC and DRUG tables using the ID number. The contribution of the medication to adverse events was classified into three categories: “suspected medicine,” “concomitant medicine,” and “interaction.” We only extracted cases that were classified as “suspected medicine” and analyzed the reports of suspected drugs and adverse events in the “Preferred Term (PT)” coded in the Medical Dictionary for Regulatory Activities (MedDRA). We compiled a cross-tabulation table based on two classifications: the presence or absence of the adverse event, and the presence or absence of the suspected medicine. Then, we calculated the reporting odds ratio (ROR) by the following formula.
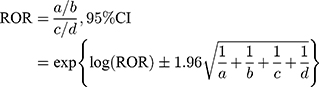
a: the number of patients with a target event when they received a target drug
b: the number of patients with non-target adverse events when they received a target drug
c: the number of patients with a target event when they received non-target drugs
d: the number of patients with non-target adverse events when they received non-target drugs
A signal was considered present when the lower limit of the 95% CI of the ROR exceeded one.
Results
The total number of adverse events associated with the use of TNF-alfa inhibitors was 34,031. Of those, 16,724, 7441, 5131, 3376, and 1359 were reported with infliximab, etanercept, adalimumab, golimumab, and certolizumab pegol, respectively (Table 1). Infliximab has been available for the longest period among the five drugs (Table S1). As shown in Table 2, characteristics of those who experienced adverse events on receiving TNF-alfa inhibitors are listed. In brief, most of the reports concerned females (65.8%), who were most frequently in their 60s (28.2%). Reasons for using TNF-alfa inhibitors included RA (71.3%), Crohn’s disease (13.0%), and ulcerative colitis (3.0%). Drug use for unknown or other indications involved 1620 reports (4.7%).
 |
Table 1 Annual Reports of Adverse Events Associated with TNF-Alfa Inhibitors from 2004 to 2016 |
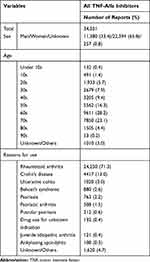 |
Table 2 Characteristics of the Patients with Adverse Events Associated with TNF-Alfa Inhibitors |
Table 3 shows the disproportionality analysis based on the top 30 adverse events associated with TNF-alfa inhibitors as a class. The most frequently reported adverse event associated with TNF-alfa inhibitors as a class was pneumonia (ROR, 5.36; 95% CI, 5.14–5.6), followed by interstitial lung disease (ROR, 2.04; 95% CI, 1.95–2.15), pneumocystis jirovecii pneumonia (ROR, 11.8; 95% CI, 11.1–12.5), herpes zoster (ROR, 6.4; 95% CI, 5.92–6.91), infusion-related reaction (ROR, 29.2; 95% CI, 26.6–32), sepsis (ROR, 3.16; 95% CI, 2.92–3.41), and pneumonia bacterial (ROR, 11.5; 95% CI, 10.5–12.5). Focusing on SOC, “infections and infestations” were most frequently reported.
 |
Table 3 The Top 30 Adverse Drug Events Associated with TNF-Alfa Inhibitors |
Next, we examined the top 10 adverse events by individual TNF-alfa inhibitor (Tables 4–8). Pneumonia was the most frequently reported for infliximab, etanercept, adalimumab, golimumab, and certolizumab pegol. Especially, the number of reports involving infliximab was the highest (1254 reports). Interstitial lung disease ranked high in infliximab, etanercept, adalimumab, golimumab, and certolizumab, and the ROR values were similar among the five TNF-alfa inhibitors. The ROR value for pneumocystis jirovecii pneumonia suggested the strongest association with infliximab. As for infusion reactions, reports were obtained for only infliximab, and the ROR value was noteworthy (ROR, 58.6; 95% CI, 53.4–64.3). Regarding malignant tumors, the associations of breast and colon cancers were suggested with etanercept and certolizumab pegol, respectively.
 |
Table 4 The Top 10 Adverse Drug Events Associated with Infliximab |
 |
Table 5 The Top 10 Adverse Drug Events Associated with Etanercept |
 |
Table 6 The Top 10 Adverse Drug Events Associated with Adalimumab |
 |
Table 7 The Top 10 Adverse Drug Events Associated with Golimumab |
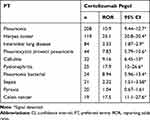 |
Table 8 The Top 10 Adverse Drug Events Associated with Certolizumab Pegol |
Discussion
This study presents profiles of adverse events associated with the use of TNF-alfa inhibitors based on the real-world data from the JADER database. In our results, the number of reports of adverse events involving TNF-alfa inhibitors during the study period were 34,031, and there is variability in the safety profile among TNF-alfa inhibitors. To the best of our knowledge, this is the first study to report the associations of five TNF-alfa inhibitors with adverse events using a spontaneous reporting database.
Among the TNF-alfa inhibitors analyzed in this study, infliximab has been clinically available for use for the longest (Table S1), and so it is suggested that infliximab is likely to be associated with more adverse events, solely based on this longer time on the market. In this study, we found that reports of infectious adverse events were common on the use of TNF-alfa inhibitors (Table 3). TNF-alfa inhibitors suppress immunity; therefore, the risk of infection may be increased.13,14 However, we found that individual agents showed a variable signal strength for multiple types of infectious adverse events. For example, the ROR value for pneumocystis jirovecii pneumonia was the highest for infliximab, whereas that for herpes zoster was the highest for certolizumab pegol. On the other hand, interstitial lung disease, which was the second most highly reported after pneumonia (Table 3), showed almost the same signal values among all TNF-alfa inhibitors. Wakao et al reported that interstitial lung disease is more often reported in Japan compared with the rest of the world.15 There may be a coding preference that contributes to the difference in the relative reporting rate of interstitial lung disease between Japan and the rest of the world. Therefore, interstitial lung disease may be more commonly reported on the use of individual TNF-alfa inhibitors, leading to small variations in signal values.
Binding and neutralizing activities against soluble TNF are common actions of TNF-alfa inhibitors; however, recent studies demonstrated that these inhibitors have additional biological effects against transmembrane TNF16–20 and Fc receptor-expressing cells.21,22 Several studies demonstrated the binding of infliximab, adalimumab, etanercept, or certolizumab to cell lines expressing transfected transmembrane TNF, and showed that the degree of cell binding was 3-fold greater with infliximab or adalimumab than with etanercept or certolizumab.19,23 In addition, CD64 modulates the efficacy of infliximab both in vitro and ex vivo, whereas the presence of this receptor has no impact on the inhibitory activity of certolizumab-pegol, which lacks the Fc fragment.21,22 In order to reduce adverse effects by preventing interaction with the Fc receptors,24 certolizumab pegol was produced without the Fc region, removing the mechanism dependent on the presence of that region.24 In addition, certolizumab pegol was a pegylated anti-TNF F(ab’)2 fragment,25 with a structure different from that of other TNF-alfa inhibitors. However, it is possible that other factors contribute to infections. Further studies will be needed to clarify this.
Different from other TNF-alfa inhibitors, infliximab is a mouse/human chimeric monoclonal TNF-alfa inhibitor of the IgG1 isotype,25 so it requires intravenous drip infusion. In our results, infusion reaction was reported only for infliximab. In addition, anaphylactic reaction and reduction in blood pressure were noted with infliximab. These results are consistent with those of previous reports.26–28
As for malignancy, our results showed that lymphoma, breast cancer, and gastric cancer were significantly correlated with TNF-alpha inhibitors as a class. Especially, reports of breast cancer were numerous with etanercept. It has been reported that the immunosuppressive effects of TNF-alpha inhibitors raise the risk of cancer,29 although it is known that RA disease activity by itself is a risk factor for developing malignancy.30
Several studies reported that TNF-alfa inhibitors are associated with central nervous system (CNS) demyelinating disorders, as well as peripheral nervous system disorders (Guillain-Barré syndrome, Miller Fisher syndrome, chronic inflammatory demyelinating polyneuropathy, multifocal motor neuropathy with conduction block, mononeuropathy multiplex, and axonal sensorimotor polyneuropathies).31,32 However, in this study, the top 30 adverse events caused by TNF-alfa inhibitors included no CNS-related adverse events.
The present study has several limitations. First, this study does not cover all known TNF-alpha inhibitors. Second, as a consequence of the self-reporting database, it is difficult to completely remove reporting bias. In addition, the data occasionally contain coding errors. Third, ROR does not provide a robust indication of the signal strength. In spontaneous reporting systems such as JADER, control populations are not included, so ROR is different from the “odds ratio” that is commonly used in epidemiological studies. In real terms, ROR indicates an increased risk of adverse event reporting, and not the risk of an adverse event itself. Finally, the JADER database did not include detailed clinical information on the patients’ clinical status.
In conclusion, the strength of the association among TNF-alfa inhibitors with adverse events is variable, and further studies are required to evaluate the identified signals.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ettehadi P, Greaves MW, Wallach D, Aderka D, Camp RD. Elevated tumour necrosis factor-alpha (tnf-alpha) biological activity in psoriatic skin lesions. Clin Exp Immunol. 1994;96:146–151. doi:10.1111/j.1365-2249.1994.tb06244.x
2. Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of crohn’s disease: results of the classic ii trial. Gut. 2007;56:1232–1239. doi:10.1136/gut.2006.106781
3. van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: A pooled analysis of 10 Phase II and iii clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(83–98):e84. doi:10.1016/j.jaad.2016.03.024
4. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (go-after study): A multicentre, randomised, double-blind, placebo-controlled, phase iii trial. Lancet. 2009;374:210–221. doi:10.1016/S0140-6736(09)60506-7
5. Mahe J, de Campaigno EP, Chene AL, Montastruc JL, Despas F, Jolliet P. Pleural adverse drugs reactions and protein kinase inhibitors: identification of suspicious targets by disproportionality analysis from vigibase. Br J Clin Pharmacol. 2018. doi:10.1111/bcp.13693
6. Mendes D, Alves C, Batel-Marques F. Safety profiles of adalimumab, etanercept and infliximab: A pharmacovigilance study using a measure of disproportionality in a database of spontaneously reported adverse events. J Clin Pharm Ther. 2014;39:307–313. doi:10.1111/jcpt.12148
7. Hosoya R, Uesawa Y, Ishii-Nozawa R, Kagaya H. Analysis of factors associated with hiccups based on the japanese adverse drug event report database. PLoS One. 2017;12:e0172057. doi:10.1371/journal.pone.0172057
8. Kose E. Adverse drug event profile associated with pregabalin among patients with and without cancer: analysis of a spontaneous reporting database. J Clin Pharm Ther. 2018;43:543–549. doi:10.1111/jcpt.12683
9. Hosohata K, Inada A, Oyama S, Furushima D, Yamada H, Iwanaga K. Surveillance of drugs that most frequently induce acute kidney injury: A pharmacovigilance approach. J Clin Pharm Ther. 2019;44:49–53. doi:10.1111/jcpt.12748
10. Hosohata K, Inada A, Oyama S, Niinomi I, Wakabayashi T, Iwanaga K. Adverse cutaneous drug reactions associated with old- and new- generation antiepileptic drugs using the japanese pharmacovigilance database. Clin Drug Investig. 2019;39:363–368. doi:10.1007/s40261-019-00754-z
11. Oyama S, Hosohata K, Inada A, et al. Drug-induced tubulointerstitial nephritis in a retrospective study using spontaneous reporting system database. Ther Clin Risk Manag. 2018;14:1599–1604. doi:10.2147/TCRM.S168696
12. Inada A, Hosohata K, Oyama S, et al. Evaluation of medication-related osteonecrosis of the jaw using the japanese adverse drug event report database. Ther Clin Risk Manag. 2019;15:59–64. doi:10.2147/TCRM.S176620
13. Lane MA, McDonald JR, Zeringue AL, et al. Tnf-alpha antagonist use and risk of hospitalization for infection in a national cohort of veterans with rheumatoid arthritis. Medicine. 2011;90:139–145. doi:10.1097/MD.0b013e318211106a
14. Minozzi S, Bonovas S, Lytras T, et al. Risk of infections using anti-tnf agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: A systematic review and meta-analysis. Expert Opin Drug Saf. 2016;15:11–34. doi:10.1080/14740338.2016.1240783
15. Wakao R, Taavola H, Sandberg L, et al. Data-driven identification of adverse event reporting patterns for japan in vigibase, the who global database of individual case safety reports. Drug Safety. 2019;42:1487–1498. doi:10.1007/s40264-019-00861-y
16. Derer S, Till A, Haesler R, et al. Mtnf reverse signalling induced by tnfalpha antagonists involves a gdf-1 dependent pathway: implications for crohn’s disease. Gut. 2013;62:376–386. doi:10.1136/gutjnl-2011-300384
17. Kaymakcalan Z, Sakorafas P, Bose S, et al. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin immunol. 2009;131:308–316. doi:10.1016/j.clim.2009.01.002
18. Nesbitt A, Fossati G, Bergin M, et al. Mechanism of action of certolizumab pegol (cdp870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis. 2007;13:1323–1332. doi:10.1002/ibd.20225
19. Scallon B, Cai A, Solowski N, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301:418–426. doi:10.1124/jpet.301.2.418
20. Shealy DJ, Cai A, Staquet K, et al. Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor alpha. mAbs. 2010;2:428–439. doi:10.4161/mabs.12304
21. Vos AC, Wildenberg ME, Duijvestein M, Verhaar AP, van den Brink GR, Hommes DW. Anti-tumor necrosis factor-alpha antibodies induce regulatory macrophages in an fc region-dependent manner. Gastroenterology. 2011;140:221–230. doi:10.1053/j.gastro.2010.10.008
22. Wojtal KA, Rogler G, Scharl M, et al. Fc gamma receptor cd64 modulates the inhibitory activity of infliximab. PLoS One. 2012;7:e43361. doi:10.1371/journal.pone.0043361
23. Mitoma H, Horiuchi T, Tsukamoto H. Binding activities of infliximab and etanercept to transmembrane tumor necrosis factor-alpha. Gastroenterology. 2004;126:
24. Levin AD, Wildenberg ME, van den Brink GR. Mechanism of action of anti-tnf therapy in inflammatory bowel disease. J Crohn’s Colitis. 2016;10:989–997. doi:10.1093/ecco-jcc/jjw053
25. Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol Ther. 2008;117:244–279. doi:10.1016/j.pharmthera.2007.10.001
26. Iwanczak BM, Ryzko J, Jankowski P, et al. Evaluation of the infliximab therapy of severe form of pediatric crohn’s disease in poland: retrospective, multicenter studies. Adv Clin Exp Med. 2017;26:51–56. doi:10.17219/acem/35802
27. Filho AG, Kinote A, Pereira DJ, et al. Infliximab prevents increased systolic blood pressure and upregulates the akt/enos pathway in the aorta of spontaneously hypertensive rats. Eur J Pharmacol. 2013;700:201–209. doi:10.1016/j.ejphar.2012.11.059
28. Choquette D, Faraawi R, Chow A, Rodrigues J, Bensen WJ, Nantel F. Incidence and management of infusion reactions to infliximab in a prospective real-world community registry. J Rheumatol. 2015;42:1105–1111. doi:10.3899/jrheum.140538
29. Setoguchi S, Solomon DH, Weinblatt ME, et al. Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:2757–2764. doi:10.1002/art.22056
30. Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: A meta-analysis. Arthritis Res Ther. 2015;17:212. doi:10.1186/s13075-015-0728-9
31. Bosch X, Saiz A, Ramos-Casals M, Group BS. Monoclonal antibody therapy-associated neurological disorders. Nat Rev Neurol. 2011;7:165–172. doi:10.1038/nrneurol.2011.1
32. Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum. 2001;44:2862–2869. doi:10.1002/1529-0131(200112)44:12<2862::AID-ART474>3.0.CO;2-W
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
