Back to Journals » Medical Devices: Evidence and Research » Volume 14
Comparative in-vitro Study of the Trachospray, a New Device for Topical Anaesthesia of the Upper Airway
Authors van Geffen GJ, Markerink H, van Barneveld M , Verhoeven F, Scheffer GJ, Bruhn J
Received 20 November 2020
Accepted for publication 29 December 2020
Published 22 January 2021 Volume 2021:14 Pages 9—14
DOI https://doi.org/10.2147/MDER.S292529
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Geert-Jan van Geffen,1 Hielke Markerink,1 Marc van Barneveld,2 Frank Verhoeven,3 Gert Jan Scheffer,1 Jörgen Bruhn1
1Department of Anesthesiology, Pain and Palliative Care, Radboud University Medical Centre (RUMC), Nijmegen, The Netherlands; 2Medspray Anesthesia BV, Enschede, The Netherlands; 3Medspray T&M, Enschede, The Netherlands
Correspondence: Geert-Jan van Geffen
Department of Anesthesiology, Pain and Palliative Care, Radboud University Medical Center, Internal Mail 717, P.O. Box 9101, Nijmegen, HB 6500, The Netherlands
Tel +31 24 36 15 24 4
Email [email protected]
Background: Obtaining complete topical anaesthesia of the airway remains a clinical challenge. Particle size is one of the most important variables for the dose deposited and the distribution of aerosols in the airways. The mass median aerodynamic diameter of the particles should be in the range of 5– 20 μm. We developed the “Trachospray” as a soft mist spray device for local anaesthetics. This in-vitro comparative test was designed to compare the performance of the new Trachospray device with two existing medical devices. The performance was determined by comparing the spray deposition patterns in the mouth, throat, trachea and lungs.
Methods: The human airway was simulated with an artificial idealized mouth and throat model, connected to a Next Generation Impactor. Four measurements were taken for each device (Trachospray, jet nebulizer and a spray pump) with 5.85% NaCl. A fifth measurement was carried out with 0.5% fluorescein solution for a visual inspection of the deposition patterns. The mass median aerodynamic diameter and geometric standard deviation of the droplets were measured.
Results: The Trachospray produced an even coverage in the mouth, hypopharynx and vocal cords, with only a small lung fraction. The jet nebulizer produced a much thinner layer coverage of the tongue and surface around the vocal chords with a high lung deposition. The spray pump produced big droplets which deposited mainly at the hypopharynx.
Conclusion: The Trachospray device deposits local anaesthetics in the targeted areas for topical anaesthesia of the airway and has promising characteristics for providing effective airway anaesthesia.
Keywords: airway model, geometric standard deviation, mass median aerodynamic diameter, medical device, topical airway anaesthesia
Introduction
To provide comfort, effective and fast topical anaesthesia of the upper airway is of paramount importance in bronchoscopy, awake fibreoptic intubations and other awake instrumentations of the airway. There are numerous techniques to anaesthetize the airway, but obtaining complete anaesthesia of the airway remains a challenge.1 Three reflexes need to be blocked: the gag reflex, glottic closure reflex and cough reflex. Topical airway anaesthesia blocks nerve conduction by spreading the local anaesthetic over the mucosa to achieve local uptake of local anaesthetics. This causes a conduction block by targeting free nerve endings in the mucosa, thereby producing a temporary loss of sensation.
The calibre and tortuosity of the airway influence the flow of air through the segment and thereby also affect aerosol particle impaction and topical anaesthesia.2 Particle size is one of the most important variables in defining the dose deposited and the distribution of drug aerosol in the airways.3 Fine aerosols with particles <3–5 μm are distributed mainly on peripheral airways and do not make a contribution to anaesthetizing the airway that is relevant for anaesthesiologists.3 Larger particle aerosols (>5 μm), which deposit more drug per unit surface, are deposited on the larger central airways.2,3 But particles that are too large deposit only proximally in the hypopharynx and also do not contribute to anaesthetizing the airway in a way relevant for anaesthesiologists.
Therefore, ideally a device should produce particles with a mass median aerodynamic diameter (MMAD) in the range of 5–20 µm.3 These aerosol characteristics are mainly determined by the drug formulation and the atomizer/nebulizer device.2
Atomization achieves a denser airway anaesthesia compared with other techniques such as nebulization.4 Nebulization may result in the administration of large doses of local anaesthetic and may produce unpredictable anaesthesia because of the loss of local anaesthetic to the atmosphere.5
With atomization, a high-powered mist of local anaesthetic is directed to the tissues to be anaesthetized. A major advantage of atomization compared to nebulization is the rapidity with which the airway can be anaesthetized. Airway anaesthesia may be obtained in 5 minutes, compared to topicalization via nebulization which may take more than 20 minutes.6
In order to obtain an effective and fast topical anaesthesia of the upper airway, we designed an aerosol-generating inhalator suitable for topical anaesthesia of the airway, the “Trachospray device”. This device is designed to obtain optimal droplet size and flow patterns in order to achieve deposition of local anaesthetic aerosols in the relevant anatomical zones to be anaesthetized.
This in-vitro comparative test is designed to compare the performance of the new Trachospray device with two existing medical devices. The performance is determined by comparing the spray deposition patterns in the mouth, throat, trachea and lungs of an artificial idealized airway model.
Methods
Equipment
This in-vitro comparative test is designed to measure the performance of three airway topical anaesthesia medical devices: Trachospray (Medspray Anesthesia BV, Enschede, The Netherlands), the Omron Jet nebulizer with mouth piece (Omron Healthcare Co., Ltd, Kyoto, Japan) and the Acoin spray pump (Combustin pharmazeutische Präparate GmbH, Hailtingen, Germany).
The Trachospray device is an innovative new soft mist spray device for local anaesthetics, featuring a patented spray nozzle unit to produce a specific droplet size. The designed mouth piece gives a controlled flow pattern that enables the reliable deposition of local anaesthetics (Figures 1 and 2).
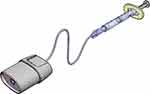 |
Figure 1 Trachospray with mouth piece, patented spray nozzle, and extension tube with finger grip. |
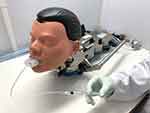 |
Figure 2 The Trachospray device connected to an Alberta Idealized Throat and a Next Generation Impactor. |
The performance of the medical devices was determined by comparing the spray deposition patterns in mouth, throat, trachea and lungs. The human mouth and throat were simulated with an artificial idealized mouth and throat model, Alberta Idealized Throat (AIT) (Copley Scientific, Colwick, Nottingham, UK), connected to a Next Generation Impactor (NGI) (Copley Scientific, Colwick, Nottingham, UK), as is usual in the pharmaceutical industry. Figure 2 shows the test set-up.
The AIT was developed as a result of extensive research into typical patient populations, including information provided by CT and MRI scans.
The NGI, or cascade impactor, is a precision instrument that separates a spray/aerosol sample on the basis of particle inertia. The air flow passes through the impactor in a sawtooth pattern. Particle sizing is achieved by successively increasing the velocity of the air stream by forcing it through a series of nozzles containing progressively reducing jet diameters.
In addition, the MMAD and geometric standard deviation (GSD) of the droplets generated by the medical devices were measured with the NGI at 15 L/min flow in combination with the AIT.
Measurements
Four measurements were performed for each device with 0.3 mL of 5.85% NaCl (representing the viscosity of 4% lidocaine). A fifth measurement for each device was taken with 0.5% fluorescein solution for a visual inspection of the deposition patterns. Each device was separately attached to the AIT. The dispensed volume was kept identical for each device, as far as possible. For the Trachospray, a continuous flow of 15 L/min was passed through the AIT and NGI, and the device was used for 10 seconds. For the Omron Jet nebulizer, a continuous flow of 15 L/min was passed through the AIT and NGI, and the device was used for 120 seconds. For the Acoin spray pump, no airflow was used and three pump strokes were sprayed, conforming to the normal use of the device.
A flow of 15 L/min is the average flow rate during inhalation through an airflow resistance (2.5 mm). This airflow resistance is comparable to the resistance due to the mouth piece of the Omron Jet nebulizer and Trachospray while tidal breathing.
The depositions on the different areas of the AIT and NGI (mouth, throat and eight lung stages) were cleaned and the liquid was diluted to a fixed volume. The concentration of each volume was measured and the total amount of deposition on each stage was calculated. In order to quantify the location of the deposited mass inside the AIT, this part was flushed in two parts. One part was defined as the mouth, while the lower part was defined as the throat.
Statistical Analysis
No formal sample size calculation was performed, owing to the lack of previous data and the pilot character of this study. But because of the huge differences in deposition pattern between the three devices in various test runs, a sample size of four measurements for each device was estimated to be a sufficient sample size. Statistical analysis of the data was performed using SPSS 25 software for Windows (IBM Corp., Armonk, NY, USA). Descriptive statistical methods were used (mean and standard deviation). The independent t-test was used to compare the results of the three devices. A p-value <0.05 was seen as statistically significant.
Results
The Trachospray soft mist spray inhaler device produced an even coverage in the mouth, hypopharynx and vocal cords (Figure 3). It was found that 67.23% (SD 2.84) of the dose was deposited in the mouth region, 18.50% (SD 2.48) was deposited in the pharynx region and 13.35% (SD 1.18) passed the throat and reached the lung. The particle size of the droplets generated by this device, as measured with the NGI in combination with the AIT, had an MMAD of 12.4 μm (SD 0.28) and a GSD of 1.55 (SD 0.01) (see also Table 1).
 |
Figure 3 Pictures taken from opened Alberta Idealized Throat model halves. |
 |
Table 1 Overview of Results |
The Omron Jet nebulizer produced a much thinner layer coverage of the tongue and surface around the vocal chords, with a high lung deposition (Figure 3). Only 1.29% (SD 0.12) of the dose of local anaesthetics was deposited in the mouth region, 1.83% (SD 0.21) was deposited in the pharynx region and 96.10% (SD 0.18) passed the throat and reached the lung. The particle size of the droplets generated by this device, as measured with the NGI in combination with the AIT, had an MMAD of 2.98 μm (SD 0.11) and a GSD of 2.03 (SD 0.02).
The Acoin spray pump with swirl nozzle produced big droplets which were deposited mainly at the hypopharynx of the AIT (Figure 3). We found that 22.68% (SD 24.93) of the dose was deposited in the mouth region, 68.76% (SD 23.86) was deposited in the pharynx region and 8.57% (SD 3.90) passed the throat and reached the lung.
Discussion
This in-vitro- study was performed to measure the performance of three airway topical anaesthesia medical devices. The Trachospray soft mist spray inhaler device produced an even coverage in the mouth, hypopharynx and vocal cords, with only a small lung deposition. The Omron Jet nebulizer produced a thin layer coverage with a high lung deposition. The Acoin spray pump with swirl nozzle produced big droplets which were deposited mainly at the hypopharynx. Owing to the mechanism of action of the Acoin spray pump, there was a large variation in the deposits, as seen in the standard deviations. Therefore, accurate dosing of the local anaesthetic fluid and reaching the target areas seem nearly impossible.
However, a limitation of this study is that no flow has been used while testing the Acoin spray pump on the AIT and NGI. In clinical use, there may be a minimal flow. However, this flow would be so negligible that it would not have affected our test results.
In many studies, atomizing, nebulizing and spraying devices were evaluated in awake fibreoptic intubations,7–9 but none has evaluated the performances of the different techniques and devices in a laboratory setting. Nor has the spreading pattern of local anaesthetic upon inhalation been evaluated. In pulmonary medicine, the AIT is widely used as an airway model for the development and evaluation of inhaled medications.10,11
In anaesthesia, the airway model (AIT with NGI) is largely unknown. There are no previous studies comparing the performance and deposition patterns of topical anaesthesia medical devices in an artificial airway model.
Based on this laboratory evaluation, we speculate that the Trachospray will provide optimal topical airway anaesthesia. The next step will be to test the Trachospray in awake volunteers as proof of concept. After anaesthetizing the airway with the Trachospray, a fibrescope will be passed through the vocal cords while measuring the resting degree of airway reactivity. In the following step, the degree of airway anaesthesia with the Trachospray will be compared to conventional methods of anaesthetizing the airway, such as pharyngeal application of lidocaine with a lidocaine spray,9 nebulized lidocaine7 or using a lidocaine spray catheter.8
Moreover, the Trachospray may provide a standardized uniform method of anaesthetizing the airway independent of its users. The clinical relevance of a fast and effective topical anaesthesia cannot be overstated. It enhances patient comfort during awake instrumentation of the airway, eg, during awake fibreoptic intubation. Even more importantly, this may change the indication for awake techniques in airway management in the non-urgent setting. Nowadays, in most institutions only patients with a known difficult airway are managed with an awake technique. But it can be hypothesized that beside patients with a known difficult airway and patients with a known easy airway, there is a grey zone with patients with a certain possibility of a difficult airway. Just hoping and relying on personal manual skills might lead to life-threatening situations, such as cannot intubate–cannot ventilate. A fast and effective topical anaesthesia of the airway could substantially lower the threshold for awake airway techniques with a fibrescope or a video laryngoscope. This may lead to higher patient safety and could also change the way in which anaesthesia is induced. A sedative dosing of an opioid, eg, low-dose sufentanil or remifentanil, with midazolam or low-dose propofol in combination with the topical anaesthesia may enable a comfortable awake airway technique in a spontaneously breathing patient as a standard technique. Then, only a low induction dose without muscle relaxant would be needed for anaesthesia induction.
In conclusion, this in-vitro study demonstrated that the Trachospray device deposits local anaesthetics in the targeted areas for topical anaesthesia of the upper airway, and thus has promising characteristics for providing effective airway anaesthesia. Further research is needed to demonstrate its results in humans.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
Equipment was provided by Medspray Anesthesia BV, Enschede, The Netherlands.
Disclosure
G.J. van Geffen, M. van Barneveld and J. Bruhn have a patent pending for the Trachospray device. G.J. van Geffen, H. Markerink and J. Bruhn report non-financial support from Medspray Anesthesia, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Doyle DJ. Airway anesthesia: theory and practice. Anesthesiol Clin. 2015;33(2):291–304. doi:10.1016/j.anclin.2015.02.013
2. Darquenne C, Fleming JS, Katz I, et al. Bridging the gap between science and clinical efficacy: physiology, imaging, and modeling of aerosols in the lung. J Aerosol Med Pulm Drug Deliv. 2016;29(2):107–126. doi:10.1089/jamp.2015.1270
3. Douglas SG, Burnett D, Strickland S, Myers TR. MBA: A Guide to Aerosol Delivery Devices for Respiratory Therapist.
4. Wieczorek PM, Schricker T, Vinet B, Backman SB. Airway topicalisation in morbidly obese patients using atomised lidocaine: 2% compared with 4%. Anaesthesia. 2007;62(10):984–988. doi:10.1111/j.1365-2044.2007.05179.x
5. Kundra P, Kutralam S, Ravishankar M. Local anaesthesia for awake fibreoptic nasotracheal intubation. Acta Anaesthesiol Scand. 2000;44(5):511–516. doi:10.1034/j.1399-6576.2000.00503.x
6. Xue FS, Liu HP, He N, et al. Spray-as-you-go airway topical anesthesia in patients with a difficult airway: a randomized, double-blind comparison of 2% and 4% lidocaine. Anesth Analg. 2009;108(2):536–543. doi:10.1213/ane.0b013e31818f1665
7. Madan K, Biswal SK, Tiwari P, et al. Nebulized lignocaine for topical anaesthesia in no-sedation bronchoscopy (NEBULA): a randomized, double blind, placebo-controlled trial. Lung India. 2019;36(4):288–294. doi:10.4103/lungindia.lungindia_348_18
8. Venkatnarayan K, Devaraj U, Krishnaswamy UM, et al. Comparison of spray catheter with “spray-as-you-go” technique for airway anesthesia during flexible bronchoscopy - a randomized trial. Lung India. 2020;37(5):384–388. doi:10.4103/lungindia.lungindia_528_19
9. Madan K, Mittal S, Mohan A. Lignocaine delivery for topical anesthesia during bronchoscopy: recent advances. Lung India. 2020;37(5):449–450. doi:10.4103/lungindia.lungindia_558_20
10. Ciciliani AM, Langguth P, Wachtel H. In vitro dose comparison of Respimat® inhaler with dry powder inhalers for COPD maintenance therapy. Int J Chron Obstruct Pulmon Dis. 2017;12:1565–1577. doi:10.2147/COPD.S115886
11. Ruzycki CA, Golshahi L, Vehring R, et al. Comparison of in vitro deposition of pharmaceutical aerosols in an idealized child throat with in vivo deposition in the upper respiratory tract of children. Pharm Res. 2014;31:1525. doi:10.1007/s11095-013-1258-2
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
