Back to Journals » Infection and Drug Resistance » Volume 16
Comparative Evaluation of LAMP and Nested PCR for the Rapid Diagnosis of Mycobacterium marinum Infection
Authors Feng Y , Wang M, Jiang H, Shi Y , Zhang W, Wang H
Received 15 January 2023
Accepted for publication 15 March 2023
Published 20 March 2023 Volume 2023:16 Pages 1601—1609
DOI https://doi.org/10.2147/IDR.S404929
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yumiao Feng,1,2,* Miaomiao Wang,2,3,* Haiqin Jiang,2 Ying Shi,2 Wenyue Zhang,2 Hongsheng Wang2
1Department of Dermatology, the Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, People’s Republic of China; 2Department of Mycobacterium, Jiangsu Key Laboratory of Molecular Biology for Skin Diseases and STIS, Institute of Dermatology & Hospital of Skin Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Nanjing, People’s Republic of China; 3Department of Dermatology, the First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongsheng Wang, Institute of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College, No. 12 Jiangwangmiao Street, Nanjing, People’s Republic of China, Tel +86 025 8547 8953, Email [email protected]
Purpose: Culture of Mycobacterium marinum is very time-consuming, taking several weeks to produce positive results. Seeking rapid and sensitive diagnostic methods for diagnosis can greatly improve patient treatment. Our study aimed to compare the rapid diagnostic abilities of polymerase chain reaction (PCR), nested PCR and loop mediated isothermal amplification (LAMP) of detecting M. marinum in skin samples from patients with M. marinum infection.
Methods: A total of 6 M. marinum strains and 6 skin samples with definite diagnosis of M. marinum infection were included in the study. We optimized LAMP performance for detection of M. marinum genomic DNA and confirmed the specificity of the primers. Then, the sensitivity of the LAMP and nested PCR assays were assessed by M. marinum strains and clinical samples.
Results: Nested PCR was 10-fold more sensitive than the LAMP assay by serial dilution of M. marinum DNA. PCR positive samples were all positive by LAMP detection of 6 clinical M. marinum strains. Out of 6 clinical skin specimens confirmed as M. marinum infection, 0 (0%), 3 (50%), 3 (50%), and 4 (66.6%) were positive by PCR, nested PCR, LAMP and culture. The LAMP shared the same sensitivity than nested PCR in M. marinum strains and clinical samples, but it was easy to perform and faster than nested PCR assay.
Conclusion: Compared with conventional PCR, LAMP and nested PCR are more sensitive and have a higher detection rate of M. marinum in clinical skin specimens. The LAMP assay proved to be more suitable for rapid diagnosis of M. marinum infection in a shorter time, especially in resource-limited settings.
Keywords: Mycobacterium marinum, diagnosis, PCR, nested PCR, LAMP
Introduction
Mycobacterium marinum is a nontuberculous mycobacterium (NTM) that lives in fresh or salt water with worldwide distribution.1 It causes infections in fish and can also cause cutaneous infections in humans.2 Human infection follows contact with fish or contaminated water and is often described as “swimming pool granuloma” or “fish tank granuloma”.3 It usually occurs in the upper extremities and presents as cutaneous nodules or plaques with or without ulceration, occasionally with a sporotrichoid pattern.4 Despite an increase in the number of cases in recent years, M. marinum infection is often unrecognized or misdiagnosed as fungal or tuberculosis infection due to nonspecific lesions and atypical histopathology. Diagnosis of M. marinum infection is usually based on isolation of M. marinum. However, culture of mycobacterium is very time-consuming, taking several weeks to produce positive results. Therefore, diagnosis of M. marinum infection remains a challenge, with a considerable time delay between onset of symptoms and diagnosis.1
Recent advances in molecular methods based on PCR technique allow rapid detection of mycobacteria species directly in the clinical sample. However, in extra-pulmonary specimens, there is a lack of sensitivity of conventional PCR techniques as they are mostly paucibacillary in nature.5 Another major limitation of single-step PCR is the presence of PCR inhibitors that inhibit the amplification.5 Nested-PCR, with two steps, can eliminate/dilute the inhibitors present in the clinical specimens and has higher sensitivity and specificity than the conventional single PCR.6 These PCR methods also have limitations and require sophisticated equipment and skills, which are inaccessible to resource-constrained areas.
Loop-mediated isothermal amplification (LAMP) offers an alternative DNA amplification method, which amplifies DNA with high specificity and efficiency under isothermal conditions.7 Compared with the PCR-based assays, LAMP is more resistant to PCR inhibitors and can be carried out using a simple water bath with a shorter reaction time. Many studies have shown that the LAMP assay is a good substitute for conventional PCR-based methods for its rapidity, sensitivity, and uniform temperature requirements, making it more suitable than conventional PCR and other PCR strategies (nested PCR and real-time qPCR), thereby providing on-site detection of a pathogen without requiring sophisticated equipment.8 This technique developed by Tsai can detect about 7–70 copies of genomic DNA of M. marinum with high sensitivity,9 but it needs to be further verified in clinical skin specimens.
Therefore, the aim of this study is to compare the performances of LAMP with nested PCR assay to determine which was more suitable for rapid diagnosis of M. marinum infection.
Materials and Methods
Clinical Skin Specimens
A total of 6 skin specimens were obtained from M. marinum infection cases admitted to the Institute of Dermatology (Jiangsu, China) from August 2021 to February 2022. The diagnosis was based on history (eg contact with fish and aquaria), clinical manifestations, histopathology and pathogen proof (by culture or molecular methods). Skin tissues were subjected to grinding processing by burnisher treatment. A part was inoculated on Lowenstein Jensen (L-J) slopes for mycobacterium culture and the remaining subsequent concentration by high-speed centrifugation for DNA extraction.
Strains and Genomic DNA Preparation
Genomic DNAs used for evaluation of primer specificity were prepared from 7 reference strains representing M. marinum and 6 mycobacterial species. Genomic DNAs of 7 mycobacteria, ie M. marinum, M. tuberculosis, M. leprae, M. bovis, M. avium, M. intracellulare, M. smegmatis, were extracted when acid-fast staining and PCR detected present mycobacteria. In addition, six clinical strains of M. marinum were selected for specificity and sensitivity validation. DNA was extracted from strains and skin biopsies using QIAamp DNA Microbiome kit (Qiagen, Germany) according to the manufacturer’s instructions. Extracted DNA was measured with a NanoDrop 2000 spectrophotometer (Thermo Scientific).
PCR and Nested PCR
PCR and nested PCR based on the amplification of the rpoB genes of M. marinum were performed using the primers listed in Table 1. The total reaction volume in the PCR was 25 μL and contained 12.5 μL 2× Taq Plus Master Mix II (Vazyme, China), 0.5 μM of each primer, and 2 μL of template DNA. The PCR amplification conditions are as follows: denaturation at 95°C for 5 min and 35 cycles of 95°C for 30s, 62°C for 50s and 72°C for 1 min, followed by a final 10 min extension at 72°C. The nested PCR was performed in a total volume of 50 μL of PCR reaction mix containing 1 μL of PCR products from first PCR, 25 μL of 1.25 U of Taq DNA polymerase mix, 2 μL of 5 pmol each of primers, and 22 μL RNase-free water. PCR was performed in a T100 thermocycler (Bio-Rad, USA). The reference strain of M. marinum was used as positive control. The products of each PCR assay were analyzed by gel electrophoresis on 1.5% agarose stained with ethidium bromide. The positive PCR products were sequenced, and the sequences were compared with the nucleotide database in GenBank at NCBI (www.ncbi.nlm.gov/blast/).
 |
Table 1 Primers Used in the Study |
LAMP
LAMP was performed using formerly reported primers targeting mrsA gene of M. marinum complex, including a restriction polymorphism between M. marinum and M. ulcerans (Table 1).9 LAMP amplification kit (Biolab, China) was used according to the manufacturer’s instruction. The 20 μL reaction mixture contained 15 μL LAMP OTG Reagent, 1.6 µM each of FIP and BIP, 0.2 µM each of F3 and B3, and 2 μL of template DNA. Reaction tubes were incubated in a water bath at 63°C for 60 min. Then, invert the reaction tube to dissolve the OTG (Orange to Green) dye, pre-adhered to the tube cover in the form of dry powder, to terminate the reaction. M. marinum DNA extract was used as the positive control, and a reaction tube with additional pure water instead of the DNA template was used as the negative control. The solution turned green in the presence of a LAMP amplicon but remained orange if no amplification occurred. The amplicon was confirmed by 1.5% agarose gel electrophoresis.
PCR Sensitivity vs LAMP Sensitivity
A 10-fold serial dilution of purified M. marinum DNA were prepared using ultrapure water. Starting DNA concentration from 50 ng/μL was quantified using a NanoDrop 2000 Spectrophotometer (Thermo Fisher, Australia). The sensitivity of nested PCR and LAMP was tested using amplification procedures mentioned above. All experiments were repeated at least three times.
Statistical Analysis
A Fisher’s exact test was performed to reveal the statistical difference using SPSS (SPSS Inc., no. 16) software.
Results
Optimization of the LAMP Assay
We optimized LAMP performance for the detection of M. marinum genomic DNA. The variable conditions included primer ratios, incubation temperatures and reaction times. Optimal LAMP assay primer ratio was found to be 1:8 (F3/B3:FIP/BIP), with the final concentrations of 0.2 µM and 1.6 µM for the F3/B3 and FIP/BIP primers, respectively. The use of the primers was found to result in more specific amplification and an anneal derivative temperature of approximately 88°C (Figure 1A). We found that optimal incubation temperature and reaction time were 63°C and 60 min, respectively (Figure 1B).
Analytical Specificity and Sensitivity of the LAMP and PCR Assay with M. marinum DNA
Primers were found to be specific, as only M. marinum isolates showed positive reactions, while other 6 common pathogenic mycobacteria and negative controls showed no amplification for LAMP (Figure 1C) and PCR (Figure 1D) assay.
The sensitivity of the nested PCR and LAMP assays was assessed by 10-fold serial dilution of M. marinum DNA. The sensitivity of PCR was 5 × 10−2 ng/μL (Figure 2A), but nested PCR was 5 × 10−5 ng/μL (Figure 2B), 1000-fold higher than that of the first reaction of nested PCR. By the naked eye, the sensitivity of LAMP assay was 5 × 10−4 ng/μL (Figure 2D). Further, the products were analyzed by 1.5% gel electrophoresis with ethidium bromide staining, and ladder-shaped bands demonstrated successful amplification. Positive reactions yielded green color and negative reactions yielded remained brown. The results were consistent with gel electrophoresis (Figure 2C). The results showed that nested PCR was 10-fold more sensitive than the LAMP assay.
Application of the LAMP and PCR Assay in Clinical M. marinum Strains
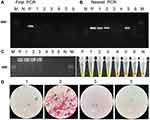
To evaluate the performance of the LAMP and PCR assay with M. marinum strains, DNA was extracted from 6 clinical M. marinum strains (Figure 3A) using extraction methods described above and then used as target template DNA for the LAMP and PCR reaction. PCR assay detected 6 out of 6 confirmed M. marinum infected cases, giving a sensitivity of 100% (Figure 3B) and further result of sequencing confirmed M. marinum (Figure 3C). LAMP assay products were detected with the naked eye and 1.5% gel electrophoresis. Six PCR positive samples were all positive by LAMP detection (Figure 3D). These results reveal a high sensitivity and specificity of the LAMP assay with PCR for diagnosis of M. marinum strains.
Application of the LAMP and PCR Assay in Clinical Skin Samples
Out of 6 skin M. marinum infection cases, 0 (0%), 3 (50%), 3 (50%), and 4 (66.7%) were positive by PCR (Figure 4A), nested PCR (Figure 4B), LAMP (Figure 4C) and culture (Figure 4D). No significant differences in positive rate were found between nested PCR and LAMP (P > 0.05), but significantly higher than that of PCR (P < 0.01). None of the samples was positive by the first amplification reaction of PCR. In 4 culture-positive samples, the rates of positive identification of the nested PCR and LAMP were 75% (3/4) and 75% (3/4). There was no amplification in one case by nested PCR and LAMP method.
Discussion
M. marinum is a slow-growing photochromatic NTM that can cause human opportunistic infection. Recently, a growing number of cases of M. marinum infection have been reported in China,2,10,11 which has attracted the attention of clinical dermatologists. M. marinum has become the most common NTM in our hospital. The majority of reported cases had an aquatic exposure. The aquatic exposure was related to aquaculture, handling fish, fish tanks or water-related activities.2,12,13 M. marinum infection is often unrecognized or misdiagnosed as fungal or tuberculosis infection due to nonspecific lesions and atypical histopathology. Although the diagnosis of M. marinum infection can be suspected clinically, when aquatic exposure is obtained, the definitive diagnosis is difficult and dependent on the isolation and identification of M. marinum.
The positive rate of culture was 66.7% in this study, which is consistent with the literature (70% to 80%).14 Cultured isolates are rarely identified by traditional biochemical method and more commonly by molecular methods, such as PCR-RFLP and gene sequencing of 16S rRNA, rpoB and hsp65. Unfortunately, sequence analysis of these conserved genes cannot differentiate M. marinum from M. ulcerans, since they are highly homologous with more than 98% genomic similarity. M. marinum seems to be an M. ulcerans ancestor.15 Divergence would have occurred along with the gain by M. ulcerans of genes encoding the virulence factor of mycolactone and of copies of the insertion sequences IS2404 and IS2606.16,17 Therefore, the differentiation between M. marinum and M. ulcerans can be achieved by PCR of mycolactone-producing genes (mlsA and mlsB) or two transposons (IS2404 and IS2606).18–20 This additional identification is not routinely performed in our laboratory, since Buruli ulcers caused by M. ulcerans are very rare in China.
PCR methods have been used for early diagnosis of M. marinum infection in some case reports by rapid detection of M. marinum in clinical specimens.11,21–23 Although it has been reported that the detection rate of nested PCR was 80% in cutaneous tuberculosis,24 relevant data are lacking in M. marinum infection. In this study, the nested PCR showed highest sensitivity (5 × 10−5 ng/μL) by serial dilution of M. marinum DNA, 1000-fold and 10-fold higher than conventional PCR and LAMP, respectively. Then, 6 skin samples confirmed as M. marinum infection were used for direct detection. The positive rate of nested PCR was 50%, slightly lower than that of culture (66.7%), but there was no statistically significant difference (P > 0.05). The sensitivity of nested PCR was no better than that of culture, which may be due to the difficulty of mycobacterium DNA extraction in paucibacillary skin specimen. Similar results have also been found in the study of M. ulcerans. Portaels et al directly detected M. ulcerans in 22 clinical specimens from 10 patients with Buruli ulcer by nested PCR and oligonucleotide-specific capture plate hybridization (PCR-OSCPH). The positive rate was 45.5% for PCR-OSCPH and 54.5% for culture (P > 0.05).25 Although the detection of M. marinum by nested PCR and by culture gave similar results in our study, the rapid availability of results by nested PCR is a distinct advantage for early diagnosis.
LAMP is a novel molecular diagnostic technique with high specificity and sensitivity and has been applied for diagnosis of mycobacterial infection, including M. tuberculosis, M. ulcerans, M. avium, M. kansasii and M. leprae.26–30 In this study, the LAMP had high sensitivity in M. marinum strains and clinical samples and shared the same sensitivity than nested PCR. In the detection of clinical specimens, the 50% positive rate of LAMP was significantly higher than conventional PCR (0%), the same with nested PCR (50%). de Souza et al compared LAMP and conventional PCR methods for the detection of M. ulcerans in clinical specimens. Fifty-nine percent of samples were positive using the LAMP, and only 14% were positive using the conventional PCR method.31 Our previous study on paucibacillary leprosy showed that LAMP detects M. leprae at a higher positive rate (50%, 15/30) than PCR (33.33%, 10/30) and comparable to q-PCR assays (56.67%, 17/30).30 LAMP assay is extremely simple using a simple water bath, and very convenient to observe the results by the naked eye. The whole LAMP assay process required approximately 60 min, significantly faster than nested PCR. Therefore, the LAMP assay has the potential for rapid diagnosis of M. marinum infection at the point-of-care. The major limitation of this study was the limited number of clinical skin samples. In future study, a larger number of samples should be tested.
Conclusion
Compared with conventional PCR, LAMP and nested PCR are more sensitive and have a higher detection rate of M. marinum in clinical skin specimens. The LAMP assay proved to be more suitable for rapid diagnosis of M. marinum infection in a shorter time especially in resource-limited settings. Further expanding the sample and more studies are needed to evaluate the diagnostic potential of LAMP and nested PCR for M. marinum infections.
Ethics Approval and Informed Consent
This study was approved by the institutional ethical committee of the Institute of Dermatology, Chinese Academy of Medical Sciences, China (2020-KY-008) and conducted in accordance with the Declaration of Helsinki. All patients signed a written informed consent for the collection of samples and subsequent analysis.
Acknowledgments
We are grateful to all the authors for their contributions to this study.
Funding
This work was supported by grants from the Suzhou Science and Technology Program (SYS2020176); Suzhou Gusu Health Talents Training Program (GSWS2020071).
Disclosure
All authors declare that they have no conflicts of interest for this work.
References
1. Bouceiro-Mendes R, Ortins-Pina A, Fraga A, et al. Mycobacterium marinum lymphocutaneous infection. Dermatol Online J. 2019;25(2):
2. Feng Y, Xu H, Wang H, Zhang C, Zong W, Wu Q. Outbreak of a cutaneous Mycobacterium marinum infection in Jiangsu Haian, China. Diagn Microbiol Infect Dis. 2011;71(3):267–272. doi:10.1016/j.diagmicrobio.2011.07.003
3. Guarneri C, Cannavò SP. ‘Fish-tank’ granuloma: a diagnostic dilemma. Intern Med J. 2009;39(5):338–339. doi:10.1111/j.1445-5994.2009.01923.x
4. Canetti D, Riccardi N, Antonello RM, Nozza S, Sotgiu G. Mycobacterium marinum: a brief update for clinical purposes. Eur J Intern Med. 2022;105:15–19. doi:10.1016/j.ejim.2022.07.013
5. Sinha P, Gupta A, Prakash P, Anupurba S, Tripathi R, Srivastava GN. Differentiation of Mycobacterium tuberculosis complex from nontubercular mycobacteria by nested multiplex PCR targeting IS6110, MTP40 and 32kD alpha antigen encoding gene fragments. BMC Infect Dis. 2016;16:123. doi:10.1186/s12879-016-1450-1
6. da Cruz HL, De albuquerque montenegro R, De araújo lima JF, et al. Evaluation of a nested-PCR for mycobacterium tuberculosis detection in blood and urine samples. Braz J Microbiol. 2011;42(1):321–329. doi:10.1590/S1517-83822011000100041
7. Paris DH, Blacksell SD, Newton PN, Day NP. Simple, rapid and sensitive detection of Orientia tsutsugamushi by loop-isothermal DNA amplification. Trans R Soc Trop Med Hyg. 2008;102(12):1239–1246. doi:10.1016/j.trstmh.2008.04.040
8. Khan M, Li B, Jiang Y, Weng Q, Chen Q. Evaluation of different PCR-based assays and LAMP method for rapid detection of Phytophthora infestans by targeting the ypt1 gene. Front Microbiol. 2017;8:1920. doi:10.3389/fmicb.2017.01920
9. Tsai MA, Wang PC, Yoshida S, et al. Establishment of loop-mediated isothermal amplification for rapid and convenient detection of Mycobacterium marinum complex. J Microbiol Methods. 2019;164:105671. doi:10.1016/j.mimet.2019.105671
10. Mei Y, Zhang W, Shi Y, et al. Cutaneous Tuberculosis and Nontuberculous Mycobacterial Infections at a National Specialized Hospital in China. Acta Derm Venereol. 2019;99(11):997–1003. doi:10.2340/00015555-3283
11. Cai L, Chen X, Zhao T, Ding BC, Zhang JZ. Identification of Mycobacterium marinum 65 kD heat shock protein gene by polymerase chain reaction restriction analysis from lesions of swimming pool granuloma. Chin Med J. 2006;119(1):43–48. doi:10.1097/00029330-200601010-00008
12. Holden IK, Kehrer M, Andersen AB, Wejse C, Svensson E, Johansen IS. Mycobacterium marinum infections in Denmark from 2004 to 2017: a retrospective study of incidence, patient characteristics, treatment regimens and outcome. Sci Rep. 2018;8(1):6738. doi:10.1038/s41598-018-24702-7
13. Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43(6):655–662. doi:10.1007/s15010-015-0776-8
14. Aubry A, Mougari F, Reibel F, Cambau E. Mycobacterium marinum. Microbiol Spectr. 2017;5:2. doi:10.1128/microbiolspec.TNMI7-0038-2016
15. Stinear TP, Jenkin GA, Johnson PD, Davies JK. Comparative genetic analysis of Mycobacterium ulcerans and Mycobacterium marinum reveals evidence of recent divergence. J Bacteriol. 2000;182(22):6322–6330. doi:10.1128/JB.182.22.6322-6330.2000
16. Stinear TP, Mve-Obiang A, Small PL, et al. Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc Natl Acad Sci U S A. 2004;101(5):1345–1349. doi:10.1073/pnas.0305877101
17. Chemlal K, Huys G, Fonteyne PA, et al. Evaluation of PCR-restriction profile analysis and IS2404 restriction fragment length polymorphism and amplified fragment length polymorphism fingerprinting for identification and typing of Mycobacterium ulcerans and M. marinum. J Clin Microbiol. 2001;39(9):3272–3278. doi:10.1128/JCM.39.9.3272-3278.2001
18. Fyfe JA, Lavender CJ, Johnson PD, et al. Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Appl Environ Microbiol. 2007;73(15):4733–4740. doi:10.1128/AEM.02971-06
19. Mve-Obiang A, Lee RE, Umstot ES, et al. A newly discovered mycobacterial pathogen isolated from laboratory colonies of Xenopus species with lethal infections produces a novel form of mycolactone, the Mycobacterium ulcerans macrolide toxin. Infect Immun. 2005;73(6):3307–3312. doi:10.1128/IAI.73.6.3307-3312.2005
20. Stinear T, Ross BC, Davies JK, et al. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J Clin Microbiol. 1999;37(4):1018–1023. doi:10.1128/JCM.37.4.1018-1023.1999
21. Enzensberger R, Hunfeld KP, Elshorst-Schmidt T, Böer A, Brade V. Disseminated cutaneous Mycobacterium marinum infection in a patient with non-Hodgkin’s lymphoma. Infection. 2002;30(6):393–395. doi:10.1007/s15010-002-2063-8
22. Galdiero M, Finamore E, Galdiero E, Baldi F, Petrillo L, Petrillo G. A case of granulomatous skin lesions caused by Mycobacterium marinum in the Campania region. New Microbiol. 2005;28(1):89–92.
23. Posteraro B, Sanguinetti M, Garcovich A, et al. Polymerase chain reaction-reverse cross-blot hybridization assay in the diagnosis of sporotrichoid Mycobacterium marinum infection. Br J Dermatol. 1998;139(5):872–876. doi:10.1046/j.1365-2133.1998.02516.x
24. Maldonado-Bernal C, Ramos-Garibay A, Rios-Sarabia N, et al. Nested Polymerase Chain Reaction and Cutaneous Tuberculosis. Am J Dermatopathol. 2019;41(6):428–435. doi:10.1097/DAD.0000000000001315
25. Portaels F, Agular J, Fissette K, et al. Direct detection and identification of Mycobacterium ulcerans in clinical specimens by PCR and oligonucleotide-specific capture plate hybridization. J Clin Microbiol. 1997;35(5):1097–1100. doi:10.1128/jcm.35.5.1097-1100.1997
26. Thapa J, Maharjan B, Malla M, et al. Direct detection of Mycobacterium tuberculosis in clinical samples by a dry methyl green loop-mediated isothermal amplification (LAMP) method. Tuberculosis. 2019;117:1–6. doi:10.1016/j.tube.2019.05.004
27. Ablordey A, Amissah DA, Aboagye IF, et al. Detection of Mycobacterium ulcerans by the loop mediated isothermal amplification method. PLoS Negl Trop Dis. 2012;6(4):e1590. doi:10.1371/journal.pntd.0001590
28. Yashiki N, Yamazaki Y, Subangkit M, Okabayashi T, Yamazaki W, Goto Y. Development of a LAMP assay for rapid and sensitive detection and differentiation of Mycobacterium avium subsp. avium and subsp. hominissuis. Lett Appl Microbiol. 2019;69(3):155–160. doi:10.1111/lam.13188
29. Chen C, Lu J, Long B, et al. Detection of Mycobacterium kansasii using a combination of loop-mediated isothermal amplification (LAMP) and lateral flow biosensors. Int Microbiol. 2021;24(1):75–82. doi:10.1007/s10123-020-00143-z
30. Jiang H, Tsang L, Wang H, Liu C. Loop-mediated isothermal amplification(LAMP) assay targeting RLEP for detection of Mycobacterium leprae in leprosy patients. Int J Infect Dis. 2021;107:145–152. doi:10.1016/j.ijid.2021.04.041
31. de Souza DK, Quaye C, Mosi L, Addo P, Boakye DA. A quick and cost effective method for the diagnosis of Mycobacterium ulcerans infection. BMC Infect Dis. 2012;12:8. doi:10.1186/1471-2334-12-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.