Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Comparative study on drug safety surveillance between medical students of Malaysia and Nigeria
Authors Abubakar AR , Ismail S, Rahman NIA, Haque M
Received 19 March 2015
Accepted for publication 6 May 2015
Published 30 June 2015 Volume 2015:11 Pages 1015—1025
DOI https://doi.org/10.2147/TCRM.S85019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Garry Walsh
Video abstract presented by AR Abubakar
Views: 205
Abdullahi Rabiu Abubakar, Salwani Ismail, Nor Iza A Rahman, Mainul Haque
Faculty of Medicine, Universiti Sultan Zainal Abidin, Kuala Terengganu, Terengganu, Malaysia
Background: Internationally, there is a remarkable achievement in the areas of drug discovery, drug design, and clinical trials. New and efficient drug formulation techniques are widely available which have led to success in treatment of several diseases. Despite these achievements, large number of patients continue to experience adverse drug reactions (ADRs), and majority of them are yet to be on record.
Objectives: The purpose of this survey is to compare knowledge, attitude, and practice with respect to ADRs and pharmacovigilance (PV) between medical students of Malaysia and Nigeria and to determine if there is a relationship between their knowledge and practice.
Method: A cross-sectional, questionnaire-based survey involving year IV and year V medical students of the Department of Medicine, Universiti Sultan Zainal Abidin and Bayero University Kano was carried out. The questionnaire which comprised 25 questions on knowledge, attitude, and practice was adopted, modified, validated, and administered to them. The response was analyzed using SPSS version 20.
Results: The response rate from each country was 74%. There was a statistically significant difference in mean knowledge and practice score on ADRs and PV between medical students of Malaysia and Nigeria, both at P<0.000. No significance difference in attitude was observed at P=0.389. Also, a statistically significant relationship was recorded between their knowledge and practice (r=0.229, P=0.001), although the relationship was weak.
Conclusion: Nigerian medical students have better knowledge and practice than those of Malaysia, although they need improvement. Imparting knowledge of ADRs and PV among medical students will upgrade their practice and enhance health care delivery services in the future.
Keywords: drug safety, underreporting, medical students, KAP, Malaysia, Nigeria
Introduction
Globally, pharmacovigilance (PV) program is one of the new areas requiring the attention of health care professionals in order to safeguard patients’ life.1,2 Majority of patients and health care workforces give more priority to remedy of the current illness but do not give much attention to the upcoming adverse drug reactions (ADRs).3 Moreover, some patients may continue their medications even after completing the dose. Other patients may even use their prescribed medicine as a self-medication.2–5 Consequently, self-medication, irrational prescribing, drug misuse, and drug abuse have been attributed to ADRs in many occasions.2–5 Investigation has shown that ADRs remain a substantial cause of morbidity and mortality among patients.6–8 The Institute of Medicine reported that more than one million avoidable adverse events occur every year, and out of this figure, 44,000–98,000 are very dangerous.9 ADRs are the fourth leading cause of death in USA.7,9–11 ADR is one of the key causes of hospital admission, extended hospital stays, and increased health care cost and delay at work, as well as patient lack of satisfaction.7,8,12,13 Drugs frequently prescribed like antibiotics, nonsteroidal anti-inflammatory drugs, analgesics, anticoagulants, and antineoplastics are commonly associated with ADRs.10,14 The World Health Organization proposed that geriatric patients, infants and neonates, and pregnant and lactating women should be closely monitored for ADRs.3,10,14–16
Spontaneous reporting (SPR) and intensive monitoring are the conventional systems used in detecting, recording, and reporting ADRs. Using SPR, a lot of success has been made as existing ADRs were identified and new ones prevented through this method.1,3,8,11,12 It is imperative to note that SPR scheme suffers a lot of underreporting incidences; similarly, data mining is associated with selection bias and limited number of subjects.1,3,8,11,12 Similarly, regulatory authorities, pharmaceutical companies, and academia are yet to establish an excellent method of ADRs reporting with the highest level of precision.3,17
In general, regulatory agencies consider only the information and outcome of premarketing clinical trial in controlled settings to approve and register a drug for human use. However, not much is known about the drug beyond the data obtained from clinical trials in controlled settings. The outcome of premarketing studies on safety, efficacy, and quality of new medicines will not basically represent the whole population of patients who will use the drugs when they are approved for marketing.3,18 Henceforth, suitable and constant post-marketing drug safety surveillance is indispensable.2,3,18,19 Several life-threatening diseases and new disease conditions that were not treated before may necessitate research for a new drug. Recent example is Ebola virus medicine; trial drugs have been used in many African countries. Once discovered, it may become necessary to give the drug a quick approval in order to save the life of patients.1
In Malaysia, before the year 2003, there was huge prevalence of ADRs which harm great number of patients nationwide.20,21 Malaysia National Adverse Drug Reaction Monitoring Centre reviewed and upgraded their guidelines with respect to SPR and reinforced them across the country.20–22 A survey from Malaysia indicated less number of ADR reports; the reasons are lack of acknowledgment of the presence of ADRs and national ADRs-reporting program.20–24
In Nigeria, previous surveys conducted in different parts of the country focused only on doctors while disregarding other health care professionals and medical students. In addition, Nigeria accounts for high prevalence of ADRs across the country; unfortunately, only 6%–10% cases were reported.25,26 The reason for low reporting comprises lack of familiarity with ADRs-reporting forms and ignorance of the reporting procedure.23,27–29 The high rate of underreporting cutting across continents over long period will diminish signal detection and become detrimental to public health.21,30 Various studies have been carried out on knowledge, attitude, and practices (KAPs) of health professionals in Nigeria; the outcome indicated poor KAPs among them.27,31 It was established that an existing PV program can advance to the next level by evaluating the baseline KAPs of major health care workers regarding ADRs monitoring prior to carrying out any treatment.1,8,17,19,23 Also, the curriculum of training medical students both in Malaysia and Nigeria with respect to ADRs and PV is insufficient and needs to be upgraded.21,22,32 The present study is conducted to compare the KAP with respect to ADRs and PV between medical students of Malaysia and Nigeria.
Research objectives
The objectives of the study are as follows:
- To assess medical students’ KAP with respect to ADRs and PV
- To determine the difference in the mean knowledge score between year IV and V medical students and between sexes
- To assess if there is difference in the mean KAP score between medical students of Malaysia and Nigeria
- To determine if there is correlation between medical students’ knowledge and practice with respect to ADRs and PV.
Materials and methods
Study population
The study was conducted among population of year IV and year V medical students selected from Universiti Sultan Zainal Abidin (UniSZA), Terengganu, Malaysia and Bayero University Kano (BUK), Kano, Nigeria. The two countries were selected for convenience as the principal author is from Nigeria and studying in Malaysia.
Study design
A cross-sectional, questionnaire-based survey involving year IV and year V medical students of the Department of Medicine, UniSZA and BUK, was carried out to evaluate their KAPs toward ADRs and PV. The questions were extracted from Kamtane and Jayawardhani12 and Reddy et al (Figure S1).33 They were scrutinized by the experts in the field, modified, and validated to suit the intended study.
Sampling method and sample size
A universal sampling method was used: the total number of medical students in UniSZA and BUK were 303 and 519, respectively. The total number of medical students in year IV and year V in UniSZA and BUK were 117 and 146, respectively. Universal sample of all medical students in year IV and V in both UniSZA and BUK was taken.
Ethical approval
The study was conducted after obtaining ethical clearance from the ethical committee of both UniSZA and BUK. This research was approved for master’s program by the ethical committee of UniSZA (memo number UniSZA.N/1/628-1 (70) dated 21 July 2014) and BUK (memo number BUK/FMEC/013-14/053).
Written consent and inclusion and exclusion criteria
All the participants were given information sheets and written consent form for their agreement to participate in the study. Only student who are studying medicine and who reach year IV and year V were included. Meanwhile, medical students who were not willing to participate were excluded.
Development of questionnaire
The questionnaire comprised four parts: Section A comprised seven demographic information. Section B comprised ten knowledge questions designed with multiple choices and yes/no options; score of 1 was allocated to the correct answer and 0 to the wrong answer. Section C consisted of seven attitude questions framed into 3-point Likert scale (3= strongly agree, 2= neutral, 1= strongly disagree). Finally, section D comprised eight practice questions with strongly agree or strongly disagree options. Score of 1 was assigned to the correct answer and 0 to the wrong answer. The questionnaire included both positive and negative questions to escape agreement bias, conformity, and affirmation in the questions pattern.
Piloting, testing, and validation of questionnaire
The questionnaire was first pilot-tested among 20 medical students who were not involved in the main survey. Their response was then tested for validity and reliability, and the overall Cronbach’s alpha was calculated as 0.69.
Data collection
The data were collected in Malaysia by self-administering the questionnaire to the medical students of UniSZA and obtaining their responses. In Nigeria, the questionnaire was administered to the medical students by lecturers of Faculty of Medicine, BUK, and their responses were obtained. The study was carried out between June 2014 and February 2015.
Data analysis
The data were coded using Microsoft excel and analyzed using the SPSS version 20.0. Descriptive statistics was carried out to evaluate the KAP score of the participants. Independent t-test was performed to determine if there was difference in the mean knowledge score on ADRs and PV between year IV and year V medical students, and between sexes within each school. Also, two-sample t-tests were performed to determine if there was difference in mean KAP score on ADRs and PV between medical students of UniSZA and BUK. Finally, Spearman’s correlation analysis was performed to assess if there was correlation between knowledge and practice of ADRs reporting. The statistical significance was determined at P<0.05 and 95% confidence interval.
Results
Demographic information
The total number of medical students who participated in Malaysia was 87, while that of Nigeria was 108. The response rate was 74% from both participants of both countries. Among Malaysian participants, 42% were in year IV, while 58% were in year V; among Nigerian participants, 63% reached year IV, and 37% reached year V. The summary of the demographic information is shown in Table 1.
  | Table 1 Demographic information |
Knowledge
Among the 87 respondents from Malaysia, 68% and 49% got the definition of ADRs and PV correct, while out of 108 Nigerian respondents, 80% and 66% got the right definition of ADR and PV, respectively. Fifty-nine percent of Malaysian medical students and 63% of Nigerian medical students recognized the right functions of PV. Similarly, among Malaysian participants, 70% identified the health care professionals responsible for reporting ADRs in a hospital, while 71% of Nigerians got the same response. Among Malaysian respondents, only 21% knew the method commonly used in ADR reporting, and only 15% were aware of any nearby PV center, while from Nigeria, they were 39% and 10%, respectively. The results are shown in Table 2.
  | Table 2 Medical students’ knowledge on ADRs and PV |
Attitude
Among Malaysian medical students, 88% strongly agreed that ADRs reporting is professional obligation, while among Nigerian medical students, 82% hold the same view. In addition, 98% of Malaysian respondents thought that ADR monitoring will benefit patients, whereas 95% of Nigerian respondents thought the same. In contrast, 78% of Malaysian respondents stated that they were not adequately trained on how to report ADRs, while 74% of the Nigerian respondents said the same. The results are shown in Table 3.
  | Table 3 Medical students’ attitude toward ADRs and PV |
Practice
Among 87 Malaysian medical students, 99% have never reported any ADRs to their superiors during clinical ward round; 94% stated that they had never received any training on how to report ADRs, while among Nigerian medical students, 96% and 86% gave the same response, respectively. In contrast, 67% of Malaysian medical students stated that even commonly seen ADRs like headache, fever, and vomiting should be reported; also, 72% stated that nonmedical person can report an ADR to a nearby health care professional, whereas among Nigerian medical students, 67% and 83% had similar idea, respectively. The results are shown in Table 4.
  | Table 4 Medical students’ practice with respect to ADRs and PV |
Difference in the mean knowledge score between years of study
The result of the independent t-test performed among Malaysian medical students indicated that there was no statistically significance difference in the mean knowledge score on ADRs and PV between year IV and year V medical students with a P-value of 0.274. That of Nigerian medical students also produced similar result with a P-value of 0.956. The results are shown in Table 5.
Difference in the mean knowledge score between sexes
Result of the independent t-test performed among Malaysian medical students indicated that there was no statistically significance difference in the mean knowledge score on ADRs and PV between sexes with a P-value of 0.126. That of Nigerian medical students also produced similar result with a P-value of 0.080. The results are shown in Table 6.
Difference in the mean KAP score between medical students of Malaysia and Nigeria
The result of an independent sample t-test has shown that there was a statistically significance difference in the mean knowledge score on ADRs and PV between medical students of Malaysia and Nigeria with a P-value of 0.000. The Nigerian medical students were found to have more knowledge. In addition, there was no statistically significance difference in the mean attitude score on PV between medical students of Malaysia and Nigeria with a P-value of 0.389. Also, there was a statistically significance difference in the mean practice score on PV between medical students of Malaysia and Nigeria with a P-value of 0.000. The Nigerian medical students were found to have more practice. The results are shown in Table 7.
  | Table 7 The result of the difference in the mean knowledge, attitude, and practice score between medical students of Malaysia and Nigeria (N=195) |
The correlation between mean knowledge score and mean practice score on ADRs and PV
Spearman’s correlation analysis performed has shown that there was positive correlation between knowledge and practice of PV at (P=0.001, r=0.229). The correlation that exists between knowledge and practice is weak with r=0.229. The closer the r is to 1, the stronger the correlation with r=1 is considered as perfect correlation. The results are shown in Table 8 and Figure 1.
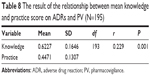  | Table 8 The result of the relationship between mean knowledge and practice score on ADRs and PV (N=195) |
  | Figure 1 Scatter plot showing correlation between knowledge and practice. |
Discussion
The response rate obtained from this research was 74% from each country. This is considered as satisfactory because it is much higher than the response (63%) obtained in another study conducted among medicine and pharmacy students in Malaysia.21
Knowledge
The mean knowledge score acquired by the medical students from Malaysia signified that their knowledge on ADRs and PV was not satisfactory. In contrast, medical students from Nigeria have demonstrated good knowledge of ADRs and PV. This is highly recommended in every medical school because ADRs reporting is part of their future professional obligation.
Definition of ADR and PV
Among the medical students of Malaysia interviewed, 68% and 49% got the correct definition of ADRs and PV, whereas 80% and 66% among the medical students of Nigeria answered correctly. The response of Malaysian medical students is similar to the result obtained in another study.21 However, outcome of Nigerian medical students is similar to another survey among pharmacy students which showed satisfactory knowledge.33
Methods of reporting
Among the medical students surveyed in Malaysia, only 21% knew the method commonly used in ADR reporting; also, only 39% of the Nigerian medical students knew this method which is comparable to result obtained in other appraisal.21,32,34
Who is to report ADRs?
Among Malaysian participants, 70% identified the health care professionals responsible for reporting ADRs in a hospital, while 71% of Nigerian participants mentioned the same, which revealed good awareness. This finding contradicts outcome of another surveys.32,33
Drugs banned due to ADRs
Seventy-seven percent and 85% of Malaysian and Nigerian medical students were aware of drugs banned due to ADRs, respectively, which is in contrast with the result of another survey among medical and dental students.34
Attitude
Research conducted has shown that the attitude of Malaysian and Nigerian medical students was good. This is imperative because imparting good attitude among medical students will help to improve the health care delivery services.
Obligation to report
Among Malaysian medical students, 87% strongly agreed that ADRs reporting is a professional compulsion, and 82% of Nigerian medical students felt the same. Moreover, current study respondents from both the countries (Malaysia, 87%; Nigeria, 82%) expressed that PV should be taught in detail. Similar results were obtained in a number of studies.35,36
Purpose of reporting
Ninety-eight percent and 95% of Malaysian and Nigerian respondents, respectively, believed that ADR monitoring and reporting will ensure better health care for their population. This figure is much higher than the result of the study of Iran.36
Practice
The practice of Malaysian medical student toward ADRs and PV was not satisfactory. It is important to incorporate and ensure practical application of PV in teaching hours of any medical and health professional schools in order to produce good doctors and health professionals.37 Similarly, Nigerian medical students have only showed moderate practice of PV.
Number of ADRs ever reported
Among study respondents, 99% and 96% from Malaysia and Nigeria, respectively, have never reported any ADRs. These findings are comparable with the result of a number of surveys.32,36 In addition, 72% of Malaysian respondents and 99% of Nigerian respondents have come across patients experiencing ADR but did not take any action which is similar to the outcome of other appraisals.32,35,36
Training on how to report ADRs
Among the Malaysian medical students surveyed, 94% stated that they have never received any training on how to report ADRs; also, 96% of Nigeria medical students had similar experience. These outcomes are similar with other research reports.21,33,34
Nature of ADRs to be reported
Among the Malaysian participants, 67% stated that even commonly seen ADRs like headache, fever, and vomiting should be reported; also, 67% of Nigerian participants felt the same, which is similar to the result obtained in another survey involving medicine and pharmacy students.21
Difference in the mean knowledge score between years of study
The result of the independent t-test performed among Malaysian medical students indicated that there was no statistically significance difference in the mean knowledge score on ADRs and PV between year IV and V medical students. That of Nigerian medical students also produced similar result. These findings are in contrast with other appraisals carried out among medical and dental students.17,34
Difference in the mean knowledge score between sexes
Result of the independent t-test performed among Malaysian medical students indicated that there was no statistically significance difference in the mean knowledge score on ADRs and PV between sexes. The Nigerian medical students also produced similar result. These findings are similar to the findings of another survey.22
Difference in the mean KAP score between medical students of Malaysia and Nigeria
The result of an independent sample t-test has shown that there was statistically significance difference in the mean knowledge score on ADRs and PV between medical students of Malaysia and Nigeria. The Nigerian medical students were found to have more knowledge. Similar outcome was obtained in a survey conducted among pharmacy students in Malaysian public schools.22 In addition, there was no statistically significance difference in the mean attitude score on PV between medical students of Malaysia and Nigeria. This result is in contrast with appraisal among pharmacy students of Malaysia.22 Finally, there was statistically significance difference in the mean practice score on PV between medical students of Malaysia and Nigeria. The Nigerian medical students were found to have more practice. This result is comparable to the findings of research conducted among medical students and prescribers.35 This can be possibly explained by that as the medical school of Nigeria is much older than that of Malaysian medical school. As time passes, there are possibilities of more growth and improvement in every aspect. Therefore, time has given more opportunity to score better among Nigerian Medical students.
The correlation between mean knowledge score and mean practice score on ADRs and PV
Correlation analysis performed has shown that there was positive correlation between knowledge and practice of PV. This finding is similar to the outcome of survey conducted.38
Conclusion
Nigerian medical students were found to have better knowledge and practice with respect to ADRs and PV than the Malaysian medical students, even though they still need improvement. The attitude of the student from the two countries was moderate and similar in every aspect. The knowledge of semifinal-year and final-year medical students on ADRs and PV is partially the same within each schools, and no difference was recorded between sexes. Positive relationship exists between knowledge and practice with respect to ADRs and PV, although the relationship found was weak. The implication of this is that when knowledge develops, practice also progresses. This has suggested the need to upgrade the curriculum of teaching medical students in both Malaysia and Nigeria in order to protect patients. Also, there is need for strong collaboration between government, teaching hospitals, and academia with regard to drug safety issues.
Limitations of the study
The research did not cover all the medical schools in Malaysia and Nigeria due to the time constraints and limited financial resources. The research did not include patients who are the main victims of ADR due to time factor and difficulties in recruiting them.
Acknowledgments
The authors are grateful to the Faculty of Medicine, UniSZA, Terengganu, Malaysia. They are also grateful to Dr Musa Aliyu, Dr Bashir AZ Chedi, and Mr Khalid M Garba from the Department of Pharmacology, Bayero University, Kano, Nigeria, for approval of this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Abubakar AR, Simbak NB, Haque M. A systematic review of knowledge, attitude and practice on adverse drug reactions and pharmacovigilance among doctors. J Appl Pharm Sci. 2014;4(11):117–127. | ||
Mahmood KT, Amin F, Tahir M, Haq IU. Pharmacovigilance – a need for best patient care in Pakistan. A review. J Pharm Sci Res. 2011;3(11):1566–1584. | ||
Abubakar AR, Simbak NB, Haque M. Drug safety surveillance: modern trends and industrial action. J Young Pharm. 2015;7(2):69–75. | ||
Abubakar AR, Chedi BAZ, Simbak NB, Haque M. Medication error: the role of health care professionals, sources of error and prevention strategies. J Chem Pharm Res. 2014;6(10):646–651. | ||
Abubakar AR, Simbak NB, Haque M. Knowledge, attitude and practice on medication use and safety among Nigerian postgraduate-students of UniSZA, Malaysia. Int J Pharm Res. 2014;6(4):104–110. | ||
Kiran LJ, Shivashankaramurthy KG, Bhooma S, Dinakar KR. Adverse drug reaction reporting among clinicians in a teaching hospital in south Karnataka. Scholars J Appl Med Sci. 2014;2(1D):399–403. | ||
Aithal S, Hooli TV, Varun HV. Knowledge and attitude about adverse drug reaction reporting among doctors at a tertiary care hospital. Int J Pharma Bio Sci. 2014;5(1):108–113. | ||
Bisht M, Singh S, Dhasmana DC. Effect of educational intervention on adverse drug reporting by physicians: a cross-sectional study. ISRN Pharmacol. 2014;2014:259476. | ||
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients. A meta-analysis of prospective studies. JAMA. 1998;279(15):1200–1205. | ||
Abubakar AR, Simbak NB, Haque M. Adverse drug reactions: predisposing factors, modern classifications and causality assessment. Res J Pharm Tech. 2014;7(9):1091–1098. | ||
Iffat W, Shakeel S, Rahim N, Anjum F, Neesar S, Ghayas S. Pakistani physicians’ knowledge and attitude towards reporting adverse drug reactions. Afr J Pharm Pharmacol. 2014;8(14):379–385. | ||
Kamtane RA, Jayawardhani V. Knowledge, attitude and perception of physicians towards adverse drug reaction (ADR) reporting: a pharmacoepidemiological study. Asian J Pharm Clin Res. 2012;5(3): 210–214. | ||
Hanafi S, Torkamandi H, Hayatshahi A, Gholami K, Javadi M. Knowledge, attitudes and practice of nurse regarding adverse drug reaction reporting. Iran J Nurs Midwifery Res. 2012;17(1):21–25. | ||
Alomar MJ. Factors affecting the development of adverse drug reactions. Saudi Pharm J. 2014;22:83–94. | ||
Mandavi, D’Cruz S, Sachdev A, Tiwari P. Adverse drug reactions and their risk factors among Indian ambulatory elderly patients. Indian J Med Res. 2012;136(3):404–410. | ||
Palaian S, Mishra P, Shankar PR, Dubey AK, Bista D, Almeida R. Safety monitoring of drugs – where do we stand? Kathmandu Univ Med J (KUMJ). 2006;4(1):119–127. | ||
Vora MB, Paliwal NP, Doshi VG, Barvaliya MJ, Tripathi CB. Knowledge of adverse drug reactions and pharmacovigilance activity among the undergraduate medical students of Gujarat. Int J Pharm Sci Res. 2012;3(5):1511–1515. | ||
Adedeji WA, Ibrahem WA, Fehintola FA. Attitude and practice of doctors toward adverse drug reactions (ADRs) reporting in a Nigerian tertiary health facility. Ann Ib Postgrad Med. 2013;1(2):77–80. | ||
Sanghavi DR, Dhande PP, Pandit VA. Perception of pharmacovigilance among doctors in a tertiary care hospital: influence of an interventional lecture. Int J Risk Saf Med. 2013;25(4):197–204. | ||
Agarwal R, Daher AM, Ismail NM. Knowledge, practices and attitudes towards adverse drug reaction reporting by private practitioners from Klang Valley in Malaysia. Malays J Med Sci. 2013;20(2):52–61. | ||
Sivadasan S, Yuong NY, Chyi NW, et al. Knowledge and perception towards pharmacovigilance and adverse drug reaction reporting among medicine and pharmacy students. WJPPS. 2014;3(3):1652–1676. | ||
Elkalmi RM, Hassali MA, Izham M, Ibrahim M, Widodo RT, Efan QMA. Pharmacy students’ knowledge and perceptions about pharmacovigilance in Malaysian public universities. Am J Pharm Educ. 2011;75(5):1–7. | ||
Palaian S, Ibrahim MI, Mishra P. Health professionals’ knowledge, attitude and practices towards pharmacovigilance in Nepal. Pharm Pract (Granada). 2011;9(4):228–235. | ||
Aziz Z, Siang TC, Badarudin NS. Reporting of adverse drug reactions: predictors of under-reporting in Malaysia. Pharmacoepidemiol Drug Saf. 2007;16:223–228. | ||
Fadare JO, Enwere OO, Afolabi AO, Chedi BAZ, Musa A. Knowledge, attitude and practice of adverse drug reaction reporting among healthcare workers in a tertiary center in Northern Nigeria. Trop J Pharm Res. 2011;10(3):235–242. | ||
Okezie OO, Fawole OI. Adverse drug reactions reporting by physicians in Ibadan, Nigeria. Pharmacoepidemiol Drug Saf. 2008;17:517–522. | ||
Oshikoya KA, Awobusuyi JO. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clin Pharmacol. 2009;9:14. | ||
Awodele O, Akinyede A, Adeyemi OA, Awodele DF. Pharmacovigilance amongst doctors in private hospitals in Lagos West Senatorial District, Nigeria. Int J Risk Saf Med. 2011;23(4):217–226. | ||
Okezie EO, Olufunmilayo F. Adverse drug reactions reporting by physicians in Ibadan, Nigeria. Pharmacoepidemiol Drug Saf. 2008;17(5):517–522. | ||
Upadhyaya P, Seth V, Moghe VV, Sharma M, Ahmed M. Knowledge of adverse drug reaction reporting in first year postgraduate doctors in a medical college. Ther Clin Risk Manag. 2012;8:307–312. | ||
Oreagba IA, Ogunleye OJ, Olayemi SO. The knowledge, perceptions and practice of pharmacovigilance amongst community pharmacists in Lagos state, south west Nigeria. Pharmacoepidemiol Drug Saf. 2011;20(1):30–35. | ||
Showande JS, Oyelola FT. The concept of adverse drug reaction reporting: awareness among pharmacy students in a Nigerian university. Int J Med Update. 2013;8(1):24–30. | ||
Reddy VL, Pasha SKJ, Rathinavelu M, Reddy YP. Assessment of knowledge, attitude and perception of pharmacovigilance and adverse drug reaction (ADR) reporting among the pharmacy students in south India. IOSR J Pharm Biol Sci. 2014;9(2):34–43. | ||
Iffat W, Shakeel S, Naseem S, Imam S, Khan M. Attitudinal survey to assess medical and dental students’ belief of ADR reporting in Pakistan. Int J Pharm Pharm Sci. 2014;6(5):279–283. | ||
Rehan HS, Vasudev K, Tripathi CD. Adverse drug reaction monitoring: knowledge, attitude and practices of medical students and prescribers. Nat Med J India. 2002;15(1):24–26. | ||
Isfahani ME, Mousavi S, Rakhshan A, Assarian M, Kuti L, Eslami K. Adverse drug reactions: knowledge, attitude and practice of pharmacy students. J Pharm Care. 2013;1(4):145–148. | ||
WHO. The Safety of Medicines in Public Health Programmes: Pharmacovigilance an Essential Tool. France: WHO Press; 2006. | ||
Qassim S, Metwaly Z, Shamsain M, Al Hariri Y. Reporting adverse drug reactions: evaluation of knowledge, attitude and practice among community pharmacists in UAE. IOSR J Pharm. 2014;4(4):17–23. |
Supplementary material
  | Figure S1 Questionnaire for comparative study on drug safety surveillance between medical students of Malaysia and Nigeria. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


