Back to Journals » Journal of Pain Research » Volume 11
Comparing the injectate spread and nerve involvement between different injectate volumes for ultrasound-guided greater occipital nerve block at the C2 level: a cadaveric evaluation
Authors Baek IC, Park K, Kim TL, O J , Yang HM, Kim SH
Received 30 April 2018
Accepted for publication 7 August 2018
Published 25 September 2018 Volume 2018:11 Pages 2033—2038
DOI https://doi.org/10.2147/JPR.S172692
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Erica Wegrzyn
In Chan Baek,1 Kyungeun Park,1 Tae Lim Kim,1 Jehoon O,2 Hun-Mu Yang,2,* Shin Hyung Kim1,*
1Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Republic of Korea; 2Department of Anatomy, Yonsei University College of Medicine, Seoul, Republic of Korea
*These authors contributed equally to this work.
Purpose: The spread patterns between different injectate volumes have not yet been investigated in ultrasound-guided greater occipital nerve (GON) block at the C2 level. This cadaveric study was undertaken to compare the spread pattern and nerve involvements of different volumes of dye using this technique.
Materials and methods: After randomization, ultrasound-guided GON blocks with 1 or 5 mL dye solution were performed at the C2 level on the right or left side of five fresh cadavers. The suboccipital regions were dissected, and nerve involvement was investigated.
Results: Ten injections were successfully completed. In all cases of 5 mL dye, we observed the deeply stained posterior neck muscles, including the suboccipital triangle space. The suboccipital and third occipital nerves, in addition to GONs, were consistently stained when 5-mL dye was used in all injections (100%). Although all GONs were successfully stained in the 1-mL dye cases, three of five injections (60%) concomitantly stained the third occipital nerves.
Conclusion: The clinical efficacy of this technique using the 5-mL injectate seems unlikely to arise from the blockade of GON alone. Instead, its efficacy likely arises from the blockade of most nerves originating from the dorsal ramus of the upper cervical spinal nerve at the suboccipital area. Even using 1 mL of injectate may not guarantee blockade of the GON alone.
Keywords: obliquus capitis inferior, third occipital nerve, suboccipital nerve, Cruveilhier plexus, suboccipital triangle region, headache
Introduction
Greater occipital nerve (GON) block has been used for the management of complex headache syndromes, including cervicogenic headache, occipital neuralgia, migraine and postdural puncture headache.1–4 The GON is a pure sensory fiber that originates from the medial branch of the dorsal ramus of C2. It has sensory distribution to the posterior part of head, vertex and inferolateral occipital area.4 However, the upper cervical spinal nerves (C1–C2–C3) converge onto second-order neurons in the trigeminocervical nucleus. Therefore, using the GON block may be an effective treatment modality for various headache syndromes.5
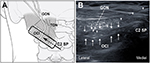
Recently, ultrasound-guided GON block at the C2 level that targets GON on the obliquus capitis inferior (OCI) muscle has been used in clinical practice.3,6,7 OCI muscle lies deep to the semispinalis capitis and trapezius muscle. It runs laterally and slightly upward from the base of spinous process of the C2 to the tip of the transverse process of the C1. The ultrasound approach may increase the success rate and reduce the risk of potential complications.6,7 The course of GON has some anatomical variation at the occipital area.8,9 Therefore, the proximal approach using ultrasound may have a higher selectivity for GON block than the landmark technique.6
Regardless of the approach, the injectate volumes used for GON block vary considerably between 1.5 and 20 mL based on the study.1,4,9,10 However, there was no consensus regarding the optimal injectate volume for the selective blockade of GON in the ultrasound approach at the C2 level. In addition, no prior studies have investigated different injectate volumes, and their effect on the spread patterns, in this technique. Therefore, the anatomical evidence may be crucial to further the understanding of these parameters.
This study uses an established cadaver model and anatomical dissection to compare the spread pattern and nerve involvement of different volumes of dye during ultrasound-guided GON block at the C2 level.
Material and methods
The institutional review board (IRB) of Severance Hospital, Yonsei University Health System exempted this cadaveric study from formal review (ref no. 2017-1349-003). This study is not human study. The anatomical study using cadavers does not require the formal review of IRB, and thus, our IRB approved the exemption from formal review for this study. All of the cadavers used in this study were legally donated to the Surgical Anatomy Education Center at Yonsei University College of Medicine (YSAEC). Five fresh cadavers were independently chosen by the scientific staff at YSAEC. The cadavers were randomized to receive either 1 or 5 mL of injectate volume for GON blocks to the right or left side of the occipital region. Five GON blocks using 1-mL dye solution and five GON blocks using 5-mL dye solution were conducted on these selected cadavers.
Ultrasound-guided GON block procedure
All of the cadavers were placed in the prone position with slight neck flexion. The blocks were performed by a single, experienced anesthesiologist specialized in regional anesthesia and pain medicine. A TE7 ultrasound unit (Mindray Bio-Medical Electronics, Shenzhen, People’s Republic of China) with a high-frequency linear probe (4–16 MHz) and an 80-mm, 22-gage needle was used in every case. The ultrasound approach in this study follows the previously described method.7 The external occipital protuberance was first identified and palpated on each patient. The probe was initially placed in the transverse view at midline to identify the external occipital protuberance. The probe was moved caudally over C1 to locate the C2 spinous process, as identified by its unique bifid appearance. Once C2 was properly identified, the transducer was moved laterally. The lateral side of the probe was aimed at the C1 transverse process to identify the OCI muscle. The GON was typically identified lying superficial to the OCI muscle (Figure 1A). An in-plane technique was used under real-time ultrasound guidance to advance the needle until the tip was visualized in the fascial plane between the OCI and semispinalis capitis in a medial-to-lateral direction (Figure 1B). After confirming the needle tip in this fascial plane, the correct spread was verified by injecting 0.5 mL of normal saline. This injection was visualized as it encompassed the GON between the OCI and semispinalis capitis muscles. After confirming the correct localization of the needle tip in all injections, a total of 1.0 or 5.0 mL of the injectate mixture was injected on each side of the cadaver. The needle was attached to an extension tube and then connected to a syringe according to the different injectate volumes. The potential injectate volumes were as follows: a 1.0 mL mixture of 0.6 mL distilled water, 0.2 mL latex solution, and 0.2 mL green-colored ink; or a 5.0 mL mixture of 3.0 mL distilled water, 1.0 mL latex solution, and 1.0 mL green-colored ink. This composition of injectate was verified using a comparative pilot test using several compositions of dye solution in our anatomy lab. This solution has a somewhat higher viscosity than does the actual local anesthetic. It was used in order to avoid excessive or false spreading, even if only minimal unintended disruptions of the anatomical structures occurred during the extensive dissection procedure. During the procedures, the key sonographic images were recorded digitally in all cadavers.
Anatomical dissection procedure
Anatomists were blinded to the injection techniques. The exact same dissection protocol was followed in all cadavers. The dissection was initiated no longer than 30 minutes after the injection. Bilateral dissections were simultaneously performed to eliminate the possibility of excess dye spreading on one side due to variable dissection start times. The skin overlying the occipital region was meticulously removed. The subcutaneous tissue was revealed. The posterior neck muscles (including the inserting parts of the trapezius, splenius capitis and semispinalis capitis into the occipital bone) were serially removed. During the dissection, the GON and third occipital nerve (TON) were identified and fixed with pins to prevent an unintended displacement. The anatomists paid particular attention to avoid additional spreading of the injectate. After the dissection, the spinous process of C2 was easily observed. The suboccipital triangle was specified by identifying its borders, which comprise the OCI, obliquus capitis superior and rectus capitis posterior major. During or after the dissection, the spreading pattern and extent were evaluated. Photographs of the specimen were taken for analysis. We described the spreading pattern of the injectate, given that we were unable to quantify it with precise dimensional measurements. Therefore, relatively well-distinguished nerve involvements are presented as percentages (0%–20%–40%–60%–80%–100%) of the total of five.
Results
A total of five fresh cadavers were included in this study. The study included three men and two women with an average age of 78.5 years. The key sonographic landmarks were readily identified in the bony structures, muscle layers, and fascial planes in the occipital region of all cadavers. We performed five injections using a 1-mL volume and five injections using a 5-mL volume on each side of the five cadavers.
The dye-spreading patterns and nerve involvement between the 1- and 5-mL dye solutions are summarized in Table 1. There was no dye within the subcutaneous layer, except for trivial leakage along the injection pathway. Most of the dye was contained within the fascial plane between the OCI and semispinalis capitis in all injections, regardless of the injectate volume. In one of the 1-mL dye injection cases, the suboccipital nerve was stained within the suboccipital triangle (20%). Although all GONs were successfully stained in the 1-mL injectate group (100%), three of five 1-mL injectate cases concomitantly stained TONs (60%; Figure 2). In one of the 5-mL injectate cases, the dye spread was observed beneath the trapezius. In one other 5-mL injectate case, there was predominant caudad spread along the semispinalis cervicis. In another of the 5-mL injectate cases, the dye spread laterally to the longissimus capitis muscle. Although there were different spread patterns to the muscle and fascial layer, we consistently observed that the deeply stained suboccipital triangle space was surrounded by the OCI, obliquus capitis superior, and rectus capitis major muscle. In addition, the suboccipital nerves in this space were stained in all 5-mL injectate cases (100%; Figure 3). Likewise, both GON and TON were deeply stained when 5 mL of dye solution was used for GON block in all of the injections (100%; Figure 3).
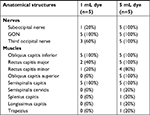  | Table 1 Comparison of dye staining between ultrasound-guided GON block with 1 mL and 5 mL of dye solution Abbreviation: GON, greater occipital nerve. |
Discussion
Ultrasound-guided GON block using 5 mL of dye consistently resulted in deep staining of the suboccipital nerve and TON in addition to the targeted GON in all injections. In contrast, when using 1 mL of dye, there was correct staining of the GON in all cases. However, 60% of the TON was concomitantly stained.
In this study, we observed that the suboccipital triangle region was deeply stained in all GON blocks using 5 mL of dye. This finding suggests that the suboccipital nerve in this area, which is the dorsal ramus of the C1 spinal nerve, is also involved. The suboccipital nerve innervates the OCI, obliquus capitis superior, rectus capitis major and semispinalis capitis muscles. There are no cutaneous, meningeal or articular branches. However, there is some communication between the dorsal rami of C1 and C2 (15%).11 These nerves may give off a cutaneous branch to the upper posterior neck and inferior scalp.11,15 It also has some interconnections with the GON, lesser occipital nerve and spinal accessory nerve.11 Therefore, the suboccipital nerve blockade can help to directly or indirectly relieve suboccipital area pain.
The communicating neural loops between the upper cervical dorsal rami are called the Cruveilhier plexus. This plexus is often known to be associated with failed surgical treatment or incomplete success of pain interventions.12 A previous anatomical study demonstrated that the interconnections between C1–C2–C3 (54%–65.4%) are more frequently observed than are those of the C3–C4 (15.4%) dorsal rami.12 In this study, we could not completely dissect the Cruveilhier plexus due to the technical limitations of the manual dissection of fresh cadavers. However, in this study, we observed that the extent of dye spread when using a 5-mL volume seemed to mostly cover the expected area of the Cruveilhier plexus.
Two cadaveric studies have found that the average distances from the GON to TON were approximately 14.9 and 12 mm, respectively, on the OCI muscle.6,13 In addition, another cadaveric study found that the TON and GON were concomitantly involved in 60% of cases with 0.3 mL volume and 100% with 0.5 mL volume when performing fluoroscopically guided TON block on the C2–C3 facet joint.10 Given the close proximity of GON and TON in the suboccipital area, 60% of TONs were involved in this study when only 1-mL volume of GON block was used on the OCI muscle. Therefore, using an injectate volume of <1 mL seems to appropriate for selective block of GON on the OCI muscle. In our previous cadaveric study, approximately 30.2 mm horizontal distance from the midline (C2 spinous process), the GON winds around the OCI muscle, then ascends vertically and turns medially at the upper border of this muscle.14 This finding indicates that the distance between the GON and TON is closest at the superomedial area of the OCI muscle.14 Therefore, the GON would be most suitable for target at the C2 level on the inferolateral area of the OCI muscle, where it is anatomically farthest away from the TON. One must consider individual anatomical variation. Regardless, one can achieve selective GON blockade under ultrasound guidance at the C2 level using a very small volume of injectate.
In a previous study by Pingree et al, the authors used 4 mL steroid mixed injectate for ultrasound-guided GON block at the C2 level. These injections resulted in significant pain reduction over the 4-week study period in patients with occipital neuralgia and cervicogenic headache.7 Zipfel et al reported ultrasound-guided intermediate site GON infiltration using a 5-mL volume of local anesthetic resulting in significant pain relief in patients with various craniofacial pain syndromes. This technique is comparable to the ultrasound approach used in this study.16 Lauretti et al used the subcompartmental technique for GON block with injected volume of either 5, 10, or 15 mL.1 This group found that there was no difference in the clinical efficacy between three different volume groups 24 weeks after the injection. Therefore, the authors suggest that 5 mL volume of injectate is sufficient for a successful block. The subcompartmental technique under fluoroscopy seems to have a similar anatomical basis with the current ultrasound-guided GON block, because it uses the same injection target area under the semispinalis capitis muscle at the C2 level.1 Interestingly, we identified the contrast filling pattern in the expected location of the suboccipital triangle space at the lateral fluoroscopic view after injection with 5-mL volume in their study.1 This was similarly observed in all of the 5-mL cases in our study. Collectively, based on our results, the previously reported clinical efficacy of the GON block at the C2 level (using 4–5 mL injectate) does not seem to result from blockade of the GON alone. Instead, we suspect that the efficacy of this block is through the blockade of most nerves originating from the dorsal ramus of the upper cervical spinal nerve in the suboccipital area.
This study has several limitations. This study used a small number of cadavers. Considering that we used cadavers in this study, changes in the tissue integrity inevitably could affect the injectate diffusion. In living subjects, the patient’s neck movement can cause the delayed diffusion of injectate. The extent of dye spread could not be precisely quantified in all anatomical structures. Therefore, our study is limited by descriptive statistics, rather than by inferential statistics.
Conclusion
This study demonstrates that ultrasound-guided GON block using 5 mL of dye solution consistently involves most major nerves in the suboccipital area. A 5-mL volume of injectate for this technique seems to be suitable for therapeutic purposes, rather than for diagnostic purposes. Even using a very small volume (1 mL) of injectate may not guarantee that only the GON will be blocked.
Acknowledgments
This work was supported by the National Research Foundation of Korea grant funded by the Korean government (MSIT; grant no. NRF-2017R1C1B5074007). The funder had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. We are grateful to Dae Won Kim, Jun Ho Kim and Jong Ho Bang for their technical support (all are staff members in the Surgical Anatomy Education Center at Yonsei University College of Medicine). Hun-Mu Yang and Shin Hyung Kim should be considered as co-corresponding authors.
Disclosure
The authors report no conflicts of interest in this work.
References
Lauretti GR, Corrêa SW, Mattos AL. Efficacy of the greater occipital nerve block for cervicogenic headache: comparing classical and subcompartmental techniques. Pain Pract. 2015;15(7):654–661. | ||
Choi I, Jeon SR. Neuralgias of the head: occipital neuralgia. J Korean Med Sci. 2016;31(4):479–488. | ||
Nair AS, Kodisharapu PK, Anne P, Saifuddin MS, Asiel C, Rayani BK. Efficacy of bilateral greater occipital nerve block in postdural puncture headache: a narrative review. Korean J Pain. 2018;31(2):80–86. | ||
Allen SM, Mookadam F, Cha SS, Freeman JA, Starling AJ, Mookadam M. Greater occipital nerve block for acute treatment of migraine headache: a large retrospective cohort study. J Am Board Fam Med. 2018;31(2):211–218. | ||
Bogduk N, Govind J. Cervicogenic headache: an assessment of the evidence on clinical diagnosis, invasive tests, and treatment. Lancet Neurol. 2009;8(10):959–968. | ||
Greher M, Moriggl B, Curatolo M, Kirchmair L, Eichenberger U. Sonographic visualization and ultrasound-guided blockade of the greater occipital nerve: a comparison of two selective techniques confirmed by anatomical dissection. Br J Anaesth. 2010;104(5):637–642. | ||
Pingree MJ, Sole JS, O’ Brien TG, Eldrige JS, Moeschler SM. Clinical efficacy of an ultrasound-guided greater occipital nerve block at the level of C2. Reg Anesth Pain Med. 2017;42(1):99–104. | ||
Loukas M, El-Sedfy A, Tubbs RS, et al. Identification of greater occipital nerve landmarks for the treatment of occipital neuralgia. Folia Morphol. 2006;65(4):337–342. | ||
Natsis K, Baraliakos X, Appell HJ, Tsikaras P, Gigis I, Koebke J. The course of the greater occipital nerve in the suboccipital region: a proposal for setting landmarks for local anesthesia in patients with occipital neuralgia. Clin Anat. 2006;19(4):332–336. | ||
Wahezi SE, Silva K, Shaparin N, et al. Currently recommended TON injectate volumes concomitantly block the GON: clinical implications for managing cervicogenic headache. Pain Physician. 2016;19(7):E1079–E1086. | ||
Tubbs RS, Loukas M, Yalçin B, Shoja MM, Cohen-Gadol AA. Classification and clinical anatomy of the first spinal nerve: surgical implications. J Neurosurg Spine. 2009;10(4):390–394. | ||
Tubbs RS, Mortazavi MM, Loukas M, D’Antoni AV, Shoja MM, Cohen-Gadol AA. Cruveilhier plexus: an anatomical study and a potential cause of failed treatments for occipital neuralgia and muscular and facet denervation procedures. J Neurosurg. 2011;115(5):929–933. | ||
Kariya K, Usui Y, Higashi N, et al. Anatomical basis for simultaneous block of greater and third occipital nerves, with an ultrasound-guided technique. J Anesth. 2018;32(4):483–492. | ||
Kim HS, Shin KJ, O J, Kwon HJ, Lee M, Yang HM. Stereotactic topography of the greater and third occipital nerves and its clinical implication. Sci Rep. 2018;8(1):870. | ||
Ouaknine G, Nathan H. Anastomotic connections between the eleventh nerve and the posterior root of the first cervical nerve in humans. J Neurosurg. 1973;38(2):189–197. | ||
Zipfel J, Kastler A, Tatu L, Behr J, Kechidi R, Kastler B. Ultrasound-guided intermediate site greater occipital nerve infiltration: a technical feasibility study. Pain Physician. 2016;19(7):E1027–E1034. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.