Back to Journals » Clinical Ophthalmology » Volume 13
Combining Porcine Xenograft Intra-Corneal Ring Segments and CXL: a Novel Technique
Authors Kanellopoulos AJ , Vingopoulos F
Received 5 September 2019
Accepted for publication 5 November 2019
Published 17 December 2019 Volume 2019:13 Pages 2521—2525
DOI https://doi.org/10.2147/OPTH.S230011
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Anastasios John Kanellopoulos.
Views: 233
Anastasios John Kanellopoulos,1,2 Filippos Vingopoulos1
1Department of Ophthalmology, LaserVision Clinical & Research Eye Institute, Athens, Greece; 2Department of Ophthalmology, New York University Medical School, New York, NY, USA
Correspondence: Anastasios John Kanellopoulos
LaserVision Clinical & Research Eye Institute, Tsocha str. 15 - 17, Athens 115 21, Greece
Tel + 30 210 7472777
Email [email protected]
Purpose: The ex-vivo feasibility of creating corneal ring segments (ICRS) from biological tissue (xenograft porcine cornea) and combining intra-corneal implantation with Corneal CrossLinking (CXL).
Methods: The ring segments from gamma-irradiated porcine donor cornea were shaped and implanted in human cadaver host cornea using a femtosecond laser for their dissection and host cornea channel preparation. Intra-channel 0.25% riboflavin solution combined with higher fluence CXL of 15 joules total energy followed their implantation. Anterior segment Optical Coherence Tomography (OCT), Scheimpflug tomography and Placido-disc topography were used to monitor the positioning and refractive effect.
Results: The novel xenograft ring segments were positioned as planned at 85% corneal depth and mid-peripheral, with documented, up to 7 diopters central cornea flattening.
Conclusion: Xenograft intracorneal ring segments combined with CXL may offer an alternative to the synthetic materials used clinically so far, aiming in reducing complications of intracorneal opaque deposit accumulation, segment migration, corneal erosion and potential extrusion. Combining CXL may enhance their refractive effect and stabilize potential or documented host ectasia.
Keywords: intrastromal corneal ring segments, gamma-radiated porcine cornea, biological tissue, xenograft corneal surgery, myopia correction, cornea transplantation
Introduction
Intrastromal corneal ring segments (ICRS) are small crescent-shaped pieces of synthetic material that are implanted deep in the corneal stroma in order to modify the corneal curvature and achieve refractive changes. Several studies have demonstrated the efficacy of ICRS in correcting low myopia, in post-laser in situ keratomileusis corneal ectasia, and in keratoconic eyes.1–5 When combined with a CXL procedure, these segments can support a keratoconic cornea and improve vision, while CXL prevents the ectasia progression.5
Polymethyl-methacrylate (PMMA), a biocompatible material widely used in ophthalmology, is currently used to produce the above-mentioned ring segments (PMMA used in INTACS-Addition technology, CA, USA). Despite PMMA’s biocompatibility, various complications of PMMA ICRS have been reported in the literature.9
Allograft tissue ICRS have been reported as a feasible alternative.10 A xenograft biological tissue-derived ICRS that would be devoid of biocompatibility complications has not been introduced yet. The purpose of this paper is to introduce the idea of using porcine cornea in order to create ring segments and to test this preliminary idea by implanting them in a human cadaver cornea (Ex vivo).
Materials and Methods
This study received prior approval from the Laservision Clinical and Research Institute, ethics committee, and did not involve study in live human or animal subjects. The use and disposal of all human and porcine cadaver tissue were according to the declaration of Helsinki.
The dissection of ICRS from freeze-dried gamma-irradiated porcine corneas (CRMI, Wanchai, Hong Kong) as well as the subsequent implantation to a human cadaver cornea took place in our institutions laboratory facilities, and were performed by one surgeon (AJK).
This novel method aims to create ICRS out of porcine corneas (xenografts) instead of PMMA or human allograft donor tissue. The xenograft was dissected with the FS200 femtosecond laser. Two ring segments were dissected from the xenograft, in order to cover a total of 300 degrees. Each segment parameters were:
Arc length: 150 degrees,
Cross-section shape: square,
Thickness (mm): 0.45,
Inner radius: 6.8mm,
Outer radius 8.10mm.
An ex-vivo human cadaver cornea was gamma irradiated and placed in a single-use artificial chamber (Katena Products, Parsippany, NJ, USA) in order to conduct the pre-and post-operative tomography with the Scheimpflug and OCT devices, and to conduct the xenograft ICRS implantation and CXL combined procedures.
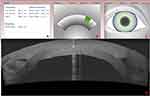
The WaveLight FS200 femtosecond laser (Alcon, Ft. Worth, TX) was used to create the circular channel into which the ICRS would be implanted. The settings are pre-set and illustrated in Figure 1. Specifically for the lamellar channel creation: the outer diameter dissection was set at 8.0mm; the inner diameter at 5.5mm; the depth at 500 micrometers. The vertical incision was set at 1.5mm length, 1.5mm width, and arc position of 0 degrees, as also illustrated in Figure 1. The increased precision of this laser allowed the creation of a well-defined circular insertion channel, preventing digressions to false pathways within the cornea during the ring segment insertion. The insertion point was marked manually on the cornea, and the INTACS channel maker was applied into the femto laser-created channels, in order to complete the path for the implantation. The corneal channel had the following parameters: outer diameter 8 mm, inner diameter 5.5 mm, width 2.5 mm and depth 500 um. The platform length and width were 1.5 mm and 0-degree of incision position. The parameters used on the femtosecond laser are illustrated in Figure 1, images A through C. The thinnest pachymetry of the cornea was 750 µm.
Both rings were implanted in a similar fashion to INTACS,5 with the assistance of forceps. The porcine ICRS were visualized with the operating room slit lamp assessed to be well positioned.
Following implantation, an enhanced corneal crosslinking (CXL) procedure was applied. The channels containing the porcine segments were filled with VibeX Xtra 0.25% riboflavin solution (Avedro, Waltham, Massachusetts), which is a much more highly concentrated solution than the one described in the classic Dresden protocol.11 Once the riboflavin solution was instilled in the channels, higher-fluence UV radiation (30 mW/cm2) for total 7.2 joules was delivered on the corneal (cadaver cornea with the xenograft ICRS implanted) surface with the KXL I device (Avedro, Waltham, MA, USA). Once the riboflavin solution was instilled in the channels, higher-fluence UV radiation (30 mW/cm2) for total 7.2 joules was delivered on the corneal (cadaver cornea with the xenograft ICRS implanted) surface with the KXL I device (Avedro, Waltham, MA, USA).
The Fourier-domain Anterior Segment – Optical Coherence Tomography (AS-OCT) system RTVue 100 (Optovue Inc., Fremont, CA, USA), Scheimplug tomography (Pentacam Scheimpflug imaging device Oculyzer II, WaveLight AG, Erlangen, Germany) and Placido-disc topography (Alcon/WaveLight Allegro Topolyzer Vario, WaveLight AG, Erlangen, Germany) were used to visualize and assess the positioning and refractive changes following the CXL procedure as seen in image D of Figure 1.
Results
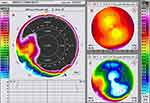
Pre-operative cadaver cornea keratometry was 44.8 Diopters in the cornea apex and revealed 1.6 Diopters of astigmatism: steep keratometry 44.8 Diopters at 45 degrees steep axis, and flat keratometry 43.2 diopters at 135 degrees flat axis. Postoperatively: The position of the ICRS was established. The anterior segment OCT showed well-positioned ICRS with an intact central corneal region (Figure 1D). The Scheimpflug comparative tomography images revealed a significant flattening of up to 7 Diopters (7.21 Diopters flattening) on the corneal apex after the procedure as illustrated in the anterior curvature maps in Figure 2, documenting the flattening achieved in the left image 2A, the pre-op anterior curvature in image 2B upper right, and the post-xenograft ICRS implantation combined with high fluence CXL in image 2C lower right. Specifically the post-operative keratometries were: Cornea apex: 35.7 diopters; steep keratometry 35.7 at 30 degrees; flat keratometry 30.5 diopters at 120 degrees.
Discussion
The use of intralamellar homoplasty in the cornea, in order to achieve refractive flattening and is old, and the first report dates back from 1966!12
Intracorneal ring segments (ICRS) were initially introduced and used clinically for an alternative corneal-based refractive procedure in order to reduce myopia and/or astigmatism. Following initial clinical acceptance for this purpose, its use in healthy, myopic eyes has subsided and instead they have been used extensively in corneal ectasia as a therapeutic intervention. Specifically patients ill-suited for interventions involving corneal ablation, as in thinned keratoconic corneas. These ring segments were implanted in the corneal stroma and their role was to flatten the steep part of the cornea and improve visual acuity.1,2 With ICRS implantation, no elimination of corneal tissue is required and the changes induced in the corneal shape are reversible – the cornea nearly returns to its original state in case the segments are explanted.7
Currently, ICRS are commonly used to flatten misshapen areas of irregular corneas, which has been proven to be a beneficial treatment for patients with keratoconus and post-LASIK keratectasia. These small crescent-shaped implants function by flattening the central corneal curvature while maintaining clarity in the central optic zone.4 When used for keratoconus (or keratectasia), they are often coupled with corneal crosslinking (CXL) to enhance the biomechanical characteristics of the corneal stroma along with the ICRS-mediated correction the visual impairment.5
The biocompatible PMMA material that is currently used to create ICRS is not devoid of complications. These mainly concern the actual stability of the ring segments in the cornea, their movement as well as exposure through the axial wound. Significant corneal thinning, potential risks of intrastromal ring segment corneal extrusion, infection, corneal melt and corneal infiltrate have also been reported by us and other groups.6,8,9 These complications may relate to the difference in biomechanical properties between the rigid and impermeable PMMA material used when compared to the host stromal tissue consisting mainly of collagen. Biological tissue-made ICRS, such as allograft or even xenograft corneal stroma, would potentially be more stable and allow nourishment flow through the cornea stroma of the cornea, as they would resemble a biomechanically supporting biological implant in the cornea.
In this ex-vivo experimental work, two ICRS resembling in parameters the “largest” commercially available INTACT were dissected from a porcine cornea and then implanted in a normal, not keratoconic, human cadaver host cornea. The procedure was followed by an enhanced CXL protocol. Images from OCT showed satisfactory positioning of the segments while the tomography showed significant flattening of the central 6mm of the cornea.
As a single-case presentation, this study does not describe elaborative data accomplished with this technique. It does though demonstrate feasibility in an ex-vivo setting and potential application in-vivo and in potentially ectatic corneas such as keratoconus. These results should enhance the continuation of experimental work in order to proceed in clinical trials and the formation of a new protocol, which will hopefully produce better ICRS with improved biocompatibility and fewer complications.
Disclosure
A.J. Kanellopoulos is a Consultant for Alcon, Avedro, Allergan, i-Optics, Keramed, Zeiss, and ISP Surgical. The authors report no other conflicts of interest in this work.
References
1. Rapuano CJ, Sugar A, Koch DD, et al. Intrastromal corneal ring segments for low myopia. Ophthalmology. 2001;108:1922–1928. doi:10.1016/S0161-6420(01)00804-1
2. Schanzlin DJ, Abbott RL, Asbell PA, et al. Two-year outcomes of intrastromal corneal ring segments for the correction of myopia. Ophthalmology. 2001;108:1688–1694. doi:10.1016/S0161-6420(01)00692-3
3. Chan CCK, Sharma M, Wachler BSB. Effect of inferior-segment intacs with and without C3-R on keratoconus. J Cataract Refract Surg. 2007;33:75–80. doi:10.1016/j.jcrs.2006.09.012
4. Zare MA, Hashemi H, Salari MR. Intracorneal ring segment implantation for the management of keratoconus: safety and efficacy. J Cataract Refract Surg. 2007;33:1886–1891. doi:10.1016/j.jcrs.2007.06.055
5. Kamburoglu G, Ertan A. Intacs implantation with sequential collagen cross-linking treatment in postoperative LASIK ectasia. J Refract Surg. 2008;24:726–729. doi:10.3928/1081597X-20080901-16
6. Hofling-Lima A, Branco BC, Romano A, et al. Corneal infections after implantation of intracorneal ring segments. Cornea. 2004;23:547–549. doi:10.1097/01.ico.0000126434.95325.24
7. Kanellopoulos A, Pe LH, Perry HD, et al. Modified intracorneal ring segment implantations (INTACS) for the management of moderate to advanced keratoconus: efficacy and complications. Am J Ophthalmol. 2006;141:604. doi:10.1016/j.ajo.2006.01.059
8. Coskunseven E, Kymionis GD, Tsiklis NS, et al. Complications of intrastromal corneal ring segment implantation using a femtosecond laser for channel creation: a survey of 850 eyes with keratoconus. Acta Ophthalmol (Copenh). 2011;89:54–57. doi:10.1111/j.1755-3768.2009.01605.x
9. Mitchell B, Kanellopoulos A, Font R. Post intrastromal corneal ring segments insertion complicated by candida parapsilosis keratitis. ClinOphthalmol. 2013;7:443–448.
10. Jacob S, Patel S, Agarwal A, et al. Corneal Allogenic Intrastromal Ring Segments (CAIRS) combined with corneal cross-linking for keratoconus. J Refract Surg. 2018;34:296–303. doi:10.3928/1081597X-20180223-01
11. Choi M, Kim J, Kim EK, Seo KY. Comparison of the conventional dresden protocol and accelerated protocol with higher ultraviolet intensity in corneal collagen cross-linking for keratoconus. Cornea. 2017;36:523–529. doi:10.1097/ICO.0000000000001165
12. Blavatskaya ED. Intralamellar homoplasty for the purpose of relaxation of refraction of the eye. Arch Soc Am Ophthalmol Optom. 1968;6:311–325.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.