Back to Journals » Patient Preference and Adherence » Volume 12
Comparison between prefilled syringe and autoinjector devices on patient-reported experiences and pharmacokinetics in galcanezumab studies
Authors Stauffer VL, Sides R, Lanteri-Minet M, Kielbasa W, Jin Y, Selzler KJ , Tepper SJ
Received 12 April 2018
Accepted for publication 17 July 2018
Published 17 September 2018 Volume 2018:12 Pages 1785—1795
DOI https://doi.org/10.2147/PPA.S170636
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Johnny Chen
Video abstract presented by Virginia L Stauffer.
Views: 6633
Virginia L Stauffer,1 Ryan Sides,1 Michel Lanteri-Minet,2,3 William Kielbasa,1 Yan Jin,1 Katherine J Selzler,1 Stewart J Tepper4
1Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, IN, USA; 2Pain Department, CHU Nice, Nice, France; 3Université Cote d’Azur, FHU InovPain, CHU de Nice, Nice, France; 4Geisel School of Medicine at Dartmouth, Hanover, NH, USA
Purpose: The aim of this study was to compare the usability and patient-rated experiences of an autoinjector with a prefilled syringe in patients with migraine, who self-administered galcanezumab, and to compare pharmacokinetic parameters between these devices.
Materials and methods: Patient-rated experiences with an investigational autoinjector and a prefilled syringe were compared in an open-label, 12-month study of once-monthly injections of galcanezumab 120 or 240 mg (NCT02614287). Patient-rated ease of usability was assessed with the Subcutaneous Administration Assessment Questionnaire (SQAAQ) and compared between devices. Positive responses on the SQAAQ were rated as “agree or strongly agree” to 12 statements. Tolerability was assessed by the frequency of injection-site-related adverse events (AEs) by device and injection location. In a separate study, galcanezumab pharmacokinetics in healthy subjects was compared between the devices (NCT02836613).
Results: In the open-label clinical trial, 179 patients used both the prefilled syringe and autoinjector at least once. The majority of patients (91%–97%) had positive responses on the SQAAQ to the use of autoinjector across the items assessed. There were 23 injection-site-related AEs with the first self-administered injection with the prefilled syringe (N=7) or autoinjector (N=16; P=0.061), with the most common AE for either device being injection-site pain. There were no significant between-device differences in injection-site-related AEs. For pharmacokinetics, the 90% CI for the ratio (autoinjector/prefilled syringe) of geometric least-square means for the galcanezumab area under the curve (AUC) concentration and maximum concentration (Cmax) was between 0.8 and 1.25, indicating no statistically significant difference in the galcanezumab concentrations regardless of the device used.
Conclusion: The ease of usability with either device was comparable, and there were no significant differences in tolerability between the prefilled syringe and autoinjector with the first self-administration; however, the analysis was not powered to detect a clinically significant difference. Galcanezumab pharmacokinetics were comparable between devices.
Keywords: galcanezumab, devices, self-administered injections, SQAAQ, pharmacokinetics
Introduction
Migraine is a chronic debilitating neurologic disease most prevalent between the ages of 25 and 55 years,1 affecting approximately one out of seven Americans annually.2 Migraine attacks are also a major cause of absenteeism and decreased productivity at work and reduced quality of life.3
During attacks, most patients with migraine have moderate-to-severe pain and reduced ability to function.4 At the onset of a migraine attack, most patients use medications that are taken for acute control of symptoms. However, approximately 38% of patients with migraine would benefit from preventive therapies to reduce migraine burden and potential progression with long-term neurologic effects.5 Once preventive therapy has been initiated, the recommended minimum duration of treatment is 3–6 months for patients with episodic migraine,6 but many patients may require longer treatment to maintain reduced migraine attack frequency.7
Oral migraine preventive medications are preferred by many patients, because they are convenient and easy to use. However, studies have demonstrated that adherence to these agents is poor due to adverse events (AEs).8,9 Non-oral delivery approaches, such as self-administered subcutaneous injections, might facilitate adherence.8 Autoinjector devices have been developed which make injection easy for patients or their caregivers compared to manual injection with prefilled syringes.10,11 Specifically, for patients with migraine, there are multiple delivery devices available for the subcutaneous formulation of sumatriptan for the acute treatment of migraine. Andre et al12 conducted a study to compare migraine patients’ device preferences of two simulated injections using three different sumatriptan subcuautoinjectors. This human factor study determined that patients preferred the autoinjector device that they rated as the easiest and most intuitive to use.12
Galcanezumab is a humanized monoclonal antibody that selectively binds to and blocks the physiological activity of calcitonin gene-related protein (CGRP), which is implicated in migraine pathogenesis.13 Neutralizing CGRP modulates neurogenic inflammation and prevents vasodilation and is a therapeutic approach for the preventive treatment of migraine. In Phase II and III studies, subcutaneous injections with galcanezumab vs placebo significantly reduced the number of migraine headache days (MHDs) per month over a 12-week period.14–18 In Phase III, double-blind studies, injections were administered by site personnel using prefilled syringes.
A single-use autoinjector for self-administration of galcanezumab was developed based on previous human factor research in autoimmune disease and was engineered to be simple to use and easy to operate. Previous studies in patients with psoriasis used a similar autoinjector device in three human factor studies, and the results supported the clinical utility of this autoinjector device for successful administration of ixekizumab.19 In short, the summative study or usability study in patients with various autoimmune disorders was designed to evaluate the proportions of patients who performed successful injections with or without supplemental device training. All patients in the untrained arm performed successful injections when provided the autoinjector and instructions for use, while two patients in the trained arm failed to deliver a dose.19
To better understand the longer-term effectiveness with galcanezumab and to provide data when patients self-injected, a 12-month, Phase III, open-label study (NCT02614287) was conducted.20 In addition to safety and effectiveness, this study provided overall experience of at-home self-administration of galcanezumab with a prefilled syringe and autoinjector device. Further, a separate study (NCT02836613) was conducted to compare the pharmacokinetics (PK) of galcanezumab when administered using a prefilled syringe or an autoinjector in healthy subjects. Results from both studies are reported in this paper.
Materials and methods
Injection devices and formulation
Galcanezumab was supplied as an injectable solution in a 1 mL, single-dose, disposable prefilled syringe and as a 1 mL, single-dose, disposable investigational autoinjector. A dose of galcanezumab 120 mg was contained in a single dose administered by either device. A 240 mg dose of galcanezumab consisted of two 1 mL injections using either device. Both devices have a 27 G needle and contain the same formulation. Galcanezumab injection is a sterile, preservative-free, clear, and colorless to slightly yellow solution for subcutaneous use. Each milliliter is composed of galcanezumab (120 mg); L-histidine, USP (0.5 mg); L-histidine hydrochloride monohydrate (1.5 mg); polysorbate 80, USP (0.5 mg); sodium chloride, USP (8.8 mg); and water for injection, USP. The pH range is 5.3–6.3.
The investigational autoinjector has an ergonomic shape, grip, and dose button, which facilitates handling the device in different ways (Figure 1). It has a lock to prevent misfiring, an automated insertion, and the body is clear, allowing the injection site and drug solution to be visualized and thereby confirm that the full dose is delivered. Audible clicks confirm the start and completion of the dose. It also has a wide base, which eliminates the need to pinch up the skin prior to use.
  | Figure 1 Galcanezumab autoinjector. |
Device instructions for use
The patients were trained by the site personnel in two ways prior to the initial self- or caregiver injection administration. First, the site personnel reviewed the instructions for use for each device with the patients. Next, the site personnel administered the first dose of galcanezumab 240 mg (2–120 mg prefilled syringes) and provided verbal instruction during injection administration. When the autoinjectors were introduced later in the study, the site personnel were provided demonstration autoinjector devices for training purposes, and they also reviewed the autoinjector instructions for use with the patients. The step-by-step injection tasks performed for the autoinjector and prefilled syringe are listed in Table 1.
  | Table 1 Instructions for use: injection with prefilled syringe or autoinjector |
Phase III, open-label, safety study
A multisite, randomized, long-term, open-label study was conducted to assess the safety, tolerability, and effectiveness of subcutaneous galcanezumab 120 and 240 mg administered once a month for 12 months for the prevention of migraine with or without aura. This study was conducted in 28 sites across five countries in North America and Europe. The primary outcomes of the study were published previously.20 Therefore, this paper addresses a secondary objective of the study, which was to compare the ease of usability and tolerability of the prefilled syringe and autoinjector.
Patients with migraine were randomized 1:1 to two dose regimens of galcanezumab 120 or 240 mg once a month. Patients randomized to the 120 mg dose received an initial loading dose of 240 mg (two injections of 120 mg each) that was administered by site personnel (at first monthly dosing visit), who were trained health care professionals. Subsequent once-monthly doses (starting at the second monthly dosing visit) were either self- or caregiver administered at subsequent office visits or at home (with conducted telephone visits). Injections could be given in one of the four locations: abdomen, thigh, arm, or buttocks. Overall, up to 12 once-monthly dose regimens were administered.
Usability of both devices was evaluated using the Subcutaneous Administration Assessment Questionnaire (SQAAQ),19 which is a novel 12-item, self-administered questionnaire that has not yet been psychometrically validated. The SQAAQ items assess ease of use of the device and patient/caregiver confidence while using the device to administer a subcutaneous injection of the drug. Each item was answered on a 7-point Likert scale ranging from “strongly disagree” to “strongly agree”. For each item, the number and percentage of patients or their caregiver who answered “strongly agree” or “agree” were summarized across the number of patient visits with the use of the prefilled syringe or autoinjector. Of note, patients completed only one SQAAQ per dose regimen (ie, patients randomized to galcanezumab 240 mg with two injections completed one SQAAQ questionnaire).
AEs related to the injection site were analyzed between the prefilled syringe and autoinjector. Because this was not a crossover study and patients did not use each device type the same number of times, a descriptive analysis of patients who used both devices focused on comparisons between both the first injection for each device type and the first three injections for each device type. Injection-site-related AEs were summarized for each device according to dose for patients who reported a specific AE with one device, but not the other. Location of injection-site-related AEs was analyzed starting at the second dosing visit, when patients could self-administer. In addition, the frequency of AEs related to the injection site, including pain, was summarized by the location of injection. Injection-site-related AEs were analyzed by first injection at either location as reported by patients in one location, but not another.
Categorical safety analyses of AEs related to the injection site were performed using the McNemar test.
PK assessment
A three-center, open-label, double-arm, randomized, parallel-group study was conducted in healthy subjects to determine the PK of galcanezumab 240 mg after subcutaneous administration of a galcanezumab solution via a prefilled syringe and an autoinjector. Galcanezumab was supplied as an injectable solution in a 1 mL, single-dose, prefilled syringe or autoinjector; each device was designed to deliver galcanezumab 120 mg. One dose of 240 mg comprised two 1 mL injections. The clinical research unit staff administered galcanezumab into the arm, abdomen, or thigh of the subjects according to the randomization schedule. All subjects provided written informed consent prior to any study procedures. For most sites, the study was approved by Quorum Institutional Review Board in the USA and for sites in other countries by the site-affiliated local institutional review board (IRB).
Subjects were healthy males or females aged between 18 and 65 years. Subjects were admitted to the clinical research unit and administered with galcanezumab on the same day. Subjects were discharged from the clinical research unit following completion of all study procedures scheduled within the initial 24 hours post dose. Subjects returned for a series of outpatient visits for PK blood sampling and safety assessments, including the injection-related AEs, for up to approximately 20 weeks after galcanezumab administration. Details for analyzing the serum samples have been described previously.21
The PK parameters of galcanezumab estimated included the maximum concentration (Cmax), time to maximum concentration (tmax), and the area under the galcanezumab concentration–time profile from time zero to infinity (AUC). Galcanezumab AUC and Cmax were log transformed and analyzed using an analysis of variance model. The model included fixed effects for injection device, investigational site, and injection site. The least-square (LS) means for the prefilled syringe and autoinjector and the 90% CIs for the difference in the means were estimated from the ANOVA model and were back transformed from the log scale to provide estimates of the geometric LS means and 90% CIs for the ratios of the geometric LS means. The tmax of galcanezumab for the prefilled syringe and autoinjector was analyzed using a Wilcoxon rank sum test. An estimate of the median difference and 90% CIs were calculated. The geometric mean and 90% CI for AUC and Cmax and the median number of days for tmax were reported.
The sample size of 160 subjects (80 per device arm) provided approximately 90% power to demonstrate that the 90% CI of the ratio (autoinjector/prefilled syringe) of geometric means of Cmax and AUC fell within 0.8–1.25,22 assuming that the true ratio was 0.95 and 12.5% of subjects would not contribute to the end point.
Results
Phase III, open-label, safety study
Baseline characteristics of the 270 patients who participated in the open-label safety study have been reported previously.20 Of the 179 patients exposed to both devices, the average age of patients was 43 years, most were female (84%), white (79%), not Latino or Hispanic (84%), and 63% of the patients were from North America. On average, these patients had 11 MHDs per month, a migraine diagnosis for 22 years, and 18% had failed two or more prior preventive treatments. All 179 patients switched from the prefilled syringe to the autoinjector and home administered at least one dose of galcanezumab with the autoinjector; of whom, 143 used the autoinjector for three consecutive dose administrations. There were 91 patients who did not use the autoinjector because they completed or discontinued the study before the autoinjector was available. Overall study completion rate was 78%.
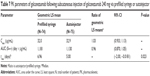
Over 90% of the patients (combined dose groups) reported positive experiences with the first use of the autoinjector, and these ratings remained very positive with subsequent use (Figure 2). A higher percentage of “agree/strongly agree” responses on the SQAAQ items were reported with the autoinjector than the prefilled syringe (Table 2). However, responses regarding the knowledge (94% both devices) and confidence (95% both devices) that the dose was complete were similar. Both the autoinjector and prefilled syringe were evaluated for multiple outcomes. Beginning with the autoinjector at the two galcanezumab doses, 120 and 240 mg, ease to inject dose was selected by 97% of those using the autoinjector to self-inject 120 mg and 94% of those injecting 240 mg. For the prefilled syringe, ease to inject dose was reported by 90% of those injecting the 120 mg dose and 88% of those injecting the 240 mg dose. Knowledge that dose was complete for those using the autoinjector was reported by 95% with the 120 mg dose and 93% with the 240 mg dose. Knowledge that the dose was complete was reported by 95% using the prefilled syringe for the 120 mg dose and 94% for the 240 mg dose. Confidence that the dose was complete was reported by 96% of those using the autoinjector for the 120 mg dose and 94% for the 240 mg dose.
  | Table 2 SQAAQ “agree/strongly agree” responses in the Phase III, open-label, safety study |
The device complaints or malfunctions in the study were low. Of the 1,397 dose regimens that were administered with the prefilled syringe, there were no product complaints related to the function of the prefilled syringe. Of the 534 dose regimens for the autoinjector device, there were four reports or 0.75% of dosing regimens that resulted in an autoinjector malfunction. The malfunctions reported included leaky device, clicks not occurring, needle not advancing, and device being already unlocked.
There were 29 patients using the prefilled syringe and 38 patients using the autoinjector who reported at least one injection-site-related AE (N=179) at the first injection, while after three self-administered injections with the same device, injection-site-related AEs (N=143) were reported in 26 patients using the prefilled syringe and 28 patients using the autoinjector. For the majority of the patients, the reported AEs were considered mild to moderate, with one patient in each device group who reported injection-site pain as severe after the first injection. After three injections using the same device, the injection-site-related pain was reported as severe in none of the patients using the prefilled syringe and two patients using the autoinjector. Of the patients who experienced an injection-site-related AE using both devices, 23 patients (combined galcanezumab doses) reported events that occurred with the first injection with one device but not the other (prefilled syringe 3.9%, autoinjector 8.9%; P=0.061; Table 3), and 18 patients reported an injection-site-related AE after the first three self-administrations of one device, but not the other (prefilled syringe 5.6% and autoinjector 7.0%; P=0.637; Table 4). After the first injection, the most common AE for either device was pain, which was reported after the first injection in 1.7% patients using the prefilled syringe and 4.5% patients using the autoinjector; however, the pain after the first three injections was similar between devices (prefilled syringe, 0.7%; autoinjector, 2.1%).
Injection site location
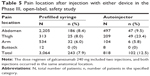
Starting the second dosing visit of the 12-month study, patients were able to begin self-injections of galcanezumab 120 or 240 mg using either the prefilled syringe or the autoinjector; however, not all patients chose to perform the self-administrations. There were 1,462 total dose regimens that were self-administered by patients with exposure to both device types (autoinjector N=473 [87% of which were self-administered]; prefilled syringe N=989 [70% of which were self-administered]). The total dose regimens were administered at the following locations: 70% in the abdomen, 13% in the thigh, 16% in the arm, and <1% in the buttock. Of the dose regimens administered by the prefilled syringe, 72% were in the abdomen, 10% in the thigh, 17% in the arm, and <1% in the buttock, while the autoinjector was administered 61% in the abdomen, 26% in the thigh, 15% in the arm, and <1% in the buttock.
Patients reported any associated injection pain by location (abdomen, arm, buttock, or thigh) and device type (Table 5). After injections with the autoinjector, more patients (23.4%) reported pain in the thigh, followed by the abdomen (9.5%). Lower pain was reported with the prefilled syringe, and it was similar for both thigh and abdomen (8.0% and 8.4%, respectively). A total of 53 patients (galcanezumab 120 mg, N=21; 240 mg, N=32) reported at least one injection-site-related AE that occurred at the abdomen or thigh (only first injection at each location was considered; Table 6). A significantly greater number of patients reported an injection-site reaction occurring at the thigh (41.5%) compared to the abdomen (20.7%; P=0.008). There was a similar incidence rate between both injection site locations (abdomen vs thigh) for other reported injection-site-related AEs.
PK assessment
One hundred and sixty healthy subjects, 79 males and 81 females, participated in the PK and tolerability study. Subjects were 38.4 years of age on average, 55.6% were white and 38.8% were African American. One hundred and fifty-six subjects completed the study.
The galcanezumab serum concentrations were similar for the prefilled syringe and autoinjector (Figure 3, Table 7). The 90% CIs for the ratios of geometric LS means for AUC and Cmax were contained within the interval of 0.8–1.25, indicating no statistically significant difference in the galcanezumab concentrations regardless of the device used. A statistically significant difference (P=0.023) in median tmax for the autoinjector (5 days) compared to the prefilled syringe (7 days) was observed, with substantial overlap in the range of tmax values observed across device arms. The site of injection (arm, thigh, abdomen) did not influence Cmax (P=0.514) or AUC (P>0.964).
Injection-site-related AEs were more frequent with the autoinjector than with prefilled syringe administration, respectively: pain (n=14 and n=2), erythema (n=6 and n=2), bruising (n=3 and n=3), induration (n=3 and n=1), and pruritus (n=3 and n=1).
Discussion
Preventive treatment of migraine may be long-term, many months or years, to prevent the progression of episodic migraine to the more chronic form.23 Adherence to treatment with daily oral medications can be problematic due to AEs,7,8 and once monthly, at-home injections of a biologic developed specifically to treat migraine could improve treatment adherence. The utility, usability, and PK of the two injection devices for the subcutaneous administration of galcanezumab were evaluated.
Overall assessment of patient/caregiver experience with the autoinjector was very positive, with over 90% of the responses being “agreed” and “strongly agreed” for each item on the SQAAQ for patients who used the autoinjector at least once. Notably, 94% of patients across dose groups agreed/strongly agreed that the device was easy to use the first time, and 92% agreed/strongly agreed that they were confident that the dose was complete after the first use. The frequency of the positive responses on the SQAAQ increased with the second and third use. Overall, the positive patient/caregiver experience with the autoinjector in this study is consistent with those of the ixekizumab autoinjector device that also has high acceptance and convenience and which was the model for the development of this autoinjector.19
The occurrence of injection-site-related AEs with the first use of either device was twofold higher with the autoinjector than with the prefilled syringe, which may be due to patients being used to self-injecting with the prefilled syringe. However, when we looked at patients who self-injected with the first three-dose regimens with the prefilled syringe compared to the first three-dose regimens with the autoinjector, the difference in the occurrence with injection-site-related AEs was no longer twofold higher but similar, likely due to patients becoming more comfortable in using the autoinjector.
Patients were given the option to administer the injections of either device into various anatomical regions, including abdomen, arm, buttock, or thigh. Most patients chose to inject in the abdomen or thigh, likely because those regions are easier to reach during self-administration. There was more associated pain with the autoinjector compared to the prefilled syringe, especially when administered at the thigh. This is possibly due to the rate at which the dose is administered in the autoinjector, quicker than the prefilled syringe, which allows the patient to control the rate of administration.
The results of the PK assessment showed that galcanezumab concentrations were similar regardless of the device used or the location of the injection, indicating that the device did not alter the bioavailability of galcanezumab. The median tmax was different by only 2 days across the devices, and it is likely to not be of any clinical consequence for patients, given that galcanezumab is an antibody drug intended to be given once monthly as a preventive treatment for migraine. Furthermore, substantial overlap of tmax values across the devices was observed.
In current clinical practice, patients with migraine attacks are likely to be accustomed to injectable devices, since common treatments, including sumatriptan for acute treatment of migraine and onabotulinumtoxinA for chronic migraine prevention, are administered as injections. However, unlike galcanezumab, onabotulinumtoxinA treatment requires multiple injections every 12 weeks and has to be administered by a trained heath care professional in an office setting. In contrast, galcanezumab injections by either the prefilled syringe or autoinjector can be self-administered once monthly by the patient at home as a preventive treatment.
There were a few limitations of this study. The SQAAQ questionnaire is not a validated measure; however, it helps provide insight into the patient-reported experiences using both devices. The open-label safety study was not placebo controlled, so the reported AEs cannot be compared to an inactive control, and this study was not powered or randomized to demonstrate a statistical difference between the two devices. In addition, the safety study was 12 months long, but the injection-site-related AEs and location of injection site were analyzed at only the first injection and first three injections with any one device. Further, this was not a predesigned crossover study; hence, patients did not use both devices for the same amount of time and were not instructed to use one device and then the other to establish a preference. In addition, the majority of patients using the autoinjector self-administered in the thigh instead of the abdomen, which was the preferred location for the prefilled syringe, possibly because the instructions for use in the device picture (Figure 1) showed that the autoinjector needed to be held in a vertical position, which would be easier to administer in the thigh while sitting. These differences in the preferred injection location of each device could have confounded the results, since the patients using the autoinjector were more likely to report AEs than the patients using the prefilled syringe. Finally, due to timing of when the autoinjector was available during the study, none of the patients used the autoinjector as the first device.
Conclusion
The prefilled syringe and autoinjector administration of galcanezumab was similar in tolerability, and the two devices were successfully used by patients and caregivers, both of whom felt that the device was easy to use, and they were confident using it. The PK of galcanezumab was similar irrespective of the subcutaneous delivery devices evaluated. It is worth noting that although there were no statistical differences between the two devices with regard to injection-site-related AEs, there may be clinical differences that this analysis was not adequately powered to detect. Since the results of both devices are similar in these two studies, patients may prefer to have both options available to decide which device is better suited to their personal preferences and experiences in future clinical practice.
Acknowledgments
The authors would like to thank the patients and study subjects who participated in these two clinical trials as well as the full galcanezumab study team. They also thank Alex King, MD, for his contributions as principal investigator for the PK study; Jonna Ahl, PhD, for her contributions to the first draft of this disclosure; David Williams, MS, and Qiuling Shan, MS, for their statistical analysis assistance for the PK assessment study and the open-label safety study, respectively, and Tianle Hu, PhD, for his review of the statistical content.
Author contributions
VLS participated in the study design, data acquisition, interpretation of data, and drafting of the manuscript. RS participated in the statistical analyses, interpretation of data, and drafting of the manuscript. ML-M participated in the data acquisition and interpretation of data. WK participated in the study design, data acquisition, and interpretation of data. YJ participated in the study design and interpretation of data. KJS participated in the interpretation of data and drafting of the manuscript. SJT participated in interpretation of data. All the authors have revised the manuscript critically for important content, approve the final version to be published, and agree to be accountable for all aspects of the work.
Disclosure
VLS, RS, WK, YJ, and KJS are full-time employees and minor shareholders of Eli Lilly and Company. ML-M in the past 5 years has received honoraria for advisory boards and has been in speaker panels or investigation studies from Allergan, Amgen, Astellas, ATI, BMS, Boehringer, Boston Scientific, CoLucid, Convergence, GlaxoSmithKline, Grunenthal, Eli Lilly, Medtronic, Menarini, MSD, Novartis, Pfizer, Reckitt Benckiser, Saint-Jude, Sanofi-Aventis, Teva, UCB, and Zambon. SJT in the last year has research grants paid to Dartmouth without personal compensation from Alder, Allergan, Amgen, ATI, Dr Reddy’s, ElectroCore, eNeura, Scion Neurostim, Teva, and Zosano. He has received honoraria for consultation from Acorda, Alder, Allergan, Amgen, ATI, Cefaly, Charleston Laboratories, DeepBench, Dr Reddy’s, ElectroCore, Eli Lilly, eNeura, GLG, Guidepoint Global, Impax, Neurolief, Pfizer, Scion Neurostim, Slingshot Insights, Supernus, Teva, and Zosano. He has stock options in ATI, receives royalties for textbooks from Springer, and receives salary from Dartmouth-Hitchcock Medical Center and the American Headache Society. The authors report no other conflicts of interest in this work.
References
Hazard E, Munakata J, Bigal ME, Rupnow MF, Lipton RB. The burden of migraine in the United States: current and emerging perspectives on disease management and economic analysis. Value Health. 2009;12(1):55–64. | ||
Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55(1):21–34. | ||
Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache. 2007;47(3):355–363. | ||
Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–349. | ||
Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337–1345. | ||
Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55(6):754–762. | ||
Vanderpluym J, Evans RW, Starling AJ. Long-term use and safety of migraine preventive medications. Headache. 2016;56(8):1335–1343. | ||
Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55(suppl 2):103–122. | ||
Hepp Z, Dodick DW, Varon SF, Gillard P, Hansen RN, Devine EB. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478–488. | ||
Kivitz A, Cohen S, Dowd JE, et al. Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther. 2006;28(10):1619–1629. | ||
Rothrock JF, Freitag FG, Farr SJ, Smith EF. A review of needle-free sumatriptan injection for rapid control of migraine. Headache. 2013;53(suppl 2):21–33. | ||
Andre AD, Brand-Schieber E, Ramirez M, Munjal S, Kumar R. Subcutaneous sumatriptan delivery devices: comparative ease of use and preference among migraineurs. Patient Prefer Adherence. 2017;11:121–129. | ||
Vermeersch S, Benschop RJ, van Hecken A, et al. Translational pharmacodynamics of calcitonin gene-related peptide monoclonal antibody LY2951742 in a capsaicin-induced dermal blood flow model. J Pharmacol Exp Ther. 2015;354(3):350–357. | ||
Dodick DW, Goadsby PJ, Spierings EL, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13(9):885–892. | ||
Skljarevski V, Oakes TM, Zhang Q, et al. Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: a randomized clinical trial. JAMA Neurol. 2018;75(2):187–193. | ||
Stauffer VL, Dodick DW, Zhang Q, Carter J, Ailani JX, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: results from the EVOLVE-1 study, a randomized phase 3 placebo-controlled clinical trial. JAMA Neurol. Epub 2018 May 29. | ||
Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim B-K, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;47:033310241877954. | ||
Detke H, Wang S, Skljarevski, et al. A Phase 3 Placebo-Controlled Study of Galcanezumab in Patients with Chronic Migraine: Results from the 3-month Double-Blind Treatment Phase of the REGAIN study. 59th Annual Scientific Meeting American Headache Society® June 8–11, 2017 Westin Boston Waterfront Boston, MA. Headache: The Journal of Head and Face Pain. 2017;57:1311–1337. | ||
Callis Duffin K, Bukhalo M, Bobonich M, et al. Usability of a novel disposable autoinjector devise for ixekizumab: results from a qualitative study and an open-label clinical trial, including patient-reported experience. Med Devices (Auckl). 2016;9:361–369. | ||
Stauffer VL, Sides R, Camporeale A, et al. A Phase 3, Long-Term, Open-Label Safety Study of Self-Administered Galcanezumab Injections in Patients with Migraine. Cephalalgia. 2017;37(suppl 1):330. [Abstract PO-01-184] | ||
Oakes TMM, Skljarevski V, Zhang Q, et al. Safety of galcanezumab in patients with episodic migraine: a randomized placebo-controlled dose-ranging phase 2b study. Cephalalgia. 2018;38(6):1015–1025. | ||
U.S. Food and Drug Administration/Center for Drug Evaluation and Research. Guidance for Industry: Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs – General Considerations. 2014. Available from: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm389370.pdf. Accessed March 26, 2018. | ||
Lipton RB, Fanning KM, Serrano D, Reed ML, Cady R, Buse DC. Ineffective acute treatment of episodic migraine is associated with new-onset chronic migraine. Neurology. 2015;84(7):688–695. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.