Back to Journals » International Journal of Nanomedicine » Volume 15
Comparative Study of Antibacterial Effects of Titanium Dioxide Nanoparticles Alone and in Combination with Antibiotics on MDR Pseudomonas aeruginosa Strains
Authors Ahmed FY, Farghaly Aly U, Abd El-Baky RM, Waly NGFM
Received 16 January 2020
Accepted for publication 18 April 2020
Published 14 May 2020 Volume 2020:15 Pages 3393—3404
DOI https://doi.org/10.2147/IJN.S246310
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Fatma Y Ahmed,1 Usama Farghaly Aly,2 Rehab M Abd El-Baky,1,3 Nancy G F M Waly1
1Department of Microbiology and Immunology, Faculty of Pharmacy, Minia University, Minia 61519, Egypt; 2Department of Pharmaceutics, Faculty of Pharmacy, Minia University, Minia 61519, Egypt; 3Department of Microbiology and Immunology, Faculty of Pharmacy, Deraya University, Minia 11566, Egypt
Correspondence: Usama Farghaly Aly
Faculty of Pharmacy, Minia University, Minia, Egypt
Tel +201001108688
Email [email protected]
Introduction: The efficacy of several antimicrobial agents has been hindered due to the increasing frequency of multidrug-resistant (MDR) Pseudomonas aeruginosa strains. So, the need for new antibacterial drugs or drug combinations is urgent. Recently, desirable antibacterial effects were reported for many metals nanoparticles such as TiO2 nanoparticles (TDNs).
Purpose: This study aims to investigate the prevalence of MDR P. aeruginosa and assess the efficiency of TDN in the treatment of MDR P. aeruginosa-associated infections.
Materials and Methods: The synthesis of TDN by the sol-gel method was carried out. Particle size measurements and morphology were done using dynamic light scattering (DLS) and high-resolution transmission electron microscopy (HR-TEM). To investigate the physical and chemical changes of drugs due to the combination, the tested drugs, both alone and in combination with TDN, were subjected to differential scanning calorimetry (DSC), infrared (IR) spectroscopy, and X-ray diffraction studies. Antimicrobial susceptibility was detected by agar disc-diffusion assay. The minimum inhibitory concentration (MIC) of TDN and the tested antibiotics were assessed by the agar dilution method. Checkerboard analysis was performed to determine the combined effect of TDN and the tested antibiotics against 25 MDR P. aeruginosa strains.
Results: TDNs were prepared with an average particle size of 64.77 ± 0.14 nm with an accepted polydispersity index (PDI) value of 0.274 ± 0.004. TEM showed that the particles were shaped into irregular spheres. Twenty-five P. aeruginosa isolates that were absolutely resistant to cefepime (100%), highly resistant to ceftriaxone (96%), amikacin (80%), and ciprofloxacin (76%) were selected. Superior antibacterial activity of TDN was observed against the selected 25 MDR P. aeruginosa isolates. The combination of TDN and cefepime were found to show synergistic activity against all tested isolates followed by ceftriaxone (96%), amikacin (88%), and ciprofloxacin (80%).
Conclusion: Using TDN in combination with antibiotics can help in the treatment of MDR P. aeruginosa-associated infections. So, preparation of topical pharmaceutical dosage forms containing a combination of these antibiotics and TDN can be useful against MDR P. aeruginosa.
Keywords: MDR P. aeruginosa, titanium dioxide nanoparticle, checkerboard assay
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen that causes a variety of diseases ranging from mild to severe life-threatening infections and considered as one of the main causes of nosocomial or hospital-acquired infections.1–3 P. aeruginosa infections are difficult to eradicate by many antimicrobial agents due to its intrinsic resistance (low outer membrane permeability, efflux mechanisms, and inactivity of the antibiotics by enzymes), acquired resistance (mutations in genes targeted by the antibiotics), adaptive resistance (biofilm formation),4 and various virulence factors (extracellular proteases, toxins).5 Treatment of these infections has become a great challenge because of the ability of this bacterium to resist a variety of antibiotics, including aminoglycosides, quinolones, and β-lactams. So, combination therapy may help in the reduction of the mortality rate of patients with severe P. aeruginosa infections.4
The emergence of multidrug-resistant (MDR), Extensive Drug-Resistant (XDR), and pandrug-resistant bacteria increased the need for the development of alternative strategies to treat bacterial diseases. One of these strategies is the use of nanoscale materials.6 The nanoparticles used as antimicrobial agents due to its ability to penetrate bacterial membranes, disrupt biofilm formation, and be good carriers of antibiotics.4 Metallic nanoparticles are attracting a great deal of attention because of their selectivity and potential success in the biological and pharmaceutical applications.7,8 Many metal oxides nanoparticles showed good antimicrobial activity such as the following: TiO2, ZnO, MgO, CuO, SiO2, and CoO. They are less toxic and heat resistant and exhibit marked effectiveness against resistant strains of some microorganisms. Besides, they may act as mineral elements supplement that is essential to human cells.9 Titanium oxide nanoparticles showed good inhibitory activity against bacterial growth due to its small nanometer scale and potent oxidizing power.3 This study was conducted to determine the prevalence of MDR P. aeruginosa that causes many diseases and to assess the efficiency of TDN alone and in combination with different antibiotics in the treatment of MDR P. aeruginosa.
Materials and Methods
Reagents
Titanium Tetrachloride (TiCl4, 99.5%) was obtained from Loba chemie, India.
Ceftriaxone sodium, amikacin sulfate, cefepime hydrochloride, and ciprofloxacin were obtained from Pharco B company, Egypt.
Synthesis of TiO2 Nanoparticles (TDN)
TDN was prepared by the sol-gel method. The procedures were carried out according to the previously described method with slight modifications.10 Briefly, 5 mL of titanium tetrachloride were added to 50 mL of absolute Ethanol; the mixture was stirred for 30 min using a magnetic stirrer till a yellow sol phase was formed. While stirring, 200 mL of distilled water were added until the solution became colorless and clear. The solution was further stirred for 45 min while the temperature was kept at 25–30°C. The formed TDN was collected by centrifugation at 10,000 rpm for 15 min, washed by distilled water several times, and then dried at 50°C for 30 h.
Characterizations of TiO2 Nanoparticles (TDN)
Dynamic Light Scattering (DLS) Analysis and Zeta Potential Measurement
A liquid sample was diluted several times using Milli-Q water purifier and analyzed using DLS (Zetasizer Nano-ZS instrument, Worcestershire, United Kingdom). By placing samples in the cuvette, both particle sizes and the zeta potentials of the synthesized nanoparticles were determined. The samples were prepared for analysis at room temperature (25°C) and measured at 37°C in triplicates. Results were presented as mean ± SD (standard deviation).
Transmission Electron Microscopy
The morphological characteristics of TDN were investigated by a transmission electron microscope (TEM, Model 100 CX II, Tokyo, Japan).
Compatibility Study
Preparation of Physical Mixtures (PM)
Physical mixtures of each of the tested drugs with TDN at weight ratio 1:1 were prepared by gentle mixing in a mortar using a pestle.
Differential Scanning Calorimetry (DSC) Study
The DSC patterns were obtained by heating the samples from 30–600°C at a scanning rate of 10°C/min under a stream of nitrogen gas at a flow rate of 40 mL/min using SDT (simultaneous DSC\TGA) Q600, USA. Samples of 4 mg were accurately weighed and encapsulated into flat-bottomed aluminum pans with crimped-on lids. The procedures involved heating of the sample contained in the aluminum pan and a similar empty reference pan at the predetermined heating rate. The differential heat flow between the sample and the reference was recorded and presented graphically.
Infrared (IR) Spectroscopy Study
IR spectroscopy was used to detect chemical interactions between TDN and the tested drugs using FTIR Perkin Elmer Spectrum One, UK. It was carried out using the potassium bromide disk method. Samples, 1–2 mg each, were mixed with potassium bromide, compressed at a pressure of 6 ton/cm2 into discs, and scanned over the range of 400–4000 cm-1 using a blank pellet of potassium bromide as a reference.
X-Ray Diffraction Study
Powdered samples of TDN, tested drugs, and physical mixtures were analyzed using X-ray. The X-ray diffractograms were obtained using Panalytical X’PERT PRO, Holland. A single-crystal graphite monochromator was employed. The target was CuK∝ radiation, operating at a current of 40 mA and 40 kV. Diffractograms were obtained using continuous scan mode with 2 θ values ranging from 5 to 80 degrees at a rate of 2 degrees/minute.
Isolation and Identification of Pseudomonas aeruginosa Isolates
One hundred clinical specimens were examined for P. aeruginosa (50 urine and 30 wound exudate specimens and, five sputa, ear discharge, burn swab, and eye discharge specimens). All specimens were collected from different hospitals in Minia governorate, Egypt. All specimens were examined for the presence of P. aeruginosa by the conventional microbiological procedures11 and confirmed by biochemical reactions.
Antimicrobial Susceptibility Testing
Pseudomonas aeruginosa isolates underwent antimicrobial susceptibility test in six antibiotic discs using the Kirby-Bauer disc diffusion method.12 The antibiotics tested were ciprofloxacin (CIP, 5 μg), amikacin (AK, 30 μg), ceftriaxone (CRO, 30 μg), levofloxacin (LEV,10 μg), imipenem (IPM, 10 μg), and cefepime (FEP, 30 μg) discs (Oxoid, England).
Determination of Minimum Inhibitory Concentrations (MICs) of the Tested Antibiotics and TND
Minimum Inhibitory Concentrations (MIC) were expressed as the lowest antibacterial agent concentrations that caused a total inhibition of bacterial growth for 24 hours. The MICs of cefepime, ceftriaxone, amikacin, ciprofloxacin, and TDN were determined for 25 MDR P. aeruginosa isolates using the agar dilution method. Overnight cultures of the tested isolates in a Mueller-Hinton Broth (MHB) were adjusted to a cell density of 107 CFU/mL. Then, the bacterial culture spots were applied to the surface of a dry Muller-Hinton Agar (MHA) containing the tested antibiotics and TDN of concentrations ranged from 1 to 1024 μg/mL using a multi-inoculator. Plates were incubated at 37 °C for 18–24 h and examined for growth. Spots showing no growth were defined as MIC.13
Determination of the Synergistic Effect of TDN with Antibiotics by Checkerboard Assay
The effect of combinations of TDN with the tested antibiotics at sub-MIC values was examined by checkerboard titration tests. The MICs of antibiotics and TDN in combination were determined by the agar dilution method against 25 MDR strains, and the fractional inhibitory concentration index (FICI) was determined as described before.14
Results and Discussion
Characterizations of TDN
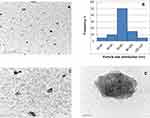
In this study, TDN was prepared using the sol-gel method. The average particle size determined by DLS was 64.77 ± 0.14 nm. This small size of nanoparticles provides a large surface area that enhances the interactions with the microbes and increases the range of antimicrobial activities.15 The poly dispersive index (PDI) was 0.274 ± 0.004, which is lower than 0.3, indicating the homogenous population of the particle size16 as shown in Figure 1. According to literature, this value suggests the stability of the prepared TDN with storage as zeta potential value was 23.8 mV. As TDN suspension has large positive or negative zeta potential, particles in suspension will tend to repel from each other which prevents aggregation and agglomeration of the particles.17 The morphology was investigated by a high-resolution transmission electron microscope (Figure 2). Images showed that the prepared nanoparticles were nearly spherical in shape of irregular edges with little aggregations at different magnifications (Figure 2A, C, and D). Particle size histogram showed that the maximum distribution of particle sizes was in the range of 60–80 nm (Figure 2B), and that agrees with DLS results. Many nanoparticle shapes with nonuniform size and superficial agglomeration were reported for TiO2, which appeared to be a normal reaction during the sol-gel method synthesis.18,19
 |
Figure 1 Particle size distribution of the formed titanium dioxide nanoparticles measured by dynamic light scattering analysis. |
Diffraction Scanning Calorimetry Studies
DSC is a significant tool to predict the physicochemical changes associated with drug interactions of the tested antibacterial agents and TDN. Drug interactions may be detected through shifts in peaks onset, area, or even disappearance of peaks. Sometimes the appearance of new peaks can be considered evidence of an interaction. All tested drugs and TDN showed dehydration peaks at different temperatures that appeared also in their corresponding Physical Mixtures (PM) (usually below 100°C) as shown in Figure 3.
The DSC thermogram of TDN showed a broad endothermic peak around 80°C to120°C due to dehydration. Besides, a second small broad endothermic peak appeared on the curve at 330°C which may have occurred due to the crystallization of the amorphous TiO2.20 Concerning the amikacin system, the drug showed its melting point endothermic peak at 256°C, per other reports mentioned elsewhere.21 There were no significant changes in the Physical Mixture (PM) suggesting the absence of a physicochemical interaction between the drug and the prepared TDN (Figure 3A). For the ciprofloxacin system, the drug showed its melting point endothermic peak at its proper position at 315°C, very close to the reported value22–24 without any considerable changes in peak position in Figure 3B.
Figure 3C shows the DSC thermograms of the ceftriaxone system. The drug produced its melting point endothermic peak at 181°C,25 identical to the DSC thermogram of the PM. Cefepime produced its characteristic peak at 185°C which is corresponding to its melting point. The peak appeared nearly in the same position in PM without any considerable changes26 (Figure 3D).
Infrared (IR) Spectroscopy Studies
FTIR was applied to assess the chemical composition and quality of the synthesized TDN as shown in Figure 4. FTIR spectrum of the synthesized TDN showed a small band in 1624 cm−1 which is characteristic to o-Ti-o bond, a broadband at 3385 cm−1, which is related to O-H stretching in agreement with previous studies27,28 that confirm the formation of TDN. To estimate the fundamental interactions of TDN and the tested drugs, Physical Mixtures (PM) were examined employing the FTIR spectrophotometer for detecting typical absorption bands. Figure 4A shows the broadband at 1638 cm−1 corresponding to N–H bending vibration of primary aromatic amines of amikacin sulfate. This band appeared in PM in the same position, suggesting the absence of interaction between drugs and TDN.29
For ciprofloxacin, the major character bands in the IR spectrum included sharp and small bands at 1624 cm−1 and 1668 cm−1 respectively (corresponding to the vibration absorption of CH2 on benzene ring), a sharp band at 1710 cm−1 (due to stretching vibration attributed to a carbonyl group), and small bands at 1399 and 944 cm−1 (corresponding to O-H).30 Nearly all the previous bands appeared in the PM (Figure 4B). The FT-IR spectrum of ceftriaxone (Figure 4C) showed characteristic bands at 3429 cm−1 (N–H) stretching of the H-bonded amide group, 1742 cm−1 β-lactam C=O stretching vibrations, and 1610 cm−1 oxime C=N stretching vibrations. All bands appeared very close to the reported data.25 Characteristic bands of ceftriaxone appeared in the same position in the PM. Cefepime showed main characteristic bands at 2935 cm−1, 1773.29 cm-1, and 1655 cm−1 corresponding to O-H stretching, β-lactam C=O stretching, and amide C=O, respectively. That is close to the reported value.26 Bands appeared in the PM in their proper positions without any significant shift (Figure 4D). Data of IR and DSC supported the absence of interaction between tested drugs and TDN.
X-Ray Diffraction Studies
The X-ray diffractogram of TDN, the tested drugs, and their combinations in the PM are shown in Figure 5. TDN showed less crystalline or amorphous structure which is somewhat similar to a brookite polymorph. Growth of brookite under acidic conditions has been consistently reported when the water-soluble Ti complexes are replaced by TiCl4. Also, for most of the reported work, it was noticed that the use of a water-based growth environment has been a common denominator on the growth of brookite nanostructures.31 Regarding amikacin, the X-ray diffractogram revealed a crystalline structure of the drug with major diffraction peaks at 2 θ values of 17.57°, 18.08°, and 33.84°. The drug retained its crystalline structure in the PM without detectable changes in its characteristic peaks (Figure 5A). The same results were observed in ciprofloxacin PM. The major diffraction peaks at 2 θ values of the drug appeared at 19.18°, 26.4°, 18.8°, 29°, 25.66°, 23°, and 19.7° without significant changes (Figure 5B). Concerning ceftriaxone, the X-ray diffractogram showed major diffraction peaks at 2 θ values 21.09°, 22.66°, 23.67°, 18.80°, and 18.28° which revealed the high crystallinity of the drug.
The diffractogram of the PM with TDN showed that all characteristic peaks of the drug appeared at the same two theta values without any considerable changes (Figure 5C). Concerning cefepime, the X-ray diffractogram of the drug revealed a high degree of crystallinity with major diffraction peaks at 2 θ values of 27.72°, 23.18°, 19.37°, 16.7°, and 22.63°. No detectable changes were observed in the position of peaks in the PM (Figure 5D).
From the previous data, it could be concluded that the four above mentioned drugs kept their crystalline structure in the PM with TND, which confirms the DSC and FTIR. The physicochemical compatibility between the investigated drugs and the prepared TDN allows for the combination and preparation of pharmaceutical dosage forms containing each drug with nanoparticles to get the benefits of adding TDN to the antibiotics to overcome the big problem of MDR bacteria such as P. aeruginosa.
Prevalence of Pseudomonas aeruginosa in Clinical Specimens
In this study, 67 P. aeruginosa strains were isolated from 100 different clinical specimens.
This result was similar to other results reported from Iraq (69%)32 and lower than others.13,33,34 Geographic climatic and hygienic factors may play important roles in the relative variability of the incidence rate. Prevalence of Pseudomonas aeruginosa isolates according to the type of clinical specimen were 39 (78%) from urine, 20 (66.7%) from wound exudate, 5 (100%) from ear discharge, 2 (40%) from eye discharge, and 1 (20%) from sputum specimens. P. aeruginosa had the highest incidence rate in ear infections (100%). No P. aeruginosa were isolated from burn specimens. Appiah-Korang et al (2014) showed that Pseudomonas species were the most common bacteriologic cause of ear discharge since it is a widespread environmental organism that is usually found in warm and humid environments and is known to colonize the external auditory channel which is consistent with our findings.35 In contrast, Mahmoud et al36 and Abed Al-Azzawi et al32 found that P. aeruginosa was mostly isolated from burn specimens (32.3%, 65%, respectively).
Antibiotic Susceptibility of P. aeruginosa Isolates
Recently, Egypt has been considered among the countries that suffer from high rates of antimicrobial resistance. The present study showed a high resistance for the tested antibiotics. Sixty strains (89.5%) were found to be resistant to ceftriaxone, 40 (59.7%) to cefepime, 21 (31.34%) to amikacin, 20 (29.8%) to imipenem, 17 (25.4%) to ciprofloxacin, and 8 (12%) levofloxacin.
A similarly high rate of resistance has been reported in many previous studies in Egypt.37–39
The rate of resistance to the third and fourth generation cephalosporins indicates the limited treatment choices in the hospitals of our area and the wide misuse of bactericidal antibiotics in the treatment of any infection. This leads to the accumulation of antibiotic resistance and cross-resistance between antibiotics and the appearance of multidrug-resistant (MDR) forms of P. aeruginosa.40,41 However, Diab et al (2013) reported the lowest resistant rate to ceftazidime (46%) in comparison to other used antibiotics.42
Minimum Inhibitory Concentrations (MICs) values of ceftriaxone, cefepime, amikacin, and ciprofloxacin were detected against selected 25 MDR P. aeruginosa strains.
The selected 25 P. aeruginosa strains showed high resistance for the tested antibiotics (resist to three or more antibiotics) with different antibiotic resistance patterns and clinical specimens (13 urine,9 wound exudate, and three ear discharge specimens) as shown in Table 1. P. aeruginosa strains isolated from eye discharge and sputum specimens (2 and 1, respectively) were more susceptible to the tested antibiotics.
 |
Table 1 Minimum Inhibitory Concentrations of Titanium Dioxide Nanoparticles and Different Antibiotics Against 25 Pseudomonas aeruginosa Isolates |
Determined Minimum Inhibitory Concentrations MICs of the studied antibiotics were compared to breakpoint values reported by CLSI as the basis for calculating the response. Tables 1 and 2 represent MICs of different antibiotics and antibiotic susceptibilities of the 25 MDR P. aeruginosa isolates. Results showed that all 25 MDR P. aeruginosa isolates were resistant to cefepime (100%) with MICs ranging from 32 to 512μg /mL. Ninety-six percent of strains were resistant to ceftriaxone with MICs ranging from16 to >1024 μg/mL. Eighty percent were resistant to amikacin with MICs of 16 to >1024 μg/mL, and 76% were resistant to ciprofloxacin with MICs 4 to 32 μg/mL. Our results showed a spread of multidrug-resistant strains due to indiscriminate use of antibiotics, lack of awareness, patient noncompliance, and nonavailability of antimicrobial testing facilities.43
 |
Table 2 Antibiotic Susceptibility of Pseudomonas aeruginosa Isolates |
Susceptibility of P. aeruginosa Isolates to TDN
Antimicrobial NPs offer many distinctive advantages in reducing acute toxicity, overcoming resistance and lowering cost when compared to conventional antibiotics. Titanium dioxide nanoparticles have received considerable attention as effective antimicrobial agents. The antimicrobial activity of TDN alone was tested at different concentrations against 25 MDR P. aeruginosa strains. Depending on the isolates’ sensitivity, the MICs of TDN were ranged from 8 to 64 μg/mL. Where three strains (12%) had MIC less than 1 μg/mL (Table 1).
The mechanism by which the tested nanoparticles and the bacterial cells interact depends on the interaction between the negative charges of the bacterial cells and the positive charge of metal oxides. As a result, electromagnetic skirmishes were created between bacterial cells and metal oxide surfaces. Furthermore, nanoparticles release ions that can react with the –SH group of proteins that control the transporting of material, reducing their permeability.7,9,44
Many studies detected antifungal and antibacterial activities of TDN as Duymaz et al45 and Alhadrami et al.46 Thomas (2016) showed that antimicrobial activity of toothpastes and mouthwashes against dental plaque-causing organisms were enhanced by adding TDN.47 Recently many studies reported that the wound dressing with TDN enhanced wound healing due to its antimicrobial and cell growth stimulation features.48–52
In contrast to our findings, Kotlhao et al (2017) reported that TDN had no antibacterial activity against Pseudomonas aeruginosa53 which may be due to the reduction in porosity of Gram-negative cell wall or existence of efflux pump that causes a decrease in intracellular concentrations of these compounds, resulting in a drastic reduction of biocidal activity.54
The Effect of TDN and Antibiotics on MDR P. aeruginosa Isolates
The antimicrobial resistance of the 25 MDR P. aeruginosa against various antibiotics was found to be reduced in the presence of TDN. The checkerboard titration method revealed that there was a marked decrease in MICs of commercial antibiotics in combination with TDN nanoparticles. This case was called the synergistic effect. The synergistic effect between TDN and the antibiotics was evaluated by calculating the FIC index. The effect of TDN in combination was found to be synergistic.
Present results showed that using antibiotics in combination with nanoparticles increases the therapeutic activity against the resistant strains. The presence of antibiotics with TDN increases the concentration of antibiotics at the site of infection and the binding of bacteria to antibiotics.55
Many studies have shown that combining TDN potentiate the antimicrobial action of different classes of antibiotics.2,7-9 FICI is an indicator of the degree of interaction between TDN with commercial antibiotics for the 25 MDR P. aeruginosa strains. The FICI of the combination of TDN plus cefepime shows synergistic effect to all isolates (100%), ceftriaxone; showing synergistic effect against 24 strains (96%) and additive effect against one strain (4%); ciprofloxacin showing synergistic effect against 20 strains (80%) and additive effect against three (12%) and two strains were not evaluated using the combination of TDN and ciprofloxacin as both were sensitive to ciprofloxacin; amikacin, showing synergistic effect against 22 strains (88%) and additive effect against three (12%) isolates as shown in Table 3. Masoumi et al (2018) reported the additive effect of nano-TiO2 and nano-ZnO combinations (FIC = 0.95) against both Acentobacte baumannii and K. pneumoniae strains; whereas, this combination showed an indifferent effect against P. aeruginosa isolates (FIC > 2).54
 |
Table 3 Checkerboard Results of Combination of Titanium Dioxide Nanoparticles and Antibiotics to 25 MDR Pseudomonas aeruginosa Isolates |
Conclusion
The addition of TDN to the tested antibiotics enhances the therapeutic activity of these antibiotics. So, the preparation of topical pharmaceutical dosage forms containing a combination of antibiotics and TDN will help in the eradication of MDR P. aeruginosa.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jain SN, Vishwanatha T, Reena V, et al. Antibiotic synergy test: checkerboard method on multidrug resistant Pseudomonas aeruginosa. Int Res J Pharm. 2011;2(12):196–198.
2. Arora B, Murar M, Dhumale V. Antimicrobial potential of TiO2 nanoparticles against MDR Pseudomonas aeruginosa. J Exp Nanosci. 2015;10(11):819–827. doi:10.1080/17458080.2014.902544
3. Vincent MG, John NP, Narayanan PM, Vani C, Murugan S. In vitro study on the efficacy of zinc oxide and titanium dioxide nanoparticles against metallo beta-lactamase and biofilm producing Pseudomonas aeruginosa. J Appl Pharm Sci. 2014;4(7):41–46. doi:10.7324/JAPS.2014.40707
4. Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37(1):177–192. doi:10.1016/j.biotechadv.2018.11.013
5. Yang YX, Xu ZH, Zhang YQ, Tian J, Weng LX, Wang LH. A new quorum-sensing inhibitor attenuates virulence and decreases antibiotic resistance in Pseudomonas aeruginosa. J Microbiol. 2012;50(6):987–993. doi:10.1007/s12275-012-2149-7
6. Hajipour MJ, Fromm KM, Akbar Ashkarran A, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511. doi:10.1016/j.tibtech.2012.06.004.,
7. Roy AS, Parveen A, Koppalkar AR, Prasad MVNA. Effect of nano-titanium dioxide with different antibiotics against methicillin-resistant staphylococcus aureus. J Biomater Nanobiotechnol. 2010;01(01):37–41. doi:10.4236/jbnb.2010.11005
8. Abdulrahman NBA, Nssaif ZM. Antimicrobial activity of zinc oxide, titanium dioxide and silver nanoparticles against mithicillin-resistant Staphylococcus aureus Isolates. Tikrit J of Pure Sci. 2016;21(3):49–53.
9. Kareem PA, Alsammak EG. The effect of silver and titanium dioxide nanoparticles on Klebsiella pneumoniae isolates multi resistant to antibiotics and observed by scanning electron microscopy. Cihan Univ Sci J. 2017;2017(Special–2):284–297. doi:10.24086/cuesj.si.2017.n2a26
10. Lusvardi G, Barani C, Giubertoni F, Paganelli G. Synthesis and characterization of TiO2 nanoparticles for the reduction of water pollutants. Materials. 2017;10(10):1–11. doi:10.3390/ma10101208
11. Collee JG, Fraser AG, Marmion BP, Fraser AG, Simmons A. Mackie & McCartney Practical Medical Microbiology.
12. Wayne P Clinical and Laboratory Standards Institute: performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI document M100-S20. 2010.
13. Gad GF, El-Domany RA, Zaki S, Ashour HM. Characterization of Pseudomonas aeruginosa isolated from clinical and environmental samples in Minia, Egypt: prevalence, antibiogram and resistance mechanisms. J Antimicrob Chemother. 2007;60(5):1010–1017. doi:10.1093/jac/dkm348
14. Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52(1):1. doi:10.1093/jac/dkg301
15. Ravishankar RV, Jamuna BA. Nanoparticles and their potential application as antimicrobials. Formatex. 2011;197–209.
16. Danaei M, Dehghankhold M, Ataei S, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):1–17. doi:10.3390/pharmaceutics10020057
17. Halimi SU, Abu Bakar NF, Ismail SN, Hashib SA, Naim MN. Electrospray deposition of titanium dioxide (TiO2) nanoparticles. AIP Conf Proc. 2014;1586(February 2015):57–62. doi:10.1063/1.4866730
18. Catauro M, Tranquillo E, Poggetto GD, Pasquali M, Era AD, Ciprioti SV. Influence of the heat treatment on the particles size and on the crystalline phase of TiO 2 synthesized by the Sol-Gel method. Materials. 2018;11(2364):1–11. doi:10.3390/ma11122364
19. Anaya-esparza LM, Montalvo-gonz E. Synthesis and characterization of TiO 2-ZnO-MgO mixed oxide and their antibacterial activity. Materials. 2019;12(698):1–12. doi:10.3390/ma12050698
20. Baszczuk A, Jasiorski M, Winnicki M. Low-temperature transformation of amorphous Sol–Gel TiO2 powder to anatase during cold spray deposition. J Therm Spray Technol. 2018;27(8):1551–1562. doi:10.1007/s11666-018-0769-0
21. Padhi B, Chougule M, Misra A. Aerosol performance of large respirable particles of amikacin sulfate produced by spray and freeze drying techniques. Curr Drug Deliv. 2009;6(1):8–16. doi:10.2174/156720109787048267
22. Prabhu NB, Marathe AS, Jain S, et al. Comparison of dissolution profiles for sustained release resinates of BCS class i drugs using USP apparatus 2 and 4: a technical note. AAPS PharmSciTech. 2008;9(3):769–773. doi:10.1208/s12249-008-9110-4
23. Pushpamalar J, Zakiah H, Thenapakiam S, Saravanan M. Radiation cross-linked carboxymethyl sago pulp discs for sustained drug delivery: ciprofloxacin uptake and physicochemical characterization. J Appl Pharm Sci. 2018;8(1):017–020. doi:10.7324/JAPS.2018.8103
24. Widyastuti I, Ainurofiq A, Soewandhi SN. Effects of thermal energy, mechanical energy, and solvent on ciprofloxacin hydrochloride monohydrate physicochemical properties. Rasayan J Chem. 2019;12(4):1973–1984. doi:10.31788/RJC.2019.1245426
25. Maghrabia AE, Boughdady MF, Meshali MM. New perspective enteric-coated tablet dosage form for oral administration of ceftriaxone: in vitro and in vivo assessments. AAPS PharmSciTech. 2019;20(306):1–12. doi:10.1208/s12249-019-1512-y
26. Ferdous S, Sultan MZ, Bashar T, Rahman A, Islam MS. In vitro and in vivo studies of drug-drug interaction between metformin and cefepime. Pharm Anal Acta. 2015;06:3. doi:10.4172/2153-2435.1000348
27. Shahab MU, Tabish TA, Zaman B, Tariq Z, Kamran M. Characterization and synthesis of nanosized TiO2 particles. Int Eng. 2005;3:
28. Chatterjee A, Nishanthini D, Sandhiya N, Abraham J. Biosynthesis of titanium dioxide nanoparticles using Vigna radiata. Asian J Pharm Clin Res. 2016;9(4):85–88.
29. Sharma UK, Verma A, Prajapati SK, Pandey H, Pandey AC. In vitro, in vivo and pharmacokinetic assessment of amikacin sulphate laden polymeric nanoparticles meant for controlled ocular drug delivery. Appl Nanosci. 2015;5(2):143–155. doi:10.1007/s13204-014-0300-y
30. Tan Z, Tan F, Zhao L, Li J. The synthesis, characterization and application of ciprofloxacin complexes and its coordination with copper, manganese and zirconium ions. J Cryst Process Technol. 2012;2(2):55–63. doi:10.4236/jcpt.2012.22008
31. Hezam M, Qaid SMH, Bedja IM, Alharbi F, Nazeeruddin MK, Aldwayyan A. Synthesis of pure brookite nanorods in a nonaqueous growth environment. Crystals. 2019;9(11):1–8. doi:10.3390/cryst9110562
32. Al-Azzawi SNA, Abdullah RM. Study of the resistance of P. aeruginosa isolated from wounds and burns for some disinfects and antiseptic from some baghdad hospitals. J Pharm Sci Res. 2018;10(6):1481–1484.
33. Olayinka AT, Onile BA, Olayinka BO. Prevalence of Multi-Drug Resistant (MDR) Pseudomonas aeruginosa isolates in surgical units of Ahmadu Bello University Teaching Hospital, Zaria, Nigeria: an indication for effective control measures. Ann Afr Med. 2004;3(1):13–16.
34. Kamaria PA, Aring BJ, Sinha M. Incidence of multidrug resistant Pseudomonas aeruginosa isolated from burn patients Tertiary Care Hospital, Jamnagar, Gujarat, India. IOSR J Dent Med Sci. 2016;15(07):31–34. doi:10.9790/0853-150773134
35. Appiah-Korang L, Asare-Gyasi S, Yawson AE, Searyoh K. Aetiological agents of ear discharge: a two year review in a teaching hospital in Ghana. Ghana Med J. 2014;48(2):91–95. doi:10.4314/gmj.v48i2.6
36. Mahmoud A, Zahran W, Hindawi G, Labib A, Galal R. Prevalence of multidrug-resistant Pseudomonas aeruginosa in patients with nosocomial infections at a University Hospital in Egypt, with special reference to typing methods. J Virol Microbiol. 2013;2013:1–13. doi:10.5171/2013.290047
37. Zafer MM, Al-Agamy MH, El-Mahallawy HA, Amin MA, Ashour MSED. Antimicrobial resistance pattern and their beta-lactamase encoding genes among Pseudomonas aeruginosa strains isolated from cancer patients. Biomed Res Int. 2014;2014:1–8. doi:10.1155/2014/101635
38. Hassuna NA, Mohamed AHI, Abo-Eleuoon SM, RiZk HAWA. High prevalence of multi-drug resistant Pseudomonas aeruginosa recovered from infected burn wounds in children. Arch Clin Microbiol. 2015;16(4):1–7.
39. Abbas HA, El-Ganiny AM, Kamel HA. Phenotypic and genotypic detection of antibiotic resistance of Pseudomonas aeruginosa isolated from urinary tract infections. Afr Health Sci. 2018;18(1):11–21. doi:10.4314/ahs.v18i1.3
40. Al-Agamy MH, Shibl AM, Yaki SA, Tawfik AF. Antimicrobial resistance pattern and prevalence of metallo-β-lactamases in Pseudomonas aeruginosa from Saudi Arabia. African J Microbiol Res. 2011;5(30). doi:10.5897/ajmr11.1024
41. Yayan J, Ghebremedhin B, Rasche K. Antibiotic resistance of Pseudomonas aeruginosa in pneumonia at a single university hospital center in Germany over a 10-year period. PLoS One. 2015;10(10):1–20. doi:10.1371/journal.pone.0139836
42. Diab M, Fam N, El-said M, El-dabaa E, El Defrawy I, Saber M. Occurrence of VIM-2 metallo-β-lactamases in imipenem resistant and susceptible Pseudomonas aeruginosa clinical isolates from Egypt. African J Microbiol Res. 2013;7(35):4465–4472. doi:10.5897/AJMR2013.6181
43. Ansari A, Salman SM, Yaqoob S. Original research article antibiotic resistance pattern in Pseudomonas aeruginosa strains isolated at Era s Lucknow Medical College and Hospital, Lucknow, India. Int.J.Curr.Microbiol.App.Sci. 2015;1(1):48–58.
44. Castro-Alarcón N, Herrera-Arizmendi JL, Marroquín-Carteño LA, Guzmán-Guzmán IP, Pérez-Centeno A, Santana-Aranda MA. Antibacterial activity of nanoparticles of titanium dioxide, intrinsic and doped with indium and iron. Microbiol Res Int. 2016;4(4):55–62.
45. Duymaz B, Yigit ZV, Şeker MG, Dündar F. Antibacterial properties of sol-gel derived TiO2 nanoparticles. Acta Phys Pol A. 2016;129(4):872–874. doi:10.12693/APhysPolA.129.872
46. Alhadrami HA, Al-Hazmi F. Antibacterial activities of titanium oxide nanoparticles. J Bioelectron Nanotechnol. 2017;2(1):1–5. doi:10.13188/2475-224x.1000007
47. Thomas RA. Analysis of antimicrobial activity of titaniumdioxide nanoparticles on aerobic and anaerobic dental isolates. Int J Sci Res Sci Eng Technol. 2016;2(4):2394–4099.
48. Chen Y, Yan L, Yuan T, Zhang Q, Fan H. Asymmetric polyurethane membrane with in situ generated Nano-TiO2 as wound dressing. J Appl Polym Sci. 2011;119:1532–1541. doi:10.1002/app.32813
49. Ismail NA, Amin KAM, Abdul Majid FA, Razali MH. Gellan gum incorporating titanium dioxide nanoparticles biofilm as wound dressing: physicochemical, mechanical, antibacterial properties and wound healing studies. Mate Sci Eng C. 2019;103:109770. doi:10.1016/j.msec.2019.109770
50. Nikpasand A, Parvizi MR; Study. Evaluation of the effect of titatnium dioxide nanoparticles/gelatin composite on infected skin wound healing; an animal model. Bull Emerg Trauma. 2019;7(4):366–372.
51. Amer AM, Abd El Maksoud AI, Abdeen MA, et al. Potency of titanium dioxide nanoparticles on skin wound healing in rats. Res J Pharmaceutical Biol Chem Sci. 2018;9(6):909–923.
52. Javanmardi S, Ghojoghi A, Divband B, Ashrafi J. Titanium dioxide nanoparticle/gelatin: a potential burn wound healing biomaterial. Wounds. 2018;30(12):372–379.
53. Kotlhao K, Madiseng MDT, Mtunzi FM, et al. The synthesis of silver, zinc oxide and titanium dioxide nanoparticles and their antimicrobial activity. Adv Mater Proc. 2017;2(8):479–484. doi:10.5185/amp.2017/803
54. Masoumi S, Shakibaie MR, Gholamrezazadeh M, Monirzadeh F. Evaluation synergistic effect of TiO2, ZnO nanoparticles and amphiphilic peptides (Mastoparan-B, indolicidin) against drug-resistant Pseudomonas aeruginosa, Klebsiella pneumoniae and acinetobacter baumannii. Arch Pediatr Infect Dis. 2018;6(3):0–7. doi:10.5812/pedinfect.57920
55. Allahverdiyev AM, Kon KV, Abamor ES, Bagirova M, Rafailovich M. Coping with antibiotic resistance: combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev Anti Infect Ther. 2011;9(11):1035–1052. doi:10.1586/eri.11.121
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.