Back to Journals » Infection and Drug Resistance » Volume 15
Comparative Proteomic Analysis Reveals Antibacterial Mechanism of Patrinia scabiosaefolia Against Methicillin Resistant Staphylococcus epidermidis
Authors Liu X, An L, Ren S, Zhou Y, Peng W
Received 22 November 2021
Accepted for publication 21 January 2022
Published 6 March 2022 Volume 2022:15 Pages 883—893
DOI https://doi.org/10.2147/IDR.S350715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xin Liu,1 Lili An,2 Shuaijun Ren,2 Yonghui Zhou,1 Wei Peng1
1College of Basic Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, 550025, People’s Republic of China; 2Dermatology Department, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, 550025, People’s Republic of China
Correspondence: Xin Liu, College of Basic Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, 550025, People’s Republic of China, Tel +8618886056643, Email [email protected]
Purpose: As a kind of opportunist pathogen, Staphylococcus epidermidis (MRSE) can cause nosocomial infections and easily evolve into resistant bacteria. Among these, methicillin-resistant Staphylococcus epidermidis (MRSE) exhibit significantly higher rates. Our previous study showed that Patrinia scabiosaefolia (PS) possessed strong antibacterial activity against MRSE. However, the mechanism of PS against MRSE is not clear.
Methods: Here, a tandem mass tag-based (TMT) proteomic analysis was performed to elucidate the potential mechanism of PS against MRSE. We compared the differential expression proteins of MRSE under PS stress.
Results: Based on a fold change of > 1.2 or < 1/1.2 (with p value set at < 0.05), a total of 248 proteins (128 up-regulated proteins, 120 down-regulated proteins) were identified. Bioinformatic analysis showed that proteins including arginine deiminase (arcA), ornithine carbamoyltransferase (arcB) and carbamate kinase (arcC), serine–tRNA ligase (serS), phenylalanine–tRNA ligase beta and subunit (pheT), DltD (dlt), d-alanyl carrier protein (dlt), accumulation-associated protein (SasG), serine-aspartate repeat-containing protein C (SdrC) and hemin transport system permease protein HrtB (VraG) played important roles in mechanism of PS against MRSE.
Conclusion: In summary, these results indicated that arginine deiminase pathway (ADI) pathway, protein synthesis, cell wall synthesis, biofilm formation and uptake of iron were related to mechanisms of PS against MRSE. Our findings provide an insight into the the mechanism of PS against MRSE, and may be valuable in offering new targets to develop more anti-MRSE drugs.
Keywords: methicillin-resistant Staphylococcus epidermidis, Patrinia scabiosaefolia, proteomic
Introduction
Staphylococcus epidermidis (S. epidermidis) is a commensal Gram-positive, coagulase-negative bacterium, which colonize on the human skin.1 In recent years, S. epidermidis has gained substantial interest because it has become the most important cause of nosocomial infections.2 It is listed as one of the most often isolated bacterial in nosocomial bloodstream infections, cardiovascular infections, and infections of the eye, ear, nose, and throat by the 1998 National Nosocomial Surveillance System Report (Hospital Infections Program, National Center for Infectious Diseases, Center for Disease Control and Prevention, Public Health Service, US Department of Health and Human Services, Atlanta, GA, 1998).2 Alarmingly, its specific antibiotic resistance genes and biofilm formation make its treatment very complicated, which present a danger to human health.3 Similar to methicillin resistant Staphylococcus aureus (MRSA), methicillin-resistant S. epidermidis (MRSE) exhibit β-lactam resistance. Recently, hospital isolates of MRSE is as high as 75–90%.4 The emergence of MRSE infections emphasizes the need for antimicrobial drug design to yield new treatments before the pathogens become resistant to the last-resort antibiotics.
Since ancient times, Traditional Chinese Medicine (TCM) have a long history of use for the treatment of infectious diseases.5 There are some TCM compounds referred to as nonantibiotics from medicinal plants, which possess moderate to powerful antibacterial activities, such as berberine.6 These TCM either have direct antimicrobial activities or increase the efficacy of an antibiotic.7 In our previous study, Patrinia scabiosaefolia (PS) possesses strong antibacterial against MRSE (MIC:5mg/mL). PS has a long application story in China, which is also named “Bai Jiang Cao” recorded in the “Sheng Nong’s Herbal Classic”. Pharmacology researches showed that PS possesses sedative, antibacterial, antivirus, anti-tumor activities, and protective effects of liver and gallbladder. Meng et al reported that the extracts of PS oral liquid had significant antibacterial properties against Staphylococcus aureus, while they had different degrees of inhibitory effects on Staphylococcus albus, Streptococcus B, Escherichia coli, and Pseudomonas aeruginosa.8 However, the mechanism of PS against MRSE is not known.
Proteomics has been rapidly employed to explore the mechanisms of TCM.8,9 With recent advancement in proteomics, researchers have been to explore the mechanism of bacterial resistance mechanisms.10,11 It is useful in revealing the potential targets and analyzing major components of physiological pathways.12 Therefore, a tandem mass tag-based (TMT) proteomic analysis was performed to elucidate the potential mechanism of PS against MRSE.
Materials and Methods
Determination of Minimal Inhibitory Concentration with Patrinia scabiosaefolia and Methicillin Against Methicillin-Resistant Staphylococcus epidermidis
Methicillin-resistant Staphylococcus epidermidis (MRSE) was previously induced and preserved in our lab. Minimal inhibitory concentration (MIC) assay of Patrinia scabiosaefolia (PS) and methicillin were done as previously reported.10 Briefly, MRSE was grown overnight at 37 °C.The overnight cultures were diluted in sterile physiological saline, which correspond to 1×108 colony-forming units/mL. After that, the above cultures were diluted again with TSB till a culture concentration of 1×106 colony-forming units/mL was obtained. Finally, 100 mL of samples were added to a 96-well plate containing serial dilutions of PS (0.039–80mg/mL) or methicillin (0.0625–128μg/mL) in culture medium. Control bacterial culture and medium were cultivated in the absence of PS or methicillin. The MIC was defined as the lowest concentration of PS and methicillin to visually inhibit growth. The above assays were repeated 3 times.
TMT-Based Quantitative Proteomics Analyses
TMT-based quantitative proteomics analyses were implemented as Cao described.13 Total proteins from MRSE treated with 1/2 MIC PS (2.5 mg/mL) and untreated were extracted by the method of trichloroacetic acid (TCA)-acetone precipitation. The extracted protein were quantified by Bradford assays, using BSA as a standard. Then the protein reduction, alkylation, digestion, TMT labeling and sample cleanup were continued. Protein samples (100 μg) were solved in 100 mM TEAB to total volume 100 μL and were then treated with 10 mM tris (2-carboxyethyl) phosphine (TCEP) for 1 h at 55 °C. Afterward, they were alkylated at room temperature in the dark with 17 mM iodoacetate for 30 min. Finally, the alkylated samples were mixed with about six-time volume of ice-cold acetone and put under −20 °C overnight. The alkylated proteins were digested by Trypsin. After centrifugation at 10,000 ×g for 20 min, the supernatants were collected and freeze-dried and subsequently labeled with the tandem mass tag (TMT). The labeled samples were mixed at equal amounts and were then desalted with C18 spin tips and freeze-dried. All prepared samples were stored at −80 °C until LC–MS/ MS analysis. Furthermore Liquid chromatography–mass spectrometry (LC–MS/MS) analyses were carried out. The parameters for Ion Trap analyzer were normal mass range, rapid scan rate, and centroid data type. Finally, protein identification, quantification, classification and interaction prediction were analyzed. SEQUEST HT search engine configured with Proteome Discoverer 1.4 workflow (Thermo Fisher Scientific, Bremen, Germany) was used for mass spectrometer data analyses. The latest B. napus protein databases (downloaded from http://www.uniprot.org/ and http://www.genoscope.cns/) were configured with SEQUEST HT for searching the datasets. For quantitative analysis, a protein must have at minimum one unique peptide match with TMT ratios. A ≥1.2 or ≤1/1.2 -fold cutoff value was used to identify up-regulated and down-regulated proteins with a p value of <0.05.
Bioinformatics Analysis
Based on Gene Ontology (GO) terms, differentially expressed proteins (DEPs) were analyzed by using the online OmicsBean bioinformatics resource (http://www.omicsbean.com), which is a multi-omics data analysis tool that can be applied to dynamic results.10 The cellular component, biological process and molecular function for upregulated and downregulated proteins were enriched according to GO annotation. In addition, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway of altered proteins was categorized utilizing the same resource. In view of differential expression degree, downregulated or upregulated proteins were further divided into two groups, including Q1 (<0.769), Q2 (0.769–0.833) or Q3 (1.2–1.3>), Q4 (>1.3). The DEPs from main GO and kegg cluster were received by the above cluster analysis, and protein-protein interaction was further analyzed by string (http://www.string-db.org). Finally, the main proteins related to the mechanism of PS against MRSE were inferred.
The Quantitative RT-PCR (qRT-PCR)
In order to verify the above inference, the selected differential proteins were validated at the mRNA level by qPCR analysis. Four proteins were chosen from the altered proteins (Table 1). Meanwhile, 16sRNA was used as an internal control and the primers used for the target genes are listed in Table 2. Total RNA was extracted using Total RNA extraction (Omega, Beijing, China) and cDNA synthesis (Takara, Dalian, China) of MRSE with and without PS were done according to the manufacturer’s instructions. qRT-PCR was carried out using SYBR Premix Ex Taq on StepOne Real-Time PCR System. The reaction conditions were 94°C for 10 min followed by 40 cycles of amplification at 94°C for 15s and 60°C for 60s.10 The assays were repeated 3 times.
 |
Table 1 Proteins Chosen for Investigation of DEPs in MRSE Under PS Stress |
 |
Table 2 Primers for RT-PCR |
Each containing three replicates for all genes and the relative fold changes were calculated using the 2−ΔΔCt method as described. Significance of the differences between mean values was determined with Student’s t-test.
Statistical Analysis
Values were expressed as means ± SDs. The statistical differences among the different groups were compared by 1-way ANOVA, significant means were separated using Tukey’s Honest significant difference and p < 0.05.
Results
Minimal Inhibitory Concentration of Patrinia scabiosaefolia and Methicillin Against Methicillin-Resistant Staphylococcus epidermidis
The methicillin MIC against MRSE was 32 μg/mL. According to CLSI, the MIC of methicillin indicated resistance. Meanwhile, the PS MIC of against MRSE was 5 mg/mL. Recently, there was no standard to determine the TMC antibacterial activity. According to the literature about TMC antibacterial activities, PS showed anti-MRSE activity.14,15
A Global View of Quantitative Proteomics Analyses Employing TMT-Labeling Based Approach
To gain a global view of PS against MRSE, proteomes of MRSE inoculated with and without PS were comparatively identified using TMT-label based quantitative proteomics approach. A total of 11,891 unique peptides and 1855 proteins were identified and annotated in all samples (Supplementary Table S1). Based on a fold change of >1.2 or < 1/1.2 (with p value set at <0.05), 248 proteins were considered as differentially expressed proteins (DEPs) (Supplementary Table S2). 128 DEPs were up-regulated and 120 DEPs were down-regulated (Figure 1). According to fold change, down-regulated DEPs were further divided into Q1 (<0.769) and Q2 (0.769–0.833). Similarly, up-regulated DEPs were further divided into Q3 (1.2–1.3) and Q4 (>1.3) (Figure 1).
 |
Figure 2 Continued. |
 |
Figure 2 Continued. |
 |
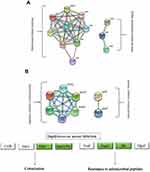
Figure 2 Go annotation and KEGG pathway of DEPs: gene ontology terms for subcellular location distribution. (A) cellular component (B) biological process (C) molecular function (D) main KEGG pathway. |
Go Annotation and KEGG Pathway Analyzed the DEPS of PS Against MRSE
Bioinformatics were used to analyze the functional clusters of DEPs of PS against MRSE. According to the Go ontology enrichment, DEPs covered a wide range of cellular components, molecular functions, and biological processes. In cellular components analysis, most of the DEPS were clustered at up-regulated (Q3), which belonged to ribosome (Figure 2A). In molecular functions analysis, most of DEPs were gathered at up-regulated (Q3 and Q4), which were related to carbon−sulfur lyase activity, cystathionine gamma−synthase activity and cystathionine gamma−lyase activity (Figure 2B). In biological processes analysis, the DEPS were averagely classified into up-regulated and down-regulated. Among these, the up-regulated DEPs were mainly clustered cellular catabolic process, peptide biosynthetic process and peptide metabolic process (Figure 2C).The down-regulated DEPs were mainly clustered at Q1, which were appropriate to cellular amino acid metabolic process and carboxylic acid metabolic process (Figure 2C). In addition, KEGG pathway analysis was applied to better understand the PS against MRSE. It can provide a comprehensive, systematic and direct understanding of cell biology and drug mechanisms of action. Generally, 21 pathways were identified as statistically significant (p < 0.05).Among these, up-regulated DEPs were mainly clustered at map03010 Ribosome, map00270 Cysteine and methionine metabolism and map00920 Sulfur metabolism. The down-regulated DEPs were mainly clustered at map00220 Arginine biosynthesis and map05150 Staphylococcus aureus infection (Figure 2D).
Interaction Network Analysis of EPs in MRSE Revealed Novel Defense Mechanisms
Based on the above Go annotation and KEGG pathway analysis, the up-regulated were major in ribosome, carbon−sulfur lyase activity, cystathionine gamma−synthase/lyase activity, cellular catabolic process, peptide biosynthetic/metabolic process, cysteine and methionine metabolism, sulfur metabolism. And then, the clustered DEPs were analyzed online by the STRING database. As shown in Figure 3A, DEPs were mainly from the two clusters including ribosome and sulfur metabolism. Similarly, the down-regulated were major in cellular amino acid metabolic process, carboxylic acid metabolic process, arginine biosynthesis and Staphylococcus aureus infection. Afterwards, DEPs apart from Staphylococcus aureus infection were analyze online by the STRING database.As shown in Figure 3B, DEPs were mainly from the two clusters, which separately constituted a related network in response to PS stress. One cluster was involved in arginine biosynthesis. Another cluster was related to protein synthesis.
Investigation of PS on Genes Expression Involved in Arginine Biosynthesis, Cell Wall Synthesis and Staphylococcus aureus Infection
To identify the veracity of proteomics data, four genes in accordance with DEPs involved in the arginine biosynthesis, cell wall synthesis and Staphylococcus aureus infection were assessed by qRT-PCR. Similar to the proteomics data, we observed an decrease in arginine deiminase (arcA), phenylalanine–tRNA ligase beta and subunit (pheT), Protein DltD (dlt) and accumulation-associated protein (SasG) (Figure 4).
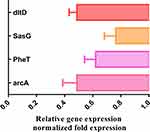 |
Figure 4 The mRNA levels of four genes were respectively analyzed by qPCR method in MRSE with and without PS. |
Discussion
S. epidermidis has ever been regarded for a long time as relatively innocuous, whereas it has now generally been accepted as a pathogen.16 And, it has become the most important cause of nosocomial infections. Nosocomial isolates of S. epidermidis are often resistant to the majority of the antimicrobials available.17 More seriously, most studies report a prevalence of methicillin resistance of around 75% occur in this species, which is often accompanied by resistance to many other antimicrobial agents belonging to different families.18 Our previous study showed PS had stronger antibacterial activity. In order to better apply PS, the mechanism of PS against MRSE should be explored. TCM, which involves the use of various herbal ingredients with multiple targets, is considered an effective alternative.19 However, it is extremely difficult to clearly identify the detailed mechanism of TCM formulas using conventional research methods. Proteomics as a powerful and systematic approach for large-scale protein analysis, is useful in revealing the potential targets or biomarkers of pharmaceutical products and analyzing major components of physiological pathways.20 And in our previous study, proteomics were already experienced in analyzing antibacterial and resistance mechanism of coagulase-negative Staphylococcus.10 So, this method was suitable to explore the mechanisms of TMC.8,9
In this study, TMT-label based quantitative proteomics approach was applied to explain the mechanism of PS against MRSE. Consequently, 128 up-regulated DEPs were identified, and mainly part of ribosome and sulfur metabolism. Ribosome was the site of protein synthesis,10 and protein were necessary for life. We inferred that the reason for the increasing translation expression can be due to the fact that the ribosome attacks the function of PS and the major role that ribosome plays in living cells.10 The expression of target subunits for survival can be seen as a strategy of MRSE against PS. Therefore, we inferred that the increasing expression ribosome can be seen as a survival strategy of MRSE against PS. In addition, sulfur is a vital element for all living organisms since it is required for the synthesis of proteins and essential cofactors.21 Our proteomics analysis showed that DEPs related to sulfur metabolism including cysteine synthase, sulfite reductase and adenylyl-sulfate kinase were up-regulated. It may be as a defensive mechanism which MRSE deploy to protect itself against the activity of PS.
In addition, 120 down-regulated DEPs were identified, and mainly belonged to arginine catabolic pathway, protein synthesis and Staphylococcus aureus infection pathway, and notably some novel potential antibacterial mechanisms of PS against MRSE were revealed. In arginine catabolic pathway, arginine deiminase (arcA), ornithine carbamoyl transferase (arcB) and carbamate kinase (arcC) were classified into arginine deiminase pathway (ADI). The ADI was one of the main arginine catabolic pathways in Gram-positive bacteria. Arginine conversion to citrulline is catalyzed by arginine deiminase, ornithine transcarbamylase phosphorolyzes citrulline to ornithine and carbamoyl phosphate, and carbamate kinase further catalyzes phosphotransfer and generates ATP, CO2, and NH 3.22 The ADI pathway can protects bacterial cells from stress conditions. In Staphylococcus aureus, the activation of the ADI pathway conveyed resistance to vancomycin.23 In addition, studies have also indicated an arcA knockout mutant Streptococcus suis weaken the survival under acidic conditions.24 Thus, the ADI pathway may play a significant role in the survival of bacteria under diverse stress conditions. In our study, arginine deiminase (arcA), ornithine carbamoyltransferase (arcB) and carbamate kinase (arcC) of MRSE were down-regulated in protein and RT-PCR under PS stress. So, We can deduce that the mechanism of PS against MRSE was related to ADI.
Next, down-regulated proteins of serine–tRNA ligase (serS), phenylalanine–tRNA ligase beta and subunit (pheT) were sorted into protein synthesis. Aminoacyl-tRNA synthetases have long been known to participate in protein synthesis25 The aminoacyl-tRNA synthetase enzymes play crucial roles in protein synthesis by catalyzing the synthesis of aminoacyl-tRNAs.26 Once these enzymes are inhibited, protein biosynthesis is halted, which in turn results in the attenuation of bacterial growth under both in vitro and infectious conditions.27 Consequently, these enzymes have been a focus of recent research for antibacterial drug. In clinical, mupirocin is currently the world’s most widely used topical antibiotic to control MRSA as a kind of inhibitor which selectively inactivates bacterial isoleucyl-tRNA synthetase.28 In our study, serine–tRNA ligase (serS), phenylalanine–tRNA ligase beta and subunit (pheT) were down-regulated at protein, which illustrated PS against MRSE by inhibiting protein synthesis.
At last, down-regulated proteins about Staphylococcus aureus infection pathway were analyzed, which were consist of Protein DltD (dlt), d-alanyl carrier protein (dlt), accumulation-associated protein (SasG), serine-aspartate repeat-containing protein C (SdrC) and hemin transport system permease protein HrtB (VraG). Among these proteins, DltD (dlt) and D-alanyl carrier protein (dlt) were part of proteins related to cell wall synthesis. The cell wall of most Gram-positive bacteria is composed of the lipoteichoic acid (LTA) and wall teichoic acid (WTA).29,30 And the dlt operon is responsible for the d-alanylation of lipoteichoic and wall teichoic acid which comprises five ORFs encoding the proteins named DltA–E.31–33 Our data showed that DltC and DltD were down-regulated, demonstrating that PS can inhibit MRSE cell wall synthesis.
In addition, serine-aspartate repeat-containing protein C (sdrC) and accumulation-associated protein (SasG) were related to biofilm formation. Biofilms are a community of microorganisms that attaches to biological and non-biological surfaces, which lead to 10–1000 times more resistant to antimicrobial agents than planktonic and recurrent infections or chronic inflammation.34 Among these, accumulation-associated protein, as a family of surface proteins, were responsible for the initial attachment with host cells and that attachment regarding as important step in biofilm formation.35 Thus, PS can control of MRSE infections by controlling attachment.
Moreover, hemin transport system permease protein HrtB was also involved in Staphylococcus aureus infection pathway. The hemin transport system in our study was belong to ABC transporter. In Staphylococcus aureus, the main iron intake is from heme-containing protein in host, and the heme from host was transported from periplasm to cytoplasm by ABC transporters.36 Iron is the essential nutrient for the growth and survival of most bacterial pathogens.36 Therefore, we induced one of mechanism of PS against MRSE by blocking the uptake of iron.
Conclusion
In summary, ADI pathway, protein synthesis, cell wall synthesis, biofilm formation and uptake of iron were related to mechanism of PS against MRSE. Our data provided a further insight into the mechanism of PS against MRSE, and may be valuable in offering new targets to develop more anti-MRSE drugs.
Acknowledgments
We thank for financial support by the earmarked funding for the Science and Technology Foundation of Guizhou Province under Grant number Qianke He Foundation -ZK[2021]General 08 and Young scientific and technological talents project of Gui zhou Department of Education under Grant number Qianjiaohe KY [2022] 269. We appreciated the supports of computer resources from the Polish National Supercomputer Center.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Ehlers MM, Wilhelmina S, Michelle L, Veronica U, Kock MM. Molecular epidemiology of Staphylococcus epidermidis implicated in catheter-related bloodstream infections at an academic hospital in Pretoria, South Africa. Front Microbiol. 2018;9:417. doi:10.3389/fmicb.2018.00417
2. Oliveira WF, Silva P, Silva R, et al. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J Hosp Infect. 2017;45:111–117. doi:10.1007/s15010-016-0941-8
3. Al-Awadi AQ, Ahmed M. Antimicrobial activity of chamomile extract against multidrug resistance Pseudomonas aeruginosa and Staphylococcus epidermidis during experimental skin infections in mice. In:
4. Lam AK, Hill MA, Moen EL, Pusavat J, Wouters CL, Rice CV. Cationic branched polyethylenimine (BPEI) disables antibiotic resistance in Methicillin-resistant Staphylococcus epidermidis (MRSE). Chem Med Chem. 2018;13(20):2240–2248. doi:10.1002/cmdc.201800433
5. Guarrera PM. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia. 2005;76(1):1–25. doi:10.1016/j.fitote.2004.09.006
6. Khayam SMU, Abdul S, Faidah HS, et al. Berberine nanoparticles with enhanced in vitro bioavailability: characterization and antimicrobial activity. Drug Des Devel Ther. 2018;12:303–312. doi:10.2147/DDDT.S156123
7. Martins M, Dastidar SG, Fanning S, et al. Potential role of non-antibiotics (helper compounds) in the treatment of multidrug-resistant Gram-negative infections: mechanisms for their direct and indirect activities. Int J Antimicrob Agents. 2008;31(3):198–208. doi:10.1016/j.ijantimicag.2007.10.025
8. Meng L, Chen S, Zhou L, Liu Z, Kang W, Kang W. Chemical constituents and pharmacological effects of Genus Patrinia: a review. Curr Pharmacol Rep. 2020;6(6):1–35. doi:10.1007/s40495-020-00240-7
9. Yao L, Yang Y, He G, Ou C, Wang L, Liu K. Global proteomics deciphered novel-function of osthole against pulmonary arterial hypertension. Sci Rep. 2018;8(1):5556. doi:10.1038/s41598-018-23775-8
10. Liu X, Wang J, Chen M, Che R, Li Y. Comparative proteomic analysis reveals drug resistance of Staphylococcus xylosus ATCC700404 under tylosin stress. BMC Vet Res. 2019;15:1. doi:10.1186/s12917-019-1959-9
11. Chang-Ro L, Hun LJ, Seung PK, Chul JB, Hee LS. Quantitative proteomic view associated with resistance to clinically important antibiotics in Gram-positive bacteria: a systematic review. Front Microbiol. 2015;6:828. doi:10.3389/fmicb.2015.00828
12. Suo T, Wang H, Li Z. Application of proteomics in research on traditional Chinese Medicine. Expert Rev Proteomics. 2016;13(9):873–881. doi:10.1080/14789450.2016.1220837
13. Cao JY, Xu YP, Cai XZ. TMT-based quantitative proteomics analyses reveal novel defense mechanisms of Brassica napus against the devastating necrotrophic pathogen Sclerotinia sclerotiorum. J Proteomics. 2016;143:265–277. doi:10.1016/j.jprot.2016.03.006
14. Luo JY, Yan D, Yang MH. Study of the anti-MRSA activity of Rhizoma coptidis by chemical fingerprinting and broth microdilution methods. Chin J Nat Med. 2014;12(5):8.
15. Chen K, Wu W, Hou X, Yang Q, Li Z. A review: antimicrobial properties of several medicinal plants widely used in Traditional Chinese Medicine. Food Qual Saf. 2021;5. doi:10.1093/fqsafe/fyab020
16. Ying D, Kg A, Ns B, Hc C, Cps A. An underestimated pathogen: staphylococcus epidermidis induces pro-inflammatory responses in human alveolar epithelial cells - ScienceDirect. Cytokine. 2019;123:154761. doi:10.1016/j.cyto.2019.154761
17. Rodríguez-Lucas C, Fernández J, Boga JA, et al. Nosocomial ventriculitis caused by a methicillin- and linezolid-resistant clone of Staphylococcus epidermidis in neurosurgical patients. J Hosp Infect. 2018;100:406–410.
18. Baos E, Candel FJ, Merino P, Pena I, Picazo JJ. Characterization and monitoring of linezolid-resistant clinical isolates of Staphylococcus epidermidis in an intensive care unit 4 years after an outbreak of infection by CFR-mediated linezolid-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2013;76(3):325–329. doi:10.1016/j.diagmicrobio.2013.04.002
19. Xing QQ, Liu LW, Zhao X, Lu Y, Liang ZQ, Liang Z-Q. Serum proteomics analysis based on label-free revealed the protective effect of Chinese herbal formula Gu-Ben-Fang-Xiao. Biomed Pharmacother. 2019;119:109390. doi:10.1016/j.biopha.2019.109390
20. Wilhelm M, Schlegl J, Hahne H, Gholami AM, Kuster B. Mass-spectrometry-based draft of the human proteome. Nature. 2014;509(7502):582–587. doi:10.1038/nature13319
21. Albanesi D, Mansilla MC, Schujman GE, Mendoza DD. Bacillus subtilis cysteine synthetase is a global regulator of the expression of genes involved in sulfur assimilation. J Bacteriol. 2005;187(22):7631–7638. doi:10.1128/JB.187.22.7631-7638.2005
22. Lin J, Luo X, Gnzle MG, Luo L. Characterization of the two nonidentical ArgR regulators of Tetragenococcus halophilus and their regulatory effects on arginine metabolism. Appl Microbiol Biotechnol. 2020;104(20):1–13. doi:10.1007/s00253-020-10868-6
23. Tan XE, Neoh HM, Looi ML, et al. Activated ADI pathway: the initiator of intermediate vancomycin resistance in Staphylococcus aureus. Can J Microbiol. 2017;63:260–264.
24. Gruening P, Fulde M, Valentin-Weigand P, Goethe R. Structure, regulation, and putative function of the arginine deiminase system of Streptococcus suis. J Bacteriol. 2006;188(2):361. doi:10.1128/JB.188.2.361-369.2006
25. Ibba M, Soll D. Aminoacyl-tRNA Synthesis. Annu Rev Biochem. 2000;69(1):617–650. doi:10.1146/annurev.biochem.69.1.617
26. Hurdle JG, O’Neill AJ, Chopra I. Prospects for aminoacyl-tRNA synthetase inhibitors as new antimicrobial agents. Antimicrob Agents Chemother. 2005;49(12):4821–4833. doi:10.1128/AAC.49.12.4821-4833.2005
27. Tao J, Wendler P, Connelly G. Drug target validation: lethal infection blocked by inducible peptide. Proc Natl Acad Sci U S A. 2000;97(2):783–786. doi:10.1073/pnas.97.2.783
28. Boyce JM. MRSA patients: proven methods to treat colonization and infection. J Hosp Infect. 2001;48(4):S9–S14. doi:10.1016/S0195-6701(01)90005-2
29. Hyyrylainen HL, Vitikainen M, Thwaite J, et al. D-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. J Biol Chem. 2000;275(35):26696–26703. doi:10.1016/S0021-9258(19)61432-8
30. Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302.
31. Perego M, Glaser P, Minutello A, Strauch MA, Leopold K, Fischer W. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. J Biol Chem. 1995;270(26):15598–15606. doi:10.1074/jbc.270.26.15598
32. Peschel A, Otto M, Jack RW, Kalbacher H, Gtz F, Götz F. Inactivation of the dlt Operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274(13):8405–8410. doi:10.1074/jbc.274.13.8405
33. Neuhaus FC, Heaton MP, Debabov DV, Zhang Q. The dlt Operon in the biosynthesis of D -alanyl-lipoteichoic acid in Lactobacillus casei. Microb Drug Resist. 1996;2(1):77–84. doi:10.1089/mdr.1996.2.77
34. Cui WQ, Qu QW, Wang JP, et al. Discovery of potential anti-infective therapy targeting glutamine synthetase in Staphylococcus xylosus. Front Chem. 2019:381. doi:10.3389/fchem.2019.00381
35. Fayad AN. The prevalence of serine-aspartate dipeptide-repeat region (sdr C and E) putative virulence gene among local isolates of Staphylococcus aureus from different sources. Univ Thi-Qar J Med. 2018;16:142–148.
36. Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 2000;54:881–941. doi:10.1146/annurev.micro.54.1.881
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.