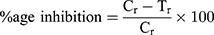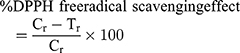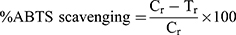Back to Journals » International Journal of Nanomedicine » Volume 15
Comparative Assessment of Antioxidant, Anti-Diabetic and Cytotoxic Effects of Three Peel/Shell Food Waste Extract-Mediated Silver Nanoparticles
Authors Das G , Shin HS , Patra JK
Received 18 August 2020
Accepted for publication 10 October 2020
Published 17 November 2020 Volume 2020:15 Pages 9075—9088
DOI https://doi.org/10.2147/IJN.S277625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Gitishree Das,1 Han-Seung Shin,2 Jayanta Kumar Patra1
1Research Institute of Biotechnology & Medical Converged Science, Dongguk University-Seoul, Gyeonggi-do, 10326, Republic of Korea; 2Department of Food Science and Biotechnology, Dongguk University-Seoul, Gyeonggi‐do, 10326, Republic of Korea
Correspondence: Jayanta Kumar Patra
Research Institute of Biotechnology & Medical Converged Science, Dongguk University-Seoul, Gyeonggi-do, 10326, Republic of Korea
Email [email protected]
Background: The natural food waste peels/shells discarded as waste materials are ample sources of natural bioactive compounds. The natural food waste mediated silver (Ag) nanoparticle (NPs) synthesis will be advantageous over chemical synthesis.
Materials and Methods: Using the various phytochemical-rich ripe P. americana peel (PAP), fresh Beta vulgaris peel (BVP), and rawArachis hypogaea shell (AHS) extracts, the bio-synthesis of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs, respectively, were carried out and its characterization was completed by standard procedures. The three biosynthesized AgNP’s multiple biological effects were accomplished by evaluating their cytotoxicity, antidiabetic, and antioxidant effects.
Results: The biosynthesis of the three generated Ag nanoparticles was confirmed through UV-vis spectrum analysis while the X-ray diffraction outlines revealed the generated AgNPs nature. The morphological structure and elemental information of the three AgNPs were obtained through SEM (scanning electron microscopy) and EDX (energy-dispersive X-ray) study. Multiple biological assays exhibited that the three generated AgNPs have significant cytotoxic, antidiabetic, and moderate antioxidant activity. In a comparative analysis, the PAP-AgNPs displayed higher anticancer potential than BVP and AHS-AgNPs, whereas AHS-AgNPs exhibited a higher antidiabetic effect with the lowest IC50 value (1.68 μg/mL) than PAP and BVP AgNPs. All three generated AgNPs displayed moderate antioxidant effects, among them BVP-AgNPs were more effective than PAP and AHS AgNPs. More than two effects of the three biosynthesized AgNPs specifies that they have ample perspective in therapeutic applications in pharmaceutical and other related industries in controlling cancer and diabetes.
Keywords: antidiabetic, antioxidant, cytotoxicity, food wastes, peels/shells, silver nanoparticles
Introduction
In various industries including agriculture, medicine, nuclear physics, etc. nanotechnology has appeared as an encouraging multidisciplinary field of research employing extensive significant aptitudes. For the management of several diseases, nanomedicines can play a significant role in developing highly effective alternative treatment approaches.1,2 Nanoparticles (NPS), and metallic NPs like silver, gold, platinum, and zinc, etc., have received extensive attention as of their unique multiple desirable properties, unique dimensions, and shape which make the NPs advantageous in green synthesis and various areas and applications.3,4 Silver NPs are non-toxic by nature and they exhibit highly desirable properties for uses in nanotechnology.4 This method employs organic or natural materials as a reducing catalyst, which promotes and enhances the stability of NPs and it can also improve the activities of nanomaterials.3,5 Ag nanoparticles are utilized for the therapeutic applications as antibacterial, antifungal, anti-inflammatory, and antiviral agent.6 For the synthesis of AgNPs, there are several methods, including chemical,7 physical, and biological methods. But the green-synthesis approach is plant-mediated synthesis, it is safe, economical, fast, and energy-efficient which instigates several scientists to use the biological technique for AgNPs synthesis. It was confirmed that amongst various biological resources plant-based biosynthesis is more considerable in the AgNPs synthesis.8–10 Currently emerging green nanoparticles are leading in the field of nanobiotechnology.11 There is a rising awareness in solid food waste management, supervision, regulations, and disposal in current times,12 correspondingly the progress of innovative techniques to manage unwanted food wastes (vegetable and fruit peels) also their usage in nanotechnology (food industries, biomedical, and agriculture).13 Fruit wastes (peels or skins and seeds) are produced in huge amounts in the fruit juice industry. These waste by-products are known to contain numerous phytochemicals, flavonoids, natural pigments, and phenolic compounds including antioxidant and metal-reducing potential.14 In the foodstuff industry intended for the advancement of health foods, phytochemicals can play a very significant role. Furthermore, phytochemicals could well be utilized in the health trade, as they are also capable to formulate drugs for medical treatments.15
Various food waste peels such as orange, pineapple, banana, dragon fruit, and papaya have been exploited for organic AgNPs synthesis.3,16 Persea americana (PA) fruit, universally known as avocado belongs to the Lauraceae family and is usually grown in Central America and Mexico and is nowadays available in the world’s all subtropical and tropical regions. PA contains abundant vitamins, dietary fiber, potassium, useful unsaturated fatty acids, and highly bioactive phytochemicals.17 The usage of PA in the cosmetics and food industries produces substantial amounts of by-products (like peels and seeds) that are highly rich in phenolics. From a commercial prospect, the employment of these byproducts would be tremendously advantageous.18 Its waste peel is rich with various phenolics and flavonoids compounds as evident from published literatures.19,20
The beetroot or Beta vulgaris (BV) counted in the Betoideae subfamily and comes under the family Amaranthaceae. In recent times, there is growing attention in the biological effect of BV and its possible usages as health-supporting and disease inhibiting health food. It is a potential source of biologically active constituents or compounds such as betalains, nitrates, phenolics, flavonoids, ascorbic acid, betacyanins, betaxanthins, and carotenoids, etc. It has antioxidant, antidiabetic, wound healing, chemo-preventive, and anti-inflammatory activity. It helps in controlling cancer, cardiovascular, and cardiometabolic disease. It also helps to reduce blood pressure.21,22 The BV peel is a source of betacyanin, micronutrients, protein, mineral vitamins, and a good source of potassium. It has also multiple potential activities.23
The peanut or Arachis hypogaea (AH) belongs to the family Fabaceae. It is an important worldwide grown food crop. The by-products of Arachis hypogaea contain various functional compounds like vitamins, minerals, fibers, polyphenols, proteins, and antioxidants. It is furthermore a tremendous source of bioactive compounds like flavonoids, phenolic acids, phytosterols, and resveratrol, etc.24 The Arachis hypogaea shell the by-product of Arachis hypogaea is rich in various bioactive compounds. It has also antioxidant activity.25 These food wastes are natural plant-derived products that are usually discarded as wastes. Hence, its utilization in the green synthesis of nanoparticles could serve as the best way for the management of food waste materials in an eco-friendly and costs effective manner.
Keeping all the above things in view, the current study reports the natural food wastes including ripe Persea americana peel (PAP), fresh Beta vulgaris peel (BVP), and raw Arachis hypogaea shell (AHS) extracts mediated AgNPs synthesis their characterization by standard methods, and comparative study of their multiple biological effects by their cytotoxic, antidiabetic, and antioxidant activity study.
Materials and Methods
Materials
Ripe P. americana peel (PAP), fresh Beta vulgaris peel (BVP), and raw Arachis hypogaea shell (AHS) were purchased from certified outlets at the Goyangsi market, the Republic of Korea. The byproduct of PA peel, BV peel, and AH shell was cleaned with tap H2O and then with DDH2O. Next, it was thoroughly dried with tissue paper and pounded. A 100 g amount of the each pounded peel was immersed (in 500 mL) of DDH2O in a flask (1000 mL) and boiled with constant stirring for approximately 30 min. The boiled mixture of PAP, BVP, and AHS was then cooled down to normal warmness then sieved using (Whatman No.1 filter paper), and then the three extracts were put in storage until further use (at 4° C).
Phytochemical (Primary) Screening of the PAP, BVP, and AHS Extracts
The PAP, BVP, and AHS extract phytochemical screening was carried out to identify the existence of active phytochemicals like tannins, flavonoids, terpenoids, saponins, phenolics, anthraquinones, carbohydrates, and cardiac steroidal glycoside as per the standard procedures.26–28
Biosynthesis and Characterization of Three Generated PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs
The PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs synthesis was accomplished by using the phytochemical-rich waste PA peel, BV peel, and AH shell through using the protocol of.29 The characterization of the three generated AgNPs was accomplished by five different standard analytical methods. These methods comprised of (UV-VIS spectral analysis, scanning-electron-microscopy, energy-dispersive X-ray spectroscopy, X-ray powder diffraction analysis, Fourier-transform-infrared spectroscopy) as per previously described standard protocols of.29,30
Assessment of the Cytotoxicity Effect of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs
The cytotoxicity consequence of the three generated PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs on the HepG2 cancer cells (Korea Cell Line Bank, Seoul, Republic of Korea) was estimated by using an EZ-Cytox kit (DoGenBio Co., Ltd., Seoul, and the Republic of Korea) following the company’s manufacture method. The check samples PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs were diluted at the concentration (1000 µg/mL) by using Dulbecco’s phosphate-buffered saline, purified by using a syringe filter (0.22µm, Millipore, Billerica, and Ma, USA). The viability of cells (dead cell %) and morphology exposed to above three synthesized (PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs) were estimated through the trypan blue exclusion experiment.31
Before the treatment of HepG2 cancer cells, the OD (optical density) of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs (suspended in DMEM) was scanned in between (300–700 nm) wavelength range. After 24 h exposition, the supernatant was substituted with a fresh medium (110 µL) consist of 10 µL of EZ-Cytox solution and kept until the melon red color changed to yellowish-orange (incubated around 20 min). Next after incubation, the samples (PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs, 100 µL each) were aliquoted in a new 96 well plate. And then by using a spectrophotometer, Spectra Max 384 Plus; Molecular Devices, Sunnyvale, CA, USA, the absorbance was documented at 450 nm wavelength. Similarly, the cell viability of the HepG2 cells exposed to the three generated PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs was valued as a result of (trypan blue exclusion analyze). In the same way, after (24 h) exposition the removed supernatant and the cells were washed with DPBS (100 µL) straightaway. Afterward, 20 µL of (1:1) fresh complete DMEM and trypan blue mixture were added to each well. Then the viability of the cell was witnessed using an inverted microscope (DMI6000B; Leica, Wetzlar, Germany).31
Assessment of Antidiabetic Effect of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs
The assessment of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs antidiabetic potential (inhibition of α-glucosidase) was assayed following a previously published standard method.32
The PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs were dissolved in methanol using a sonicated water bath. The PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs test samples (10 µg/mL each) were moved to a plate (96-well) and diluted serially (with 0.02 M sodium phosphate buffer, pH 6.9). 0.5 U/mL α-glucosidase was added to a concluding volume of 50 µL to each. Afterward kept for incubation at normal room temperature (for 10 min). The substrate was taken as P-nitrophenyl-glucopyranoside (50 µL, 3.0 mM). The reaction mixture was kept for incubation (at 37 ºC for 20 min). To stop the reaction, a 50 µL aliquot of 0.1 M Na2CO3 was added to each, then the absorbance was recorded at 405 nm. The percentage of inhibition of α-glucosidase was evaluated using the subsequent equation.
where Cr (absorbance value of the control) and Tr (absorbance of the tested sample).
Assessment of Antioxidant Potential of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs
The antioxidant effect of the three generated PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs was estimated by DPPH, ABTS, and reducing power scavenging studies by following the previously described standard protocol.30 Concisely to estimate the DPPH scavenging potential of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs, Tert-Butyl-hydroquinone (BHQ) was used as a standard reference with methanol as the reference blank. The absorbance value of the reaction solution was estimated through a UV-visible spectrum analysis (Multiskan Go; Thermo Scientific, Waltham, MA, USA, Spectrophotometer). The PAP-AgNPs, BVP-AgNPs, and AHS AgNPs scavenging (free radical) potential was evaluated using the below equation:
where Cr (absorbance value of the control) and Tr (absorbance of the tested sample).
The evaluation of the ABTS (free radical) scavenging effect of AHS-AgNPs, PAP-AgNPs, and BVP-AgNPs, the BHQ was used as a standard reference and methanol as the reference blank. The free radical scavenging effect was estimated by using a UV-visible spectrum analysis (Spectrophotometer, Multiskan GO; Thermo Scientific, Waltham, MA, USA). The scavenging effect of the standard BHQ and the tested samples PAP-AgNPs, AHS-AgNPs, and BVP-AgNPs on ABTS scavenging was evaluated by the following equation:
where Cr (absorbance value of the control) and Tr (absorbance of the tested sample).
The reducing power of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs was estimated by following a previously published standard procedure.30 For this assay, the reducing power effect is calculated as the absorbance value of the reaction mix (at 700 nm).
Statistical Analysis
The statistical investigation was undertaken through a one way ANOVA followed by Duncan’s multiple tests using SPSS statistical analysis software (Version 25.0 and IBM Crop, Armonk, NY, USA) at 5% of significance level (P < 0.05). The result data are presented as the average of three replicated values with standard deviation.
Results and Discussion
Bio-Synthesis of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs and Their Characterization
There are many chemical methods for the synthesis of NPs. The chemical methods have disadvantages of hazardous by-products, time consumption, and stability, generation of aggregated large-sized particles, and the use of toxic chemicals. These above disadvantages are directed towards the development of the synthesis of ecofriendly and green nanoparticles with less energy consuming by biological resources like plants, plant-derived products, microorganisms, and algae, etc.33 Due to the lack of toxic substances, the green synthesis process is highly reliable, whereas the chemical approaches usage chemicals, which are toxic to humans.7 In the earlier report, there is a report of no toxic effect of biosynthesized Ag nanoparticles.34 But still, it is required to conduct detailed safety research studies, that biosynthesized nanoparticles are free of side effects for humans.
From vegetable, fruit-based industries, and household kitchens generated a huge amount of natural waste peels/shells, which are 25–30% of the total product, has directed to an enormous nutritional loss with high environmental complications. The peels, rind, pomace, seeds, and shells are the common food wastes most of them are highly rich with valued active compounds like vitamins, polyphenols, flavonoids, pigments, phenolic compounds, carotenoids, antioxidants, and enzymes, etc. The usage of these low-cost agricultural wastes for the fabrication of value-added products is a novel step for food waste utilization in nanotechnology to manage surplus agricultural food waste materials. The food waste by-products are well known to have metal-reducing potential.13,14,35 These above things are encouraged to carry out the green synthesis of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs using the food wastes PAP, BVP, and AHS extracts.
In the current study, the phytochemical screening analysis of the three PAP, BVP, and AHS extracts were undertaken for the presence of several active phytochemicals like the presence of tannins, flavonoids, terpenoids, saponins, carbohydrates, phenolics, anthraquinones, and cardiac steroidal glycoside, etc. and the outcome is showed in Table 1. Silver, NPs were bio-synthesized using the food wastes of ripe PAP, BVP, and AHS are extremely rich in tannins, flavonoids, terpenoids, saponins, phenolics, anthraquinones, carbohydrates, and cardiac steroidal glycoside (Table 1). PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs synthesis was confirmed by visual evaluation of the reaction mix in terms of the alteration of color from neutral to reddish-brown (PAP and AHS-AgNPs) and light pink to dark red (BVP-AgNPs) (Figure 1A).13
 |
Table 1 Phytochemical Screening of PAP, BVP, and AHS Aqueous Extracts |
After visual validation of the reaction mixture as an alteration in the pigment of the reaction medium, the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs were exposed to spectral analyzes. The UV-VIS spectral analysis result of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs reaction solution was recorded at a constant time intervals (over 24 h). In general, due to free electrons, silver NPs shows an SPR value (surface-plasmon-resonance value) band between 440 and 558 nm.36 The reaction kinetics of the PAP, BVP, and AHS-AgNPs were followed by UV-VIS spectral analysis between 280 and 650 nm. The SPR spectra of the samples exhibited a peak at 438,436, and 448 nm wavelength, respectively (Figure 1B). This result is analogous to previously reported silver NPs synthesis37 which confirms the PAP, BVP, and AHS-AgNPs synthesis. These above results specify that the phytochemicals in PAP, BVP, and AHS extracts,17,22,38–40 act as reducing and capping substances.
The basic configuration and the morphology of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs were investigated by EDX and SEM, respectively. The SEM outcomes of the above three AgNPs were based on nanometer-scale imaging, which exposed that the AgNPs were small dots-like and nearly round-shaped entities (Figure 2), thus confirming that the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs were structurally similar to what has been stated previously.3,41 The elemental structure obtained from the EDX study revealed the existence of Ag nanoparticles. The graph of the EDX data exhibited a major peak, specifying the existence of the Ag element (Figure 2). This result is similar to what has been reported previously.42
 |
Figure 2 (A) EDX analysis of PAP-AgNPs (inset: SEM image); (B) EDX analysis of BVP-AgNPs (inset: SEM image); and (C) EDX analysis of AHS-AgNPs (inset: SEM image). |
The XRD assessment of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs revealed the physical sketch of three generated NPs and exhibited the crystalline nature and nanostructure of the PAP, BVP, and AHS-AgNPs and three/four visible peaks (Figure 3) equivalent to fccp the (face-centered cubic phase of Ag0 standard JCPDS card no. 04–0783).42 The PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs diffraction pattern exhibited clear four peaks at 2 theta angles of (38.25, 38.12, and 38.01); (46.05, 46.55, and 46.00); (64.72, 64.88, and 64.09) respectively and (76.64 and 76.88 for PAP-AgNPs, and AHS-AgNPs parallel to (111), (200), (220), and (311) was recorded (Figure 3). These peaks are possibly due to the presence of phytochemicals in the PAP, BVP, and AHS extracts. This outcome is analogous to the previous report.43
 |
Figure 3 XRD analysis of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs. |
To identify the functional groups (compounds) present in the extracts (PAP, BVP, and AHS), responsible for reducing and capping of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs the FTIR study was carried out. Alteration of the valuable compounds suggests their active participation in the bio-synthesis of the AgNPs. The FTIR data of the PAP aqueous extract and the PAP-AgNPs exhibited several peaks with varied stretching modes (Figure 4). Probably, the peak values of the PAP extract at 3321.40, 2124.22, 1094.83, and 695.14 cm−1 shifted to 3304.44, 2111.02, 1087.29, and 683.83 cm−1 for the PAP-AgNPs (Figure 4). Possibly the peak values of the BVP extract at 3313.86 cm−1, 2127.99 cm−1, 1083.52, and 695.14 cm−1. cm−1 altered to 3304.44, 2116.68 cm−1, 1087.29, and 683.83 cm−1 for BVP-AgNPs. Similarly for AHS extract the absorption peaks at 3328.95, 2124.22, 1639.69, 1102.37 and 683.83 cm−1 altered to 3304.44, 2131.76, 1632.15, 1064.67 and 687.60 for AHS-AgNPs.
 |
Figure 4 FTIR analysis of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs. |
The peak frequency of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs at 3304.44 cm−1 specifies the presence of O-H bond stretching and H–bonding owing to the functional groups like phenols and alcohols.44 Likewise, the peak values at 2111.02 cm−1, 2116.68 cm−1, and 2131.76 cm−1 (for C≡N bond) are because of nitrile functional groups, the absorption peak values at 1637.81, 1635.92, and 1632.15 cm−1 (indicative of N–H bonds) is due to primary amine functional groups. The absorption peak values at 1087.29, and 1064.67 cm−1 (C–O bond stretching) is owing to esters, alcohols, carboxylic acids, and ethers, whereas the peak at 683.83 (C-Br bond) stretching is owing to the functional group’s alkyl halide.44 Minor deviations in the peak values of the extracts (PAP, BVP, and AHS) and the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs could be credited to the capping and steadying courses during the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs synthesis.42 The analogous result has been stated previously.16
Evaluation of the Different Biological Effects of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs
Cytotoxicity Effect of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs
Cancer is a serious health condition that leads to aberrant cell division and that adversely affects various critically required tissues in the body. The configuration of NPs provides them with unique properties to target the aberrant cell development triggered by neoplastic transformation,45 and nano-drugs have been stated to be extremely active in the identification and controlling of cancer-associated diseases.13 On cytotoxicity activity, Ag nanoparticles have a size and dose-dependent effect. The cytotoxicity of biosynthesized Ag nanoparticles may be influenced by several parameters like Ag nanoparticles shape, size distribution, and surface chemistry. The variations in these elements may lead to altered in cytotoxicity reaction.10,46 It damages the cell barrier, inactivates ATPase activity, sensitizes cell signaling, and finally leads to apoptosis.47 Besides, in the conventional (chemical or physical) methods of synthesis of nanoparticle, usually highly toxic chemicals are used which are highly environmentally toxic and gave rise to numerous toxic side effects upon administration, hence these are not recommended for biomedical applications of nanoparticles, rather green synthesized nanoparticles are preferred which are environmental friendly and non-toxic in nature.48
In the current study, the resulting graph of the cell viability shown that the number of live HepG2 cells was higher as the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs concentration decreased (Figure 5A and B). HepG2 cells were detected with an inverted light microscope after they had been treated with varying concentrations of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs. In addition to less scattering of the cell lines, this revealed that their morphology was altered considerably (Figure 5A and B), and that cell death occurred in a concentration-dependent manner. At the highest concentration, the above three AgNPs were significantly toxic to HepG2 cells (Figure 5A and B). The live and spreading cells ratio augmented when the concentrations of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs were lowered (Figure 5A and B). The PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs displayed promising cytotoxicity against the HepG2 cancer cell line (Figure 5A and B). The PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs were highly active against HepG2 cancerous cells at (100 µg/mL) concentration (Figure 5A and B) and the effect on the viability of cell was concentration-dependent in HepG2 cells (Figure 5A and B). It was specified that by the mechanisms like endocytosis, phagocytosis, pinocytosis and mitochondrial respiratory chain disrupt, where the silver nanoparticles may penetrate through the cells with a result of interruption of the generation of reactive oxygen species and synthesis of adenosine triphosphate resulting in DNA damage and leads to cell death.8,10,49 Besides, Akter et al have mentioned that after entering the cell through the process of phagocytosis, diffusion, or endocytosis, the AgNPs by itself or by the ionized Ag+ generates reactive oxygen species that cause the oxidative stress. Further, the overproduction of the ROS results in the denaturation of the anti-apoptotic proteins and the initiation of expression of the pro-apoptotic proteins (Figure 6). 50 This cytotoxicity potential of the three generated AgNPs is parallel or better than the earlier reports.10,13,31,51
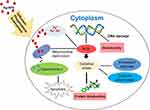 |
Figure 6 Possible uptake process and mechanism of cytotoxicity induced by AgNPs in various cell lines. Adopted from Akter et al,50 under the terms of the Creative Commons Attribution License (originally Figure 4). |
Antidiabetic Effect of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs
The potential antidiabetic activity of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs was determined by assaying α-glucosidase inhibition. The PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs inhibited α-glucosidase in a concentration-dependent method. The power of the NPs on α-glucosidase activity indicates that they have considerable antidiabetic potential, with more than 93% (PAP-AgNPs, and AHS-AgNPs) and more than 87% (BVP-AgNPs) (at 5 µg/mL of concentration) α-glucosidase inhibition (Figure 7). In comparison to PAP-AgNPs, and BVP-AgNPs the AHS-AgNPs were extremely effective and the α-glucosidase inhibition displayed more than 94% at a lower (2.5 µg/mL) of concentration (Figure 7). These above three AgNPs displayed higher activity than the earlier reported biosynthesized AgNPs.52 An analogous effect of silver NPs for α-glucosidase inhibition has been stated earlier.53 The significant antidiabetic effect of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs is evident from their considerably lower IC50 values (Table 2).
 |
Table 2 IC50 Values of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs Antioxidant and Antidiabetic Studies |
 |
Figure 7 Antidiabetic activity (α-glucosidase inhibition) of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs. |
Antioxidant Effect of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs
The combined results of the antioxidant assays of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs are presented in Figure 8. In all antioxidant assays (DPPH, ABTS, and reducing power) with three tested concentrations of PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs the effect was concentration-dependent. The antioxidant effect was higher with an increase in concentration. In the DPPH and ABTS assay, the PAP-AgNPs was more effective than BVP-AgNPs, and AHS-AgNPs (Figure 8). While for reducing power effect, the BVP-AgNPs was more effective than PAP-AgNPs, and AHS-AgNPs (Figure 8). Overall the three generated AgNPs displayed moderate antioxidant effect (Figure 8).
The PAP-AgNPs DPPH free radical activity was greater than their ABTS radical activity, which is similar to what has been found in prior research.54 The deviations in the results for the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs in the three scavenging (free radical) assays (Figure 8) could be owing to the alteration of the effect mechanisms.
The antioxidant parameters (DPPH, ABTS, and Reducing power) IC50 values are displayed in Table 2. The AgNPs displayed a moderate free radical scavenging effect in the three radical scavenging assays, which is as well evident from the IC50 values. Thus, the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs seem to have antioxidant potential, which remains in keeping with previous reports.54 This result could be owing to interference by various functional phytochemicals present in the PAP, BVP, and AHS extracts, which may have well played a vibrant role during the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs synthesis course in the process of capping and stabilization of Ag nanoparticles.55 The existing phytochemicals in the three peels/shell extracts (Table 1) act a major role in offering the antioxidant activity to the three generated AgNPs. The antioxidant activity of the above three AgNPs is lower than the previously reported AgNPs antioxidant activity.52
Conclusion
The three economical, toxic-free, and harmless bio-fabricated AgNPs (PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs) were successfully synthesized using the PAP, BVP, and AHS extracts. The phyto-component of PAP, BVP, and AHS extracts plays a considerable role as both capping and reducing agents in the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs synthesis process. The biosynthesized PAP-AgNPs, BVP-AgNPs and AHS-AgNPs displayed significant cytotoxicity and antidiabetic effect, along with moderate antioxidant potential according to the overall scavenging assays. The multi-potential consequence of the PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs suggested that this might be attributed to the phytochemicals existing in the PAP, BVP, and AHS extracts. The PAP-AgNPs, BVP-AgNPs, and AHS-AgNPs appear to be suitable candidates for controlling the related diseases such as cancer and diabetes, also it can as well employed in the pharmaceutical trades in biomedical applications.
Acknowledgment
G Das, H-S Shin, and JK Patra are grateful to Dongguk University-Seoul, Republic of Korea, for the support. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1G1A1004667), the Republic of Korea. The authors also wish to thank Prof. H. Kim and Dr. A. Ansari of the Department of Rehabilitation Medicine of Korean Medicine, Dongguk University-Seoul, Republic of Korea, and Dr. C.N. Vishnuprasad of The University of Trans-Disciplinary Health Sciences and Technology (TDU), India for experimental help. The authors also like to thank whoever helped in this research work.
Author Contributions
G. Das, H.S. Shin and, J.K. Patra made a significant contribution in the conception, study design, execution, acquisition of data, analysis and interpretation; and took part in drafting, revising, and critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1G1A1004667), the Republic of Korea.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Boomi P, Poorani GP, Palanisamy S, et al. Evaluation of antibacterial and anticancer potential of polyaniline-bimetal nanocomposites synthesized from chemical reduction method. J Cluster Sci. 2019;30(3):715–726. doi:10.1007/s10876-019-01530-x
2. Barabadi H, Tajani B, Moradi M, et al. Penicillium family as emerging nano factory for the biosynthesis of green nanomaterials: a journey into the world of microorganisms. J Cluster Sci. 2019;1–14.
3. Soto KM, Quezada-Cervantes CT, Hernández-Iturriaga M, Luna-Bárcenas G, Vazquez-Duhalt R, Mendoza S. Fruit peels waste for the green synthesis of silver nanoparticles with antimicrobial activity against foodborne pathogens. LWT. 2019;103:293–300. doi:10.1016/j.lwt.2019.01.023
4. Massironi A, Morelli A, Grassi L, et al. Ulvan as novel reducing and stabilizing agent from renewable algal biomass: application to green synthesis of silver nanoparticles. Carbohydr Polym. 2019;203:310–321. doi:10.1016/j.carbpol.2018.09.066
5. Girón-Vázquez N, Gómez-Gutiérrez C, Soto-Robles C, et al. Study of the effect of Persea americana seed in the green synthesis of silver nanoparticles and their antimicrobial properties. Results Physics. 2019;13:102142. doi:10.1016/j.rinp.2019.02.078
6. Kanagamani K, Muthukrishnan P, Shankar K, Kathiresan A, Barabadi H, Saravanan M. Antimicrobial, cytotoxicity and photocatalytic degradation of norfloxacin using Kleinia grandiflora mediated silver nanoparticles. J Cluster Sci. 2019;30(6):1415–1424.
7. El Mahdy MM, Eldin TAS, Aly HS, Mohammed FF, Shaalan MI. Evaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino rats. Experimental Toxicologic Pathology. 2015;67(1):21–29. doi:10.1016/j.etp.2014.09.005
8. Ovais M, Khalil AT, Raza A, et al. Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomedicine. 2016;12(23):3157–3177. doi:10.2217/nnm-2016-0279
9. Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Advanced Research. 2016;7(1):17–28. doi:10.1016/j.jare.2015.02.007
10. Barabadi H, Vahidi H, Kamali KD, Rashedi M, Saravanan M. Antineoplastic biogenic silver nanomaterials to combat cervical cancer: a novel approach in cancer therapeutics. J Cluster Sci. 2020;31(4):659–672. doi:10.1007/s10876-019-01697-3
11. Balachandar R, Gurumoorthy P, Karmegam N, et al. Plant-mediated synthesis, characterization, and bactericidal potential of emerging silver nanoparticles using stem extract of Phyllanthus pinnatus: a recent advance in phytonanotechnology. J Cluster Sci. 2019;30(6):1481–1488. doi:10.1007/s10876-019-01591-y
12. Omran BA, Nassar HN, Fatthallah NA, Hamdy A, El-Shatoury EH, El-Gendy NS. Waste upcycling of Citrus sinensis peels as a green route for the synthesis of silver nanoparticles. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 2018;40(2):227–236. doi:10.1080/15567036.2017.1410597
13. Oves M, Aslam M, Rauf MA, et al. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Materials Science Engineering: C. 2018;89::429–443. doi:10.1016/j.msec.2018.03.035
14. Zuorro A, Iannone A, Natali S, Lavecchia R. Green synthesis of silver nanoparticles using bilberry and red currant waste extracts. Processes. 2019;7(4):193. doi:10.3390/pr7040193
15. Rafiq S, Kaul R, Sofi S, Bashir N, Nazir F, Nayik GA. Citrus peel as a source of functional ingredient: a review. J Saudi Society Agricultural Sciences. 2018;17(4):351–358. doi:10.1016/j.jssas.2016.07.006
16. de Barros CHN, Cruz GCF, Mayrink W, Tasic L. Bio-based synthesis of silver nanoparticles from orange waste: effects of distinct biomolecule coatings on size, morphology, and antimicrobial activity. Nanotechnol Sci Appl. 2018;11:1. doi:10.2147/NSA.S156115
17. Araújo RG, Rodriguez-Jasso RM, Ruiz HA, Pintado MME, Aguilar CN. Avocado by-products: nutritional and functional properties. Trends Food Science Technology. 2018. doi:10.1016/j.tifs.2018.07.027
18. Kosińska A, Karamać M, Estrella I, Hernández T. Phenolic compound profiles and antioxidant capacity of Persea americana Mill. peels and seeds of two varieties. J Agric Food Chem. 2012;60(18):4613–4619. doi:10.1021/jf300090p
19. Tremocoldi MA, Rosalen PL, Franchin M, et al. Exploration of avocado by-products as natural sources of bioactive compounds. PLoS One. 2018;13(2):e0192577. doi:10.1371/journal.pone.0192577
20. Bhuyan DJ, Alsherbiny MA, Perera S, et al. The Odyssey of bioactive compounds in avocado (Persea americana) and their health benefits. Antioxidants. 2019;8(10):426. doi:10.3390/antiox8100426
21. Clifford T, Howatson G, West DJ, Stevenson EJ. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 2015;7(4):2801–2822. doi:10.3390/nu7042801
22. Mirmiran P, Houshialsadat Z, Gaeini Z, Bahadoran Z, Azizi F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr Metab. 2020;17(1):1–15. doi:10.1186/s12986-019-0421-0
23. Šeremet D, Durgo K, Jokić S, et al. Valorization of banana and red beetroot peels: determination of basic macrocomponent composition, application of novel extraction methodology and assessment of biological activity in vitro. Sustainability. 2020;12(11):4539. doi:10.3390/su12114539
24. Arya SS, Salve AR, Chauhan S. Peanuts as functional food: a review. J Food Sci Technol. 2016;53(1):31–41. doi:10.1007/s13197-015-2007-9
25. Adhikari B, Dhungana SK, Ali MW, Adhikari A, Kim I-D, Shin D-H. Antioxidant activities, polyphenol, flavonoid, and amino acid contents in peanut shell. J Saudi Society Agricultural Sciences. 2019;18(4):437–442. doi:10.1016/j.jssas.2018.02.004
26. Sofowora A. Medicinal Plants and Medicine In Africa. John Willey Spectrum, Ibadan Nigeria; 1993;281–285.
27. Ravikumar V, Gopal V, Sudha T. Analysis of phytochemical constituents of stem bark extracts of Zanthoxylum tetraspermum wight & arn. Research J Pharmaceutical, Biological Chemical Sciences. 2012;3(4):391–402.
28. Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Scientific World J. 2017;2017.
29. Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci. 2014;9(6):385.
30. Zhou Y, Itoh H, Uemura T, Naka K, Chujo Y, Preparation of π-conjugated polymer-protected gold nanoparticles in stable colloidal form. Chemical Communications. 2001;7:613–614. doi:10.1039/b100636n
31. Faedmaleki F, Shirazi FH, Salarian -A-A, Ashtiani HA, Rastegar H. Toxicity effect of silver nanoparticles on mice liver primary cell culture and HepG2 cell line. IJPR. 2014;13(1):235.
32. Butala MA, Kukkupuni SK, Venkatasubramanian P, Vishnuprasad CN. An ayurvedic anti‐diabetic formulation made from Curcuma longa L. and Emblica officinalis L. inhibits α‐amylase, α‐glucosidase, and starch digestion, in vitro. Starch‐Stärke. 2018;70(56):1700182. doi:10.1002/star.201700182
33. Virmani I, Sasi C, Priyadarshini E, et al. Comparative anticancer potential of biologically and chemically synthesized gold nanoparticles. J Cluster Sci. 2019;1–10.
34. Dobrucka R, Szymanski M, Przekop R. The study of toxicity effects of biosynthesized silver nanoparticles using Veronica officinalis extract. International J Environmental Science Technology. 2019;16(12):8517–8526. doi:10.1007/s13762-019-02441-0
35. Kumar H, Bhardwaj K, Sharma R, et al. Fruit and vegetable peels: utilization of high value horticultural waste in novel industrial applications. Molecules. 2020;25(12):2812. doi:10.3390/molecules25122812
36. Khalil MM, Ismail EH, El-Baghdady KZ, Mohamed D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arabian J Chemistry. 2014;7(6):1131–1139. doi:10.1016/j.arabjc.2013.04.007
37. Saravanan M, Barik SK, MubarakAli D, Prakash P, Pugazhendhi A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb Pathog. 2018;116:221–226. doi:10.1016/j.micpath.2018.01.038
38. John S, Monica J, Priyadarshini S, Sivaraj C, Arumugam P. Antioxidant and antibacterial activities of beta vulgaris l. peel extracts. International J Pharma Research Health Sciences. 2017;5(6):1974–1979.
39. Elsorady M, Ali S. Antioxidant activity of roasted and unroasted peanut skin extracts. International Food Research J. 2018;25:1.
40. Sim EW, Lai SY, Chang YP. Antioxidant capacity, nutritional and phytochemical content of peanut (Arachis hypogaea L.) shells and roots. African J Biotechnology. 2012;11(53):11547–11551.
41. Velmurugan P, Sivakumar S, Young-Chae S, et al. Synthesis and characterization comparison of peanut shell extract silver nanoparticles with commercial silver nanoparticles and their antifungal activity. J Industrial Engineering Chemistry. 2015;31:51–54. doi:10.1016/j.jiec.2015.06.031
42. He Y, Wei F, Ma Z, et al. Green synthesis of silver nanoparticles using seed extract of Alpinia katsumadai, and their antioxidant, cytotoxicity, and antibacterial activities. RSC Adv. 2017;7(63):39842–39851. doi:10.1039/C7RA05286C
43. Behravan M, Panahi AH, Naghizadeh A, Ziaee M, Mahdavi R, Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol. 2019;124:148–154. doi:10.1016/j.ijbiomac.2018.11.101
44. Coates J. Interpretation of infrared spectra, a practical approach. In: Meyers RA, editor. Encyclopedia of Analytical Chemistry, Infrared Spectroscopy, John Wiley & Sons Ltd., Chichester, 2006. doi:10.1002/9780470027318.a5606
45. Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;539(12):104–111. doi:10.1016/j.ijpharm.2018.01.034
46. Qian L, Su W, Wang Y, Dang M, Zhang W, Wang C. Synthesis and characterization of gold nanoparticles from aqueous leaf extract of Alternanthera sessilis and its anticancer activity on cervical cancer cells (HeLa). Artif Cells, Nanomed Biotechnol. 2019;47(1):1173–1180. doi:10.1080/21691401.2018.1549064
47. Tang J, Lu X, Chen B, et al. Mechanisms of silver nanoparticles-induced cytotoxicity and apoptosis in rat tracheal epithelial cells. J Toxicol Sci. 2019;44(3):155–165. doi:10.2131/jts.44.155
48. Roy N, Gaur A, Jain A, Bhattacharya S, Rani V. Green synthesis of silver nanoparticles: an approach to overcome toxicity. Environ Toxicol Pharmacol. 2013;36(3):807–812. doi:10.1016/j.etap.2013.07.005
49. Zhang L, Wu L, Si Y, Shu K. Size-dependent cytotoxicity of silver nanoparticles to Azotobacter vinelandii: growth inhibition, cell injury, oxidative stress and internalization. PLoS One. 2018;13(12):e0209020. doi:10.1371/journal.pone.0209020
50. Akter M, Sikder MT, Rahman MM, et al. A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J Advanced Research. 2018;9:1–16. doi:10.1016/j.jare.2017.10.008
51. Hemlata MPR, Singh AP, Tejavath KK. Biosynthesis of silver nanoparticles using cucumis prophetarum aqueous leaf extract and their antibacterial and antiproliferative activity against cancer cell lines. ACS Omega. 2020;5(10):5520–5528. doi:10.1021/acsomega.0c00155
52. Chinnasamy G, Chandrasekharan S, Bhatnagar S. Biosynthesis of silver nanoparticles from melia azedarach: enhancement of antibacterial, wound healing, antidiabetic and antioxidant activities. Int J Nanomedicine. 2019;14:9823. doi:10.2147/IJN.S231340
53. Govindappa M, Hemashekhar B, Arthikala M-K, Rai VR, Ramachandra Y. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Physics. 2018;9:400–408. doi:10.1016/j.rinp.2018.02.049
54. Otunola GA, Afolayan AJ. In vitro antibacterial, antioxidant and toxicity profile of silver nanoparticles green-synthesized and characterized from aqueous extract of a spice blend formulation. Biotechnology Biotechnological Equipment. 2018;32(3):724–733. doi:10.1080/13102818.2018.1448301
55. Adedapo AA, Jimoh FO, Afolayan AJ, Masika PJ. Antioxidant activities and phenolic contents of the methanol extracts of the stems of Acokanthera oppositifolia and Adenia gummifera. BMC Complement Altern Med. 2008;8(1):54. doi:10.1186/1472-6882-8-54
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.