Back to Journals » Drug Design, Development and Therapy » Volume 13
Comparative study of β-cyclodextrin, γ-cyclodextrin and 4-tert-butylcalix[8]arene ionophores as electroactive materials for the construction of new sensors for trazodone based on host-guest recognition
Authors Alrabiah H , Aljohar HI , Bakheit AH, Homoda AMA, Mostafa GAH
Received 17 January 2019
Accepted for publication 13 May 2019
Published 11 July 2019 Volume 2019:13 Pages 2283—2293
DOI https://doi.org/10.2147/DDDT.S201907
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Haitham Alrabiah,1 Haya I Aljohar,1 Ahmed Hassan Bakheit,1 Atef MA Homoda,2 Gamal Abdel-Hafiz Mostafa1,2
1Pharmaceutical Chemistry Department, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 2Micro-analytical Laboratory, Applied Organic Chemistry Department, National Research Center, Dokki, Cairo, Egypt
Background: Trazodone (TRZ) is a second-generation non-tricyclic antidepressant derived from a triazolopyridine derivative, which is mainly used to treat emotional disorders and conditions related to depressive disorders.
Purpose: This study investigated the design, development and characteristics of polyvinyl chloride (PVC) membrane sensors for trazodone HCl (TRZ).
Methods: The developed sensing membranes were constructed using β-cyclodextrin (β-CD; sensor 1), γ-cyclodextrin (γ-CD; sensor 2) or 4-tert-butylcalix[8]arene (t-BC8; sensor 3) ionophores as sensing materials in addition to ionic sites and dioctyl phthalate in the PVC matrix.
Results: Sensors 1, 2 and 3 displayed fast, stable and near-Nernstian response over a relatively wide trazodone concentration range (7.0×10−6–1×10−3, 5.0×10−5–1×10−3 and 8.0×10−6–1.0×10−3 M, respectively), with detection limits of 2.2×10−6, 1.5×10−5 and 2.42×10−6 M, respectively in the pH range of 3.0–6.0. The sensors demonstrated good selectivity for TRZ in the presence of different ionic compounds. The accuracy and precision of the proposed sensors were assessed by the determination of 40.7 μg/ml of TRZ, which showed average recoveries of 99.6%, 99.1% and 98.5% with mean relative standard deviations of 2.4%, 2.5% and 2.6% for sensor 1, 2 and 3 respectively. Molecular modeling was used to calculate the host-guest binding energy. The lowest free binding energy was −6.243, −5.752 and −5.7105 kcal/mol for 1:1 stoichiometry host-guest complexes of trazodone and β-CD, γ-CD and t-BC8, respectively, which was in-line with a Nernstian response.
Conclusion: The investigated methods can be applied for the determination of TRZ in pharmaceutical preparations. The results of investigated dosage-form of TRZ show good agreement with those using the US Pharmacopeia method.
Keywords: trazodone HCl, β-cyclodextrin, γ-cyclodextrin, tert-butylcalix[8]arene, PVC, potentiometry, molecular modeling
Introduction
Trazodone (TRZ) is a second-generation non-tricyclic antidepressant derived from a triazolopyridine derivative.1,2 Antidepressants affect mood and are mainly used to treat emotional disorders and conditions related to these disorders,3 by acting as hypnotics and anxiolytics.4 Trazodone induces selective serotonin uptake inhibition by brain synaptosomes, thus leading to the behavioral changes by the serotonin precursor, 5-hydroxytryptophan.5 Potential side effects include drowsiness, nausea, vomiting, headache, dry mouth, rashes, itching, and increased joint and muscle pain. The potential side effects and toxicity of TRZ differ significantly from those of early antidepressants and tricyclic antidepressants (TCAS).6 Increasing the dose of this medicine may exacerbate its side effects.
The importance of this drug in the treatment of such disorders and the severity of associated adverse effects renders its reliable determination very important. Different determination methods have been reported, including spectrophotometry2,7 spectrofluorometry,2,8 chemiluminescence,9 voltammetry,10–12 high-performance liquid chromatography (HPLC)- ultraviolet detection,13,14 HPLC-fluorescence1,15 and HPLC-mass spectroscopy (MS).16 However, these techniques require expensive equipment, time-consuming methods and sometimes reproducibility and derivatization procedures.
The application of potentiometric sensors in pharmaceutical and medicinal analysis17,18 has attracted increasing interest, because such sensors offer high sensitivity, reproducibility and low cost. Different potentiometric sensors have been developed for the determination of TRZ using different ion-exchange materials or ion pairs19–23 as electroactive materials. TRZ-tetraphenylborate (TPB)19–21 and TRZ-phosphomolybdate (PM)22 have been reported as ion-pairs for the determination of TRZ. Analytical characterization of these potentiometric sensors based on ion-associates or ion-exchangers is summarized in Table 1. To build on these developments, the proposed methods aimed to investigate a new material, ionophores as potentiometric sensors for TRZ. The new sensors have better detection limit, long life and higher selectivity compared with the reported sensors based on ion-pairs as sensing material.
 |
Table 1 Potentiometric method used for determination of TRZ using ion-associate PVC sensors |
β-cyclodextrin(β-CD), γ-cyclodextrin (γ-CD) and 4-tert-butylcalix[8]arene (t-BC8) were tested as ionophore sensing materials for the preparation of polyvinyl chloride (PVC) membrane sensors for TRZ.
B-cyclodextrin and γ-CD comprising seven or eight glucose units, respectively, react with many guest molecules to form inclusion complexes.24,25 The mechanisms of the investigated sensors depend upon the molecular interactions between the guest (analyte) and the host (cyclodextrins) through hydrogen bonding, electrostatic reactions, Van der Waals forces, dipole-dipole interactions, topical effects and other dispersion forces.26,27 The internal dimensions of the host cavities are appropriate to accept TRZ as a guest molecule. Figure 1 shows the chemical structures of the guest, hosts and their tridol structure.
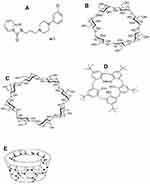 |
Figure 1 Chemical structures of trazodone (A), β-CD (B), γ-CD (C), 4t-BC8 (D), and the toroidal shape (E). |
The third tested host, t-BC8, is a cavity-shaped cyclic oligomers comprising phenol parts linked via alkylidene groups. Its reactions depend on many selection factors such as cavity size, conformation, and substitution, which induce various interactions with different compounds and permit many different analytical applications. One of the most important applications is ion-selective membranes and electrodes.28,29
The present study investigates for the first time the use of sensors incorporating β-CD, γ-CD, or t-BC8 as ionophores sensing materials in PVC matrices as selective complex for TRZ determination. The suggested sensors were effectively used for assay of TRZ with high selectivity. The investigated methods were more sensitive, accurate, reproducible, and robust relative to previously proposed ion-associated methods.
Methods
Apparatus
All potentiometric measurements were made at 25±1 °C by an “Orion pH/voltmeter (model 330)” using TRZ membrane sensors in conjunction with the Ag/AgCl reference electrode “(Orion model 90–02 double junction)” and the electrolyte was “10% (w/v) KNO3”. Adjustments of pH were performed using a combined “Ross glass pH electrode (Orion 81–02)” for all pH measurements.
Reagents and materials
All chemicals used were of analytical reagent grade. Doubly distilled water was used. “Tetrahydrofuran (THF), 2-nitrophenyl octylether (NPOE), dibutyl phthalate (DBP), dioctyl phthalate (DOP)” and “PVC powder of >99%” purity was obtained from the “Sigma-Aldrich chemical company”. Trazodone HCl was obtained from the “Sigma-Aldrich Chemical Company”, Switzerland. “β-CD, γ-CD, and 4t-BC8 were obtained from BDH Chemical, Ltd”. A pharmaceutical preparation containing TRZ HCl “Trittico, 50 mg/tablet” was acquired from a local pharmacy. A stock solution of “0.01-M TRZ was prepared by dissolving the appropriate amount of TRZ in 50 ml of water”. Working solutions of TRZ were prepared by suitable dilution of the stock in water to obtained “1×10−3, 1×10−4, 1×10−5 and 1×10−6M” concentrations. An acetate buffer (pH 3.5) was prepared using a mixture of sodium acetate and acetic acid (0.05M), and the pH was controlled by a glass electrode.
Preparation of the TRZ-PVC membrane sensors
Five mg of β-CD, γ-CD, and 4t-BC8 were thoroughly mixed with “5 mg potassium tetrakis(4-chlorophenyl)borate (KTpClPB), 190 mg PVC powder, 350 mg DOP or NPOE, and 5 ml THF in glass Petri dishes (5 cm in diameter).” All components were mixed together and the solvent was allowed to evaporate to generate the membranes. Sections of the membranes were cut using a cork borer and glued using THF solution30,31 and then attached to a laboratory-made electrode containing a mixture of TRZ and KCl (0.01 M each). The “Ag/AgCl internal reference electrode was used.” The working TRZ electrode was conditioned by insertion in the TRZ solution; it was kept in the same solution during measurements.
Procedure
The TRZ PVC membrane sensors were standardized by inserting the prepared electrodes in conjunction with the reference electrode into a measuring cell containing 10 ml of a TRZ working solution of a specific concentration after the pH was controlled using the acetate buffer at pH 3.5. For each solution, the potential was recorded after stabilizing. The recorded potential was plotted against the negative logarithm of the TRZ concentration. A straight line was obtained and the resulting curve was used to determine the unknown concentration.
Determination of TRZ in pharmaceutical formulation
Ten Trittico tablets (50 mg TRZ each) were crushed and mixed well in a mortar. Crushed powder equivalent to one tablets (50 mg) from the homogenous mixture was transferred into a 100 ml beaker and then dissolved in water using sonication for approximately 10 min before filtration (using Whatman filter paper). The resulting clear solution was collected in a standard 100 ml volumetric flask and made up with 100 ml water. An appropriate amount of this clear solution was transferred into a 50 ml volumetric flask. The pH was controlled to pH 3 using acetate buffer and made up with water to 50 ml. The concentration of the unknown sample was measured using the sensors from the previously constructed calibration graph of the potential versus -log [TRZ]. The standard addition technique32 was also used to assay TRZ.
For reconstituted TRZ powder, a laboratory tablet form was prepared by adding a mixture of starch, lactose, and magnesium stearate, to 25 mg of trazodone and mixing thoroughly. The concentration of TRZ in the synthetic laboratory tablets was determined using the developed sensors.
Molecular docking studies
To support our experimental results, a molecular docking study for trazodone with β-, γ- cyclodextrin and 4t-BC as ionophores was preferred using Molecular Operating Environment 2015.10 (Chemical Computing Group, Montreal, QC, Canada). The 3D structure of β- cyclodextrin was extracted from the protein complex of alpha-amylase (Protein Data Bank [PDB] code: 1jl8),33 and γ- cyclodextrin structure was extracted from the protein complex Ecoli Branching Enzyme (Protein Data Bank [PDB] code: 5e70),34 and the structure 4t-BC was extracted from the cytochrome in complex with sulfonato-calix[8] arene (Protein Data Bank [PDB] code: 6gd8)35 which was retrieved from the Brookhaven PDB and used as a template for building 4-tert-butylcalix[8]arene which was built by MOE Builder function via substitution of sulfonate groups with the appropriate tert-butyl. MOE Quick Prep protocol was used to add hydrogen and minimize the structure. Trazodone was drawn by MOE; its potential energy was diminished by applying the proper force field using AMBER 10 (University of California, San Francisco, CA, USA).36 The default docking protocol implemented in MOE was applied.37 Conformations of the ligand were fitted in the position with the triangle matcher method and ordered with the London ΔG scoring function. The produced positions were ranked as per their docking scores. Finally, we chose the most appropriate energy position.”
Results and discussion
Different potentiometric sensors have been developed for the determination of TRZ using different ion-exchange materials or ion pairs19–23 as electroactive materials. Reported methods have incorporated TRZ-tetraphenylborate19–21 and TRZ-phosphomolybdate22 as the sensing materials. In this investigation, we investigated for the first time a new sensing material for TRZ based on ionophores (β-CD, γ-CD, and t-BC8) as electroactive materials. The characteristics of ionophores compared with ion-pairs19–23 as electroactive material are summarized in Table 1. The ionophore methods affords an overall lower limit of detection, more sensitivity, accuracy, reproducibility, robustness and longer life compared to previously proposed ion-associated methods.
The hosts β-CD, γ-CD, and 4t-BC8 are neutral compounds that can work as cations30 or anions31 based on the additives added to the sensing materials. In this investigation, TRZ is considered a cationic additive. Therefore, KTpClPB as an anion excluder was added to the membrane composition, creating ionic sites in the PVC matrices. Thus, the ionic additives affect the behavior of the host, which works as a cation carrier. They also improve the sensor response to the analyte, yielding near-Nernstian behavior and improved selectivity.27,38
Analytical characteristics
The analytical characterization of the potentiometric method for the determination of TRZ using β-CD, γ-CD or 4t-BC8 an ionophores in a sensing membrane was carried out according to IUPAC guidelines.39 The results are presented in Table 2 comprising the analytical parameters of the proposed methods. The calibration graph equation is extracted from the plot as follows:
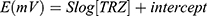
 |
Table 2 Analytical parameters for trazodone using TRZ-PVC sensors |
Where E is the potential in millivolts, S is the slope of the sensor (58.5±0.5, 55.0±0.5, and 51.5±0.5 mV) for β-CD, γ-CD or 4t-BC8, respectively), and the y-axis intercept is (111.6±0.5, −1818±0.5 and 71.6±0.5) (representing the standard electrode potential, Eo) for β-CD, γ-CD and 4t-BC8, respectively).
Effect of plasticizers
The trazodone PVC membrane sensors using β-CD, γ-CD, or 4t-BC ionophores were tested in different plasticizer to examine and characterize the behaviors of the modified membranes. The three ionophores were examined as possible electroactive materials for the construction of PVC membrane sensors with three plasticizers of DBP, DOP, and NPOE to generate different membranes. The plasticizer assists in creating a homogeneous membrane, which facilitates the transport of ions through the membrane. Sensors of β-CD, γ-CD, or t-BC8 were tested in combination with different plasticizers. DOP and NPOE showed suitable mediating properties for the construction of membranes using all tested ionophores; however, the solubility of the ionophore using DBP was minimal, and therefore DBP was excluded from further tests. Sensing membranes using DOP and o-NPOE both showed suitable sensing behaviors. Therefore, for the rest of this investigation, either DOP or NPOE is used as a plasticizer.
Working pH and the response period
The suggested TRZ sensors were tested in TRZ solutions with different pH ranges. Two different concentrations (tenfold difference) were assessed and the potential was monitored in different pH ranges, then plotted the potential versus pH. The pH profiles of the proposed TRZ sensors are presented in Figure 2. As presented in Figure 2 the slopes were 58.5±0.5, 55.0±0.5, and 51.5±0.5 mV for β-CD, γ-CD and 4t-BC8, respectively, in the pH range from 3.0 to 6.0. At higher pH more than 6 (pKa 6.74),40 the concentration of the protonated species of TRZ increases and therefore its potential decreases.
 |
Figure 2 Effect of pH of the proposed sensors (A) β- CD, (B) γ-CD and (C) 4t-BC8 sensors. |
The response times39 of the TRZ sensors were estimated by measuring the potential stabilization over time for sensor use periods reaching one month. As shown in Figure 3, the response time was stable at 25s after transfer of the electrode between two TRZ solutions with concentrations differing by tenfold. The response time for the concentration of ≥1×10−4 M was ≤25 s; for concentrations ≤1×10−5 M, the response time increases to approximately 35 s. The results show good reproducibility and lifetimes exceeded one month, during which the proposed sensors show highly reproducible responses. Thus, the reproducibility of the membrane was effective.
 |
Figure 3 Response time of the proposed (A) β- CD, (B) γ-CD, (C) 4t-BC8 sensors. |
Effect of interfering ions
According to IUPAC guidelines, the effects of interfering ions and molecules were studied using the separate-solution and mixed-solution method.39,41 The effect of interference was expressed as the selectivity coefficient  . Higher selectivity coefficient values indicate high interference effects, while lower values indicate negligible interference.
. Higher selectivity coefficient values indicate high interference effects, while lower values indicate negligible interference.
Using the separate-solution method, the selectivity coefficients were calculated according to the equation:
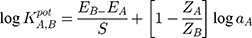
Where EA and EB are the potentials of the proposed sensors after stabilizing, ~30 s after stabilizing into solutions containing equal concentrations of TRZ and the interfering species, respectively. aA and aB are the activities of TRZ and the tested species, while ZA and ZB are the charges of trazodone and the tested species, respectively. The selectivity coefficient measured using the mixed-solution method was estimated according to the following equation:
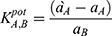
Where a`A is the known concentration of TRZ added to an unknown concentration aA. The change in potential (ΔE) was recorded. Another test experiment used a solution with the known concentration AB of the interfering ion was added to a fixed concentration of TRZ until the same potential from the first experiment was reached. The values of the selectivity coefficient are listed in Table 3. The low values indicate that the proposed method is relatively unaffected by interfering species.
 |
Table 3 Selectivity coefficients of some interfering ions, using the proposed TRZ-PVC sensors |
Validation of sensors assays
Limit of quantification and detection
According to the Nernstian equation, the relationship between concentration and potential is logarithmic:
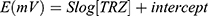
Where E is the potential and S is the slope of the calibration curve; r2 is the coefficient of regression and the intercept “represents the standard electrode potential, Eo. Each measurement was repeated five times and the average potential was plotted versus concentration. The analytical parameters of the TRZ sensors are listed in Table 2. The calibration ranges of the TRZ sensors show linearity over the ranges of 7×10−6–1×10−3, 5×10−5–1×10−3, and 8×10−6–1×10−3 M for β-CD, γ-CD, and t-BC8, respectively, in the pH range of 3–6. According to the IUPAC guidelines, the lower limits of detection (LOD), determined as the concentrations corresponding to the x-intercepts of the extrapolated linear segments of the calibration graph was 2.2×10−6, 1.5×10−5, and 2.42×10−6M for β-CD, γ-CD, and 4t-BC8, respectively.
Accuracy and precision
The accuracy and precision of the TRZ sensors were determined by assessing the repeatability of measuring 40.7 µg/ml of TRZ five times during the same day (intraday) and on different days (interday). The accuracy and precision are expressed as the recovery percentage and relative standard deviation of the measured concentration. The results obtained are listed in Table 4; the accuracy exceeded 97.6% and 96.9% for the intraday and interday measurements. The precision was 2.5% and 3.1% for intraday and interday measurements.
 |
Table 4 Intraday to interday reproducibility of trazodone using TRZ-PVC membrane sensors |
Ruggedness
The investigated sensors were examined by analysing TRZ by different analysts and different instruments.42 The precision was high with RSD of less than 3.1% for repeated measurements throughout one day and on three different days. The analysis data indicates that the investigated materials produce results with high reproducibility.
Determination of pharmaceutically formulated TRZ
To apply the proposed method for the determination of TRZ in pharmaceutical formulations, the suggested methods were tested for the determination of TRZ in solution. The accuracy and precision of the investigated sensors were assessed.
The assay of TRZ in solution was examined using TRZ sensors of β-CD, γ-CD, and 4t-BC8. The determination of 3.25–407.4 μg/ml of TRZ in solution (in five replicated experiments) by the potentiometric method showed high recoveries and precision values, as presented in Table 5. The recoveries (n=5) ranged from 97.5% to 99.0% with RSD% values of 1.9% - 3.2% for the proposed sensors.
 |
Table 5 Determinations of trazodone using the proposed PVC membrane sensors |
The analysis results of TRZ in pharmaceutical Trittico tablets using the proposed TRZ sensors are shown in Table 6. The analysis of TRZ synthetic sample by the proposed methods showed an average recovery of 98.5%, 98.0% and 98.0% using β -CD, γ-CD and 4t-BC8, respectively compared with the USP method, which showed an average recovery 97.0%. On the other hand, the relative standard deviation was 2.5%, 2.7%, 2.8%, respectively compared with 2.7% for USP method. The analysis of TRZ in Trittico tables by the proposed method showed an average recovery 98% compared with 98% for the USP method with % RSD value of 2.3%, 2.6% and 2.6% compared with USP method of 2.5%. Therefore, these results showed good agreement with data from the standard US Pharmacopeia method43
 |
Table 6 Determination of trazodone in its pharmaceutical formulation using the membrane sensors |
Molecular docking studies
The main benefit of implementing molecular modelling computations is to provide discriminant of the orientation and interaction of the drug/guest molecule with β-, γ-CD, and t-BC8 cavities, which may assist with experimental feedbacks.44 In this study, we conducted docking studies to suggest the possible interaction between trazodone and β-CD, γ- CD and t-BC8 cavity. Our investigation indicates that trazodone interacts with β-CD, γ- CD, and 4t-BC8 in a 1:1 molar ratio. This finding is agreement with the potentiometric response of the investigated sensors, which indicated a monovalent Nernstian response. The binding energies were −6.243, −5.752 and −5.7105 kcal/mol with β-CD, γ-CD, and t-BC8, respectively (Figure 4). These results agree with the potentiometric response of the investigated sensors which were 58.5mV, 55.0 mV and 51.5 mV per decade over the calibration range of the proposed sensors. Figure 4 reveals that the binding is stronger in the case of β- CD compared to γ-CD and t-BC8 which are more favorable due to the hydrophobic properties of cyclodextrin and t-BC8 pocket. This hydrophobic pocket provides a more stable complex when it sandwiches the hydrophobic and water-insoluble trazodone molecule.45 Optimal docking of TRZ:β-CD, γ-CD and t-BC8 complexes (Figure 4) revealed the binding affinity and Van der Waals force interaction within the inclusion-complex.
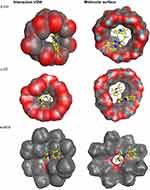 |
Figure 4 The most stable complex of trazodone with β-, γ- CD and 4t-BC8 through their cavities. |
Conclusion
Experimental evaluation of three ionophores sensing materials, β-CD, γ-CD and t-BC8, showed favorable characteristics to be used as TRZ sensors. The developed sensor membranes using these ionophores were of higher sensitivity, precision, and sensitivity compared to previously reported methods using ion pairs as electroactive materials. The optimum pH range was 3–6 representing a reasonable efficiency with fast response times of ~30 s or less. The calibration ranges for β-CD and 4t-BC8 were much wider than γ-CD. The determinations of TRZ in both bulk and dosage form showed high accuracy and precision. The quantification of TRZ by the current methods was in good agreement with standard US Pharmacopeia method. The interaction between the host (trazodone) and guest (β-CD, γ-CD and t-BC8) was assessed by molecular modelling. Optimal docking of TRZ:β-CD, γ-CD and t-BC8 complexes revealed binding affinity and Van der Waals force interactions within the inclusion-complexes.
Acknowledgment
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at the King Saud University for funding this work through the Research Group Project number RGP-1438-045.
Disclosure
All authors declared they have no conflict of interest in regard to this work.
References
1. Carda-Broch S, Gil-Aguestí MT, Rambla-Alegre M, Monferrer-Pons L, Esteve-Romero JS. Determination of trazodone in urine and pharmaceuticals using micellar liquid chromatography with fluorescence detection. J Chromatogr A. 2007;1156(1–2):254–258. doi:10.1016/j.chroma.2007.02.112
2. El-Gindy A, El-Zeany B, Awad T, Shabana MM. Spectrophotometric, spectrofluorimetric and lc determination of trazodone hydrochloride. J Pharm Biomed Anal. 2001;26(2):211–217. doi:10.1016/S0731-7085(01)00426-5
3. Davidoff G, Guarracini M, Roth E, Sliwa J, Yarkony G. Trazodone hydrochloride in the treatment of dysesthesia pain in traumatic myelopathy: a randomized, double-blind, placebo-controlled study. Pain. 1987;29(2):151–161.
4. Bryan SG, Ereshefsky L. Antidepressant properties of trazodone. Clin Pharm. 1982;1(5):406–417.
5. Chew RH, Hales RE, Yudofsky SC. What Your Patients Need to Know about Psychiatric Medications. Third edition. Arlington: American Psychiatric Publishing, Inc.; 2017.
6. Schatzberg AF, Nemeroff CB. The American Psychiatric Publishing Textbook Of Psychopharmacology. Washington, DC: American Psychiatric Pub; 2009.
7. Harikrishna K, Kumar SR, Seetharamappa J, Manjunatha DH. Sensitive extraction spectrophotometric methods for the determination of trazodone hydrochloride in pure and pharmaceutical formulations. J Serb Chem Soc. 2006;71(7):829–837. doi:10.2298/JSC0607829H
8. Yang GJ, Liu P, Qu XL, et al. Micellar‐enhanced spectrofluorimetric determination of trazodone hydrochloride in human urine and serum. Anal Lett. 2007;40(1):151–162. doi:10.1080/00032710600952598
9. Fujimori K, Sakata Y, Moriuchi‐Kawakami T, Shibutani Y. Enhanced chemiluminescence for trazodone trace analysis based on acidic permanganate oxidation in concurrent presence of rhodamine 6G. Luminescence. 2017;32(7):1240–1245. doi:10.1002/bio.3317
10. Kaçar C, Durmus Z, Kiliç E. Electrochemical behavior of trazodone at mercury and glassy carbon electrodes and voltammetric methods for its determination. Asian J Chem. 2014;26(7):1931. doi:10.14233/ajchem.2014.15559
11. Hegde RN, Shetti NP, Nandibewoor ST. Electro-oxidation and determination of trazodone at multi-walled carbon nanotube-modified glassy carbon electrode. Talanta. 2009;79(2):361–368. doi:10.1016/j.talanta.2009.03.064
12. El-Enany N, Belal F, Rizk MS. Voltammetric analysis of trazodone HCl in pharmaceuticals and biological fluids. J Pharm Biomed Anal. 2002;30(2):219–226. doi:10.1016/S0731-7085(02)00327-8
13. Nandini RP, Deeptaunshu AP. Development and validation of liquid chromatographic method for trazodone hydrochloride. J Chem Pharm Res. 2010;2(2):478–488.
14. Mercolini L, Colliva C, Amore M, Fanali S, Raggi MA. HPLC analysis of the antidepressant trazodone and its main metabolite m-CPP in human plasma. J Pharm Biomed Anal. 2008;47(4–5):882–887. doi:10.1016/j.jpba.2008.02.028
15. Li-Bo D, Rong-Hua Z, Huan-De L, Feng W, Ping-Fei F, Jiang L. Quantitative analysis of trazodone in human plasma by using HPLC-fluorescence detector coupled with strong cation exchange chromatographic column: application to a pharmacokinetic study in Chinese healthy volunteers. J Chromatogr B. 2014;944:43–48. doi:10.1016/j.jchromb.2013.11.013
16. Patel BN, Sharma N, Sanyal M, Shrivastav PS. High throughput and sensitive determination of trazodone and its primary metabolite, m-chlorophenylpiperazine, in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr B. 2008;871:44–54. doi:10.1016/j.jchromb.2008.06.046
17. Cosofret VV, Buck RP. Pharmaceutical Applications of Membrane Sensors. Boca Raton: CRC Press; 2017.
18. Gupta K, Nayak V, Agarwal A, Singhal B. Recent advances on potentiometric membrane sensors for pharmaceutical analysis. Comb Chem High Throughput Screen. 2011;14(4):284–302. doi:10.2174/138620711795222437
19. García MS, Ortuño J, Albero MI, Cuartero M. Application of a trazodone-selective electrode to pharmaceutical quality control and urine analyses. Anal Bioanal Chem. 2009;394(6):1563–1567. doi:10.1007/s00216-009-2699-7
20. Ortuño JA, García MS, Albero MI, Cuartero M. A micro-coated wire ion-selective electrode for flow-injection analysis of trazodone in pharmaceuticals,” human urine and serum. Sens Lett. 2009;7(4):615–620. doi:10.1166/sl.2009.1120
21. Khalil S. Ion-selective electrode for the determination of trazodone in tablets. Analyst. 1999;124(2):139–142.
22. Ammar R, Al-Warthan A. Ion selective PVC membrane electrodes for the determination of trazodone hydrochloride in pharmaceutical formulation. J Incl Phenom Macrocycl Chem. 2011;69(1–2):287–293. doi:10.1007/s10847-010-9846-9
23. Suzuki H, Nakagawa H, Mifune M, Saito Y. A widely applicable electrode sensitive to basic drugs based on poly (vinyl chloride) membrane plasticized with tricresyl phosphate. Chem Pharm Bull. 1993;41(6):1123–1126.
24. Frömming K-H, Szejtli J. Cyclodextrins in Pharmacy. Berlin: Springer Science & Business Media; 1993.
25. Li S, William CP. Cyclodextrins and their applications in analytical chemistry. Chemical. Reviews. 1992;92(6):1457–1470.
26. Hassan SSM, Kamel AH, Abd El-Naby H. New potentiometric sensors based on selective recognition sites for determination of ephedrine in some pharmaceuticals and biological fluids. Talanta. 2013;103:330–336. doi:10.1016/j.talanta.2012.10.067
27. Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6(2):E329–E357. doi:10.1208/pt060243
28. Diamond D. 4t-BCs as ionophores in ion-selective electrodes; The past and the future of chemical sensing. Present at the Annual Meeting of the Danish Electrochemical Society, Copenhagen, Denmark, October 9-10, 2014.
29. Khaled E, Khalil MM, Abed el Aziz GM. 4t-BC/Carbon nanotubes based screen printed sensors for potentiometric determination of gentamicin sulphate in pharmaceutical preparations and spiked surface water samples. Sens Actuators B. 2017;244:876–884. doi:10.1016/j.snb.2017.01.033
30. Abdelaziz A, Alrabiah H, Ghabbour H, Abounassif M, Mostafa GAE. Beta-and gamma-cyclodextrin ionophores as electroactive materials for construction of new polyvinyl chloride sensors for eletriptan based on host-guest recognition. Mater Express. 2018;8(2):182–188. doi:10.1166/mex.2018.1417
31. Alrabiah H, Al-majed A, Abounassif M, Mostafa GAE. Ionophore-based potentiometric pvc membrane sensors for determination of phenobarbitone in pharmaceutical formulations. Acta Pharm. 2016;66(4):503–514. doi:10.1515/acph-2016-0042
32. Ma TS, Hassan SSM. Organic Analysis Using Ion Selective Electrodes. Vol. 1&2. London: Academic press; 1982. doi:10.1016/0006-2944(75)90147-7
33. Yokota T, Tonozuka T, Shimura Y, Ichikawa K, Kamitori S, Sakano Y. Structures of thermoactinomyces vulgaris r-47 α-amylase ii complexed with substrate analogues. Biosci Biotechnol Biochem. 2001;65(3):619–626. doi:10.1271/bbb.65.619
34. Feng L, Fawaz R, Hovde S, Sheng F, Nosrati M, Geiger JH. Crystal structures of escherichia coli branching enzyme in complex with cyclodextrins. Acta Crystallogr Sect D. 2015;54(40):6207–6218.
35. Rennie M, Fox G, Pérez J, Crowley PB. Auto‐regulated protein assembly on a supramolecular scaffold. Angew Chem. 2018;130(42):13960–13965. doi:10.1002/ange.v130.42
36. Case DA, Cheatham TE, Darden T, et al. The amber biomolecular simulation programs. J Comput Chem. 2005;26(16):1668–1688. doi:10.1002/jcc.20290
37. Radwan MO, Sonoda S, Ejima T, et al. Zinc-mediated binding of a low-molecular-weight stabilizer of the host anti–viral factor apolipoprotein b mrna-editing enzyme, catalytic polypeptide-like 3g. Bioorg Med Chem. 2016;24(18):4398–4405. doi:10.1016/j.bmc.2016.07.030
38. Eugster R, Gehrig PM, Morf WE, Spichiger UE, Simon W. Selectivity-modifying influence of anionic sites in neutral-carrier-based membrane electrodes. Anal Chem. 1991;63(20):2285–2289. doi:10.1021/ac00020a017
39. Buck RP, Lindner E. Recommendations for nomenclature of ionselective electrodes (IUPAC recommendations 1994). Pure Appl Chem. 1994;66(12):2527–2536. doi:10.1351/pac199466122527
40. Gervais S, Damon S, Miloud R, et al. Trazodone composition for once a day administration, U.S. Patent 2010, 7,829,120.
41. Umezawa Y, Bühlmann P, Umezawa K, Tohda K, Amemiya S. Potentiometric selectivity coefficients of ion-selective electrodes, part I. Inorganic cations (technical report). Pure Appl Chem. 2000;72(10):1851–2082. doi:10.1351/pac200072101851
42. Miller JN, Miller JC. Statistics and Chemometrics for Analytical Chemistry. London: Pearson Education; 2005.
43. The United States Pharmacopeial Convention. Trazodone Hydrochloride Tablets: revision bulletin. Available from: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisions/trazadone-hcl-tabs-rb-notice.pdf. Accessed June 30, 2019.
44. Ahmadi P, Ghasemi JB. 3D-QSAR and docking studies of the stability constants of different guest molecules with beta-cyclodextrin. J Incl Phenom Macrocycl Chem. 2014;79(3–4):401–413. doi:10.1007/s10847-013-0363-5
45. Kfoury M, Auezova L, Fourmentin S, Greige-Gerges H. Investigation of monoterpenes complexation with hydroxypropyl-β-cyclodextrin. J Incl Phenom Macrocycl Chem. 2014;80(1–2):51–60. doi:10.1007/s10847-014-0385-7
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
