Back to Journals » Drug Design, Development and Therapy » Volume 14
Comparative Pharmacokinetics, Bioequivalence and Safety Study of Two Recombinant Human Chorionic Gonadotropin Injections in Healthy Chinese Subjects
Authors Wang J, Yang T , Mei H, Yu X, Peng H, Wang R, Cai Y
Received 17 October 2019
Accepted for publication 31 December 2019
Published 29 January 2020 Volume 2020:14 Pages 435—444
DOI https://doi.org/10.2147/DDDT.S235064
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Jin Wang,1,* Tianli Yang,1,* Hekun Mei,1 Xueming Yu,2 Hongmei Peng,3 Rui Wang,1 Yun Cai1
1Center of Medicine Clinical Research, Department of Pharmacy, PLA General Hospital, Beijing 100853, People’s Republic of China; 2Livzon MabPharm Inc, Zhuhai, Guangdong 519045, People’s Republic of China; 3Reproductive Medicine Center, Department of Obstetrics and Gynecology, PLA General Hospital, Beijing 100853, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yun Cai
Center of Medicine Clinical Research, Department of Pharmacy, PLA General Hospital, 28 Fu Xing Road, Beijing 100853, People’s Republic of China
Tel +86-10-6693-7166
Email [email protected]
Objective: To evaluate the pharmacokinetics (PK), bioequivalence and safety profile of the recombinant human chorionic gonadotropin (r-hCG) injection formulation LZM003 (test drug) comparing with that of Ovidrel® (reference drug) in healthy Chinese subjects.
Methods: This is a randomized, single-blind, single-dose, two-arm and two-period crossover Phase I study. Subjects were randomized evenly to a single dose of LZM003 or reference drug injected subcutaneously, with a 10-day or longer between-treatment washout period. PK parameters, anti-drug antibodies (ADAs), and adverse events (AEs) were assessed. The primary PK endpoints were area under the curve (AUC) of the concentration–time curve from zero to last quantifiable concentration (AUC0-t), AUC from zero to infinity (AUC0-∞), and peak concentration (Cmax). Bioequivalence was determined by assessing whether the 90% confidence intervals (CIs) for the geometric mean ratio (GMR) of LZM003 to reference drug fell within predefined margins of 80% − 125%.
Results: Forty-eight subjects (24 males and 24 females) were enrolled and one subject withdrew for personal reasons. Mean values of primary PK parameters were similar (p > 0.05) between LZM003 and the reference drug. The 90% CIs for primary PK endpoints’ GMR of LZM003 to reference drug ranged between 0.9144 and 1.1845, which were within bioequivalence margins of 80− 125%. Incidence of AEs was similar (p > 0.05) between the two groups. Neither LZM003 nor reference drug produced anti-drug antibody (ADA) in healthy subjects.
Conclusion: LZM003 and reference drug were bioequivalent. The PK and safety assessments were similar (p > 0.05) between the two formulations in healthy Chinese subjects.
Trial Registration Number: ChiCTR-IIR-16010158 (http://www.chictr.org.cn).
Trial Registration Date: December 15, 2016.
Keywords: bioequivalence, human chorionic gonadotropin, Chinese subject, pharmacokinetics
Background
Assisted Reproductive Technology (ART) refers to the technology of using medical assistance for treating infertility, including in vitro fertilization-embryo transfer (IVF-ET), intracytoplasmic sperm injection (ICSI), embryo culture, and embryo cryopreservation.1 Controlled ovarian hyperstimulation (COH) is the first stage of IVF-ET technology which requires the administration of gonadotropins such as gonadotropin-releasing hormone agonists (GnRHa), recombinant follicle-stimulating hormone (r-FSH) and human chorionic gonadotropin (hCG) to induce the maturation of a large number of follicles.2 hCG, a heterodimer glycoprotein composed of α- and β- subunits secreted by placental trophoblast cells, acts like a superagonist of luteinizing hormone (LH).3–6 The structure of β-hCG and β-LH is similar and they can bind to the same receptor.7 Clinically, hCG has been used instead of LH to produce LH pulses which can promote follicle maturation and ovulation, thus transforming ruptured follicles into corpus luteum to stimulate progesterone secretion.8,9
Recombinant hCG (r-hCG) is an intact hCG preparation produced from Chinese Hamster Ovary (CHO) cells using recombinant technology.10,11 Compared with urinary hCG (u-hCG) that is extracted from the urine of pregnant women,12–15 r-hCG shows higher purity and batch-to-batch consistency, and can be injected subcutaneously.4,16
LZM003 (test drug) was developed by Livzon MabPharm Inc. and is an r-hCG biosimilar of Ovidrel® (reference drug). (Investigator’s brochure of LZM003. Zhuhai: Livzon MabPharm Inc, Personal communication, 2016). This phase I clinical trial was implemented to assess the pharmacokinetics (PK), bioequivalence, safety and immunogenicity profiles of LZM003 by comparing with that of the reference drug after a single subcutaneous injection in healthy Chinese subjects. To support the approval of LZM003 by CFDA, bioequivalence study is required. The design17–19 was based on literature studies, and the reference drug Ovidrel was chosen.
Materials and Methods
Formulations
Test formulations of LZM003 250 μg (lot number: 201603006; production date: March 2, 2016; expiration date: February 2018 and lot number: 201610020; production date: October 25, 2016; expiration date: September 2018) and reference formulation 250 μg (lot number BA035951; expiration date: Jan 2018) were used in this study.
Subjects
All the subjects provided written informed consent forms (ICF) prior to any study-related procedure. Healthy male and female subjects aged 18–40 years old, with a body weight of 45–75 kg and a body mass index (BMI) of 19.0–26.0 kg/m2 were enrolled in this study. All subjects were judged to be healthy by physicians based on medical history, clinical examinations, clinical laboratory tests, and electrocardiography (ECG) examination. Female subjects had a normal menstrual cycle (25–34 days). The follicle-stimulating hormone (FSH), LH, prolactin (PRL), estradiol (E2) and progesterone (P) levels in female subjects (2–3 days after the first day of their recent menstrual cycle) and FSH levels in male subjects were within the normal range or, if outside the normal range, assessed by physicians as not clinically significant. Female subjects agreed to practice contraception throughout the study period and within 1 month after the end of the study.
Subjects were excluded if any of the following conditions were present: history and/or presence of gastrointestinal, renal, hepatic, cardiovascular, hematological, respiratory, nervous, psychological, metabolic or immunological abnormalities, or any other acute/chronic disease; history and/or presence of clinical-relevant allergic disease, or allergic reaction to any component of the test or reference formulations; current clinically relevant infectious disease; pregnant or lactating; history and/or presence of endocrine abnormalities; use of any prescription or nonprescription medication within 14 days before study and/or during the course of the study; medicated within 90 days from the day of drug administration in another clinical trial; the use of any LH preparation, human menopausal gonadotropin (hMG), or hCG preparation within 90 days before study enrollment; alcohol or drug abuse, or smoked >10 cigarettes per day, currently or within 90 days before study; the loss or donation of >200 mL of blood within 90 days before study enrollment; ectopic pregnancy within 90 days before study enrollment.
Ethics
This Phase I trial (registered at http://www.chictr.org.cn: ChiCTR-IIR-16010158) was approved by the Ethics Committee (EC) of People’s Liberation Army (PLA) General Hospital (C2016-044-02) and was conducted in accordance with the Guidelines of Good Clinical Practice (GCP) 20 and the Declaration of Helsinki.21
Study Design
This is a randomized, single-blind, single-dose, two-arm and two-period crossover Phase I study. Investigators were not blinded to the study group assignments, but subjects were unaware of the treatment options. Following screening, eligible subjects were randomized evenly to receive a single dose of either LZM003 or reference drug (250 μg) subcutaneously with a 10-day washout period for men, and with days according to menstrual cycle for women (female subjects were subcutaneously administered at 3–5 days of menstruation). The subjects were checked in the Hospital on the day before drug administration, and left the center 168 hrs later (ie, on day 8, 7 days after drug administration) by completing related observations and assessments. The research flow chart is presented in Figure 1.
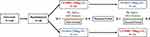 |
Figure 1 The research flow chart. Abbreviations: SC, subcutaneous injection; PK, pharmacokinetics; ADA, anti-drug antibody. |
PK Parameters
Blood samples (3 mL) from peripheral vein were collected pre-dose and at 3, 6, 9, 12, 16, 20, 24, 28, 32, 48, 72, 96, 120, 144, 168 hrs post-dose. After standing for 30 mins at room temperature, the blood samples were centrifuged (1500 g, 15 mins) at 2~8 °C to separate the serum, frozen immediately below −60 °C, and stored at −70 °C until drug concentration analysis.
Serum hCG concentrations were measured using a validated solid-phase enzyme-linked immunosorbent assay (ELISA) with the Alpco hCG ELISA kit (ALPCO Diagnostics, RN-56490/RN-56491, 25-HCGHU-E01). A standard curve (STD) was established with a lower limit of quantitation (LLOQ) of 0.20 ng/mL and an upper limit of quantitation (ULOQ) of 32.00 ng/mL. Quality controls (QC) samples were made by spiking LZM003 or the reference drug into pooled human serum (PHS) at concentrations of 24.00 (high-quality control, HQC), 5.00 (middle quality control, MQC), 0.60 (low-quality control, LQC) ng/mL. Colorimetric intensity was determined on a microplate reader at 450-nm wavelength (calibration wavelength: 620 nm).
The inter-assay accuracy and precision were calculated in at least six validation runs and displayed a coefficient of variation (CV%) values less than 20%. Overall, 130 out of 135 of reanalyzed samples (96.3%) met incurred sample reanalysis (ISR) acceptance criteria.
The serum PK parameters were determined by Bioanalytical Department, WuXi AppTec Co. Ltd, Shanghai with a non-compartmental model using WinNonlin version 6.3. The primary PK endpoints were area under the curve (AUC) of the concentration–time curve from zero to last quantifiable concentration (AUC0-t), AUC from zero to infinity (AUC0-∞), and peak concentration (Cmax). The secondary PK variables were time to Cmax (Tmax), terminal elimination half-life (t1/2), rate constant of apparent terminal elimination (λz), apparent volume of distribution (Vd), total body clearance (CL), and relative bioavailability (F) [F = AUC0-t (LZM003)/AUC0-t (reference drug) × 100%].
Immunogenicity
Blood samples (5 mL) for the measurement of anti-drug antibodies (ADAs) were collected at 10 mins pre-dose and at 168 hrs post-dose. After standing for 30 mins at room temperature, the blood samples were centrifuged (1500 g, 15 mins) at 2~8 °C to separate the serum and the serum was frozen immediately below −60 °C. The serum ADAs were analyzed by Bioanalytical Department, WuXi AppTec Co. Ltd, Shanghai.
ADAs were measured using a validated electrochemiluminescent (ECL) immunoassay method with an MSD plate reader (MESO QuickPlex SQ 120). The ECL values correspondingly reflected the levels of anti-LZM003 or anti-reference drug antibodies in samples. The immunoreaction-specific inhibition assay and titer test would be run to further confirm and measure the intensity of positive ADAs.
Safety Assessments
Safety profiles were investigated by vital signs, physical examinations, 12-lead ECG, clinical lab tests (hematology, serum biochemistry, coagulation function, hormones and routine urinalysis), adverse events (AEs) reporting, local tolerability and immunogenicity (ADAs and NAbs).
AEs were categorized and listed according to System Organ Class (SOC) and Preferred Term (PT) in the Medical Dictionary for Regulatory Activities (MedDRA, version 20.0). The severities of AEs were graded by Common Terminology Criteria for Adverse Events (CTCAE, version 4.03). All AEs were followed up until solved, stable, or subject(s) withdrew from the trial or out of contact.
Analysis Sets
The following analysis sets were employed:
- Full analysis set (FAS) for demographic and characteristic analysis that included randomized subjects receiving LZM003 and reference drug.
- Safety set (SS) for safety analysis that included subjects from FAS who had post-medication safety profiles. The analysis was based on their groups and medication cycles.
- Pharmacokinetic analysis set (PKAS) for PK analysis that included subjects from the FAS who were admittable for the calculation of Cmax, AUC0-t, AUC0-∞ and the drug absorption, distribution, metabolism and elimination and drug immunogenicity were not adversely affected.
- Bioequivalence analysis set (BEAS) for bioequivalence analysis that included subjects from PKAS who were admittable to evaluate primary PK parameters in both cycles.
- Anti-drug antibody analysis set (ADA-AS) for immunogenicity analysis that included subjects enrolled, received drugs and had immunogenicity data.
Statistical Analysis
All statistical analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc., USA). The sample size for this study was determined using a 90% power analysis performed on the basis of previous pharmacokinetic studies, which revealed 28% of %CV for Cmax.4,21 The significance level of the two one-sided t-test (TOST) was 0.05, %CV was 28%, and the dropout rate was 20%.
Demographic parameters [including age, sex, ethnic, height (cm), weight (kg), BMI (kg/m2)] were summarized by descriptive statistics. Wilcoxon rank-sum test and Fisher exact test were conducted to calculate P values between groups for continuous measurements and discrete measurements, separately.
Descriptive statistics such as mean and standard deviation, minimum, median, maximum, geometric mean and CV% were presented for PK parameters. An analysis of variance (ANOVA) model with “treatment groups, period, sequence and treatment effects” as fixed effects and “subjects within sequence effect” as a random effect was applied to the log-transformed primary PK parameters. According to the characteristics of normal theory confidence intervals (CIs), the bioequivalence test was conducted using TOST at a significance level of 5% (CIs).22 The geometric mean ratios (GMRs) and the corresponding 90% CIs between LZM003 and reference drug were estimated. If the 90% CIs for GMRs fell within predefined margins of 80–125%,22,23 the two formulations were considered bioequivalent.
Non-parametric analysis was performed to calculate the median and 90% CIs for non-transformed values of t1/2 and Tmax using the Hodges–Lehmann test. CL and Vd were summarized by formulations using descriptive statistics.
Results
Subjects
Ninety-seven out of 145 subjects who signed up for ICFs did not meet the inclusion criteria. A total of 48 subjects (24 females and 24 males) were enrolled and randomized selected to the LZM003- reference drug group and reference drug-LZM003 group evenly. The study began with 48 healthy subjects, and 47 completed the clinical study with one subject (enrolled number: 2–012, reference drug-LZM003 group) withdrew from the study after the first period of drug administration for personal reason. The subjects had the average age (mean ± SD) of 29.1 ± 4.84 years, weight (mean ± SD) of 59.85 ± 8.153 kg, and BMI (mean ± SD) of 22.30 ± 1.789 kg/m2. Demographic profiles are outlined in Table 1.
 |
Table 1 Demographic Profiles – FAS |
PK Parameters
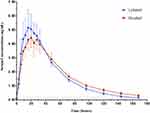
Serum hCG concentrations over time after administration of LZM003 and reference drug were analyzed based on PKAS. The mean serum concentration–time curves were superimposable between LZM003 and reference drug. The elimination of LZM003 tended to be faster than reference drug within 144~168 hrs but with no significant differences. Geometric mean (± SD) plasma concentrations of LZM003 and reference drug by log-transformation are shown in Figure 2.
PKAS included 48 (100%) subjects, and calculation of PK parameters of LZM003 included 47 subjects. After subcutaneous injection of LZM003 and reference drug, the average Cmax values were achieved at 16.00 hrs and 19.96 hrs, respectively. The mean Cmax, AUC0-t and AUC0-∞ values for LZM003 and reference drug were comparable (p > 0.05). PK parameters for the two drugs are presented in Table 2.
 |
Table 2 PK Parameters for LZM003 and Reference Drug–PKAS |
In female subjects, the mean t1/2 and Vd values in LZM003 (31.53 ± 7.52 hrs; 39.16 ± 14.08 L) group tended to be different from those in reference drug (44.41 ± 11.60 hrs; 51.66 ± 19.30 L) group. However, the mean Tmax value of LZM003 group was shorter than that of reference drug group (16.00 hrs for LZM003 and 23.99 hrs for the reference drug; p < 0.01). PK parameters by gender classification are presented in Table 3. Compared with that in males, the mean Tmax value of the LZM003 group was significantly different in females. But gender factor did not affect the bioequivalence of Tmax (Table 4). Concentration–time curves by gender classification are shown in Figures 3 and 4.
 |
Table 3 PK Parameters by Gender Classification–PKAS |
 |
Table 4 Bioequivalence Statistics for PK Parameters of LZM003 and Reference Drug–BEAS |
Bioequivalence
BEAS enrolled 47 subjects with one subject withdrew at the end of the first period. The 90% CIs for the GMRs of AUC0-∞, AUC0-t and Cmax values ranged from 0.9144 to 1.1845 and fell within the predefined range of 80.00%-125.00%, indicating LZM003 and reference drug were bioequivalent. The bioequivalence statistics for PK parameters of LZM003 and reference drug are presented in Table 4. For bioequivalence analysis of t1/2, the formulation and period factors were significantly different (p < 0.05) for the two drugs. According to non-parametric analysis, the median with 90% CIs of t1/2 was −8.94 (−11.92, −5.96), which showed that t1/2 had a significant difference (p<0.05) between LZM003 and reference drug.
Immunogenicity
No antibodies to LZM003 or reference drug were detected in all 190 serum samples. Performance of further specific inhibition assay of immunoreaction and the titer test were unnecessary.
Safety
An overview of AEs is outlined in Table 5. No serious or unexpected AEs were observed in this trial. Of all 79 treatment-emergent adverse events (TEAEs), 40 were considered drug-related. In the LZM003-reference drug group, 44 TEAEs were experienced by 21 subjects (87.5%), and 21 TEAEs experienced by 11 subjects (45.8%) were considered drug-related. In reference drug-LZM003 group, 35 TEAEs were experienced by 16 subjects (66.7%), and 19 TEAEs experienced by 11 subjects (45.8%) were considered drug-related.
 |
Table 5 Summary of Adverse Events – SS |
Difference in drug-related TEAEs between LZM003 and reference drug was significantly different (p<0.05) in the second period (34.8% vs 4.2%, P = 0.02). The drug-related TEAEs (LZM003 vs reference drug) in the first period were withdrawal bleeding (37.5% vs 4.2%), opsomenorrhea (33.3% vs 16.7%), metrorrhagia (4.2% vs 4.2%), polymenorrhea (0 vs 4.2%), sinus arrhythmia (4.2% vs 4.2%), sinus bradycardia (0 vs 4.2%), diarrhea (4.2% vs 4.2%) and abdominal pain (0 vs 4.2%). And in the second period were withdrawal bleeding (34.8% vs 0) and urine leukocyte positive (0 vs 4.2%). The incidence of withdrawal bleeding was slightly higher in subjects receiving LZM003 than those receiving the reference drug.
Discussion
The results showed that the PK profiles of single subcutaneous injection of LZM003 and reference drug in healthy Chinese subjects were similar. Neither of the drugs was immunogenic and both had similar safety profiles.
We found that both LZM003 and reference drug were of a slight difference between females and males, but with little difference in the same gender group. The absorption, distribution and elimination process of both drugs were roughly the same in males and females. But male subjects were more likely to have higher values in AUC0-∞, AUC0-t, and Cmax than female subjects. It is possible that more drugs were absorbed into males’ body circulation because more body fluids men have. The metabolic processes of LZM003 were faster than that of reference drug, because the average t1/2 and eliminating time of LZM003 were slightly shorter, and this difference was more pronounced in female subjects. The reason for this difference between the two drugs is unknown but it cannot be caused by ADAs owing to negative ADA detection.
In this trial, the t1/2 (mean ±SD) of reference drug in female subjects was 44.41 ± 11.6 hrs, which was different from the reported data. According to instructions of reference drug and previous studies on female subjects in other ethnic groups (Japanese, Korean and Caucasian),21,24 the t1/2 of reference drug was about 30 to 35 hrs. It has also reported that there were no significant differences in t1/2 of reference drug between healthy Caucasian and Japanese female subjects.24 Yet we have not found other studies which investigated the pharmacokinetics of reference drug in healthy Chinese subjects. The reason for this t1/2 difference between Chinese and other ethnic groups remains unknown. However, it is possible that pituitary down-regulation was achieved in other studies whereas it was not in our study. After pituitary gland was switched off, LH value was under the baseline and ovarian activity was suppressed in females. Because the β subunits of r-hCG and LH are the same, r-hCG is used to promote ovulation instead of LH. And we detected the β subunit of r-hCG (LZM003 and reference drug) for PK analysis. Compared with subjects who went through the down-regulation treatment, subjects in our study had a relatively high serum concentration of β subunits from endogenous LH and r-hCG injected. This might result in a relatively longer t1/2 in our study. It should also be noted that Vd was 51.66 ± 19.3 L in Chinese female subjects, higher than that reported24 in Japanese females and in Caucasian females which were 32.20 ± 24.7 L and 25.80 ± 20.3 L, respectively. We suspected that it might be related to the longer t1/2 which could cause a higher drug concentration and a wider distribution in vivo. Some PK profiles of reference drug were slightly different between male and female subjects in this study, including Tmax, t1/2, Vd and Cmax. We supposed that the differences in body weight and body mass composition might have resulted in longer Tmax and t1/2, higher Vd and lower Cmax in female subjects.
Previous studies3,21,24 have reported that some TEAEs occurred after reference drug administration in healthy subjects, including withdrawal bleeding, metrorrhagia, sinus arrhythmia, abdominal pain, diarrhea and urine leukocyte positive, yet no research has reported opsomenorrhea, polymenorrhea and sinus bradycardia. The adverse events had shown that withdrawal bleeding was more likely to happen in the LZM003 group than in the reference drug group, but all were mild (CTCAE level I). No treatment measures were taken and all bleeding were stopped naturally in the end. We suspect that fewer people enrolled in this trial which could cause great individual differences, which might be responsible for this phenomenon. And the different sensitivity to drugs might also be one of the reasons.
A Phase III study, following this trial, is ongoing which aims to determine the clinical efficacy and safety profiles for Chinese patients.
Conclusion
This study demonstrated that LZM003 was bioequivalent to reference drug in AUC and Cmax value, and the PK profiles of the reference drug were in line with the results of other ethnic groups. A single subcutaneous injection of either LZM003 or reference drug was well tolerated in healthy Chinese subjects.
Abbreviations
ADA, anti-drug antibody; ADA-AS, Anti-drug antibody analysis set; AE, adverse event; ANOVA, analysis of variance; ART, assisted reproductive techniques; AUC0-∞, area under the curve between 0 and infinity; AUC0-t, serum concentration until the last concentration observed; BEAS, bioequivalence analysis set; BMI, body mass index; CHO, Chinese hamster ovary; CI, confidence interval; CL, total body clearance; Cmax, peak of concentration; CTCAE, Common Terminology Criteria for Adverse Events; E2, estradiol; EC, Ethics Committee; ECG, electrocardiography; ECL, electrochemiluminescent; ELISA, enzyme-linked immunosorbent assay; F, relative bioavailability; FAS, full analysis set; FSH, follicle stimulating hormone; GCP, good clinical practice; GMR, geometric mean ratio; GMR, geometric mean ratio; hCG, human chorionic gonadotropin; hMG, human menopausal gonadotropin; HQC, high quality control; ICF, informed consent forms; ISR, incurred sample re-analysis; LH, luteinizing hormone; LLOQ, lower limit of quantitation; LQC, low quality control; MedDRA, Medical Dictionary for Regulatory Activities; MQC, middle quality control; NAb, neutralizing antibody s; P, progesterone; PHS, pooled human serum; PK, pharmacokinetics; PKAS, pharmacokinetic analysis set; PLA, People’s Liberation Army; PRL, prolactin; PT, Preferred Term; QC, quality control; r-hCG, recombinant hCG; SD, standard deviation; SOC, System Organ Class; SS, safety set; STD, standard curve; t1/2, terminal elimination half-life; TEAE, treatment-emergent adverse event; Tmax, time to Cmax; TOST, two one-sided t-tests; u-hCG, urinary hCG; ULOQ, upper limit of quantitation; Vd, apparent volume of distribution; λz, rate constant of apparent terminal elimination.
Data Sharing Statement
We used electronic case record form (eCRF) to collect individual identified participant data (IPD) in this trial. We are not ready to share deidentified participant data until National Medical Products Administration (NMPA) is approved for LZM003. However, the protocol of this trial is available by searching the registration number ChiCTR-IIR-16010158 at http://www.chictr.org.cn.
Disclosure
The authors report no conflicts of interest in this work.
References
1. DeAngelis AM, Martini AE, Owen CM. Assisted reproductive technology and epigenetics. Semin Reprod Med. 2018;36:221–232. doi:10.1055/s-0038-1675780
2. Zhang Y, Wu QF. The clinical research and development of human assisted reproductive technology. J Int Reprod Health Fam Plan. 2012;31(2):108–114.
3. Trinchard-Lugan I, Khan A, Porchet HC, Munafo A. Pharmacokinetics and pharmacodynamics of recombinant human chorionic gonadotrophin in healthy male and female volunteers. Reprod Biomed Online. 2002;4(2):106–115. doi:10.1016/S1472-6483(10)61927-X
4. van der Merwe R, Lugan I, Lecuelle H, Papasouliotis O, Buraglio M. The bioequivalence of liquid and freeze-dried formulations of recombinant human chorionic gonadotrophin. Curr Med Res Opin. 2004;20(3):397–402. doi:10.1185/030079904125003044
5. Wu H, Lustbader JW, Liu Y, Canfield RE, Hendrickson WA. Structure of human chorionic gonadotropin at 2.6 a resolution from MAD analysis of the selenomethionyl protein. Structure. 1994;2(6):545–558. doi:10.1016/S0969-2126(00)00054-X
6. The U.S. Multicenter Study 7927 Investigator Group, Chang P, Kenley S, Burns T, et al. Recombinant human chorionic gonadotropin (rhCG) in assisted reproductive technology: results of a clinical trial comparing two doses of rhCG (Ovidrel) to urinary hCG (Profasi) for induction of final follicular maturation in in vitro fertilization–embryo transfer. Fertil Steril. 2001;76(1):67–74. doi:10.1016/s0015-0282(01)01851-9.
7. Fournier T, Guibourdenche J, Evain-Brion D. Review: hCGs: different sources of production, different glycoforms and functions. Placenta. 2015;36:S60–65. doi:10.1016/j.placenta.2015.02.002
8. Ryan RJ, Charlesworth MC, McCormick DJ, Milius RP, Keutmann HT. The glycoprotein hormones: recent studies of structure-function relationships. FASEB J. 1988;2(11):2661–2669. doi:10.1096/fasebj.2.11.2456242
9. Ludwig M, Doody KJ, Doody KM. Use of recombinant human chorionic gonadotropin in ovulation induction. Fertil Steril. 2003;79(5):1051–1059. doi:10.1016/S0015-0282(03)00173-0
10. Thennati R, Singh SK, Nage N, et al. Analytical characterization of recombinant hcg and comparative studies with reference product. Biologics. 2018;12:23–35. doi:10.2147/BTT.S141203
11. Ovidrel®. PreFilled Syringe (Choriogonadotropin Alfa Injection) [Prescribing Information]. Rockland: EMD Serono, Inc; 2018.
12. Hugues JN. Comparative use of urinary and recombinant human chorionic gonadotropins in women. Treat Endocrinol. 2004;3(6):371–379. doi:10.2165/00024677-200403060-00005
13. Zegers-Hochschild F, Fernández E, Mackenna A, Fabres C, Altieri E, Lopez T. The empty follicle syndrome: a pharmaceutical industry syndrome. Hum Reprod. 1995;10(9):2262–2265. doi:10.1093/oxfordjournals.humrep.a136281
14. Morse JH, Lustbader JW, Harrington JW, Canfield RE. Heterogeneity of proteins in commercial preparations of Human Chorionic Gonadotropin (hCG) demonstrated by western blotting. Am J Reprod Immunol Microbiol. 1988;17:134–140. doi:10.1111/j.1600-0897.1988.tb00217.x
15. Bassett R, De Bellis C, Chiacchiarini L, et al. Comparative characterisation of a commercial human chorionic gonadotrophin extracted from human urine with a commercial recombinant human chorionic gonadotrophin. Curr Med Res Opin. 2005;21(12):1969–1976. doi:10.1185/030079905X75005
16. Chan CC, Ng EH, Tang OS, Yeung WS, Lau EY, Ho PC. A prospective, randomized, double-blind study to compare two doses of recombinant human chorionic gonadotropin in inducing final oocyte maturity and the hormonal profile during the luteal phase. J Clin Endocrinol Metab. 2005;90(7):3933–3938. doi:10.1210/jc.2004-2169
17. European Medicines Agency. Guideline on similar biological medicinal products containing monoclonal antibodies [Draft]. 2010. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-similar-biological-medicinal-products-containing-monoclonal-antibodies_en.pdf.
18. China Food and Drug Administration. Guideline for the study of bioavailability and bioequivalence of chemicals in human body. 2005. Available from: http://samr.cfda.gov.cn/directory/web/WS01/images/u6Rp9KpzuWxrzByMvM5cn6zuA+9PDtsi6zcn6zu+1yNCn0NTR0L6vLzK9da4tbzUrdTyLnBkZg==.pdf.
19. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Veterinary Medicine. Guideline for industry bioequivalence: blood level bioequivalence study. 2016. Available from: https://www.fda.gov/media/89840/download.
20. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised guideline. Integrated Addendum to ICH E6(R1): guideline for Good Clinical Practice E6 (R2). 2016. Available from: https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf.
21. Cha YJ, Kim KA, Oh TY, Park JY. Pharmacokinetics and safety profile of DA-3803, a proposed biosimilar of recombinant human chorionic gonadotropin, in healthy subjects. BioDrugs. 2015;29(3):199–205. doi:10.1007/s40259-015-0128-3
22. World Medical Association. World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
23. Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15(6):657–680. doi:10.1007/BF01068419
24. Bagchus W, Wolna P, Uhl W. Single-dose pharmacokinetic study comparing the pharmacokinetics of recombinant human chorionic gonadotropin in healthy Japanese and Caucasian women and recombinant human chorionic gonadotropin and urinary human chorionic gonadotropin in healthy Japanese women. Reprod Med Biol. 2018;17(1):52–58. doi:10.1002/rmb2.12066
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.