Back to Journals » Cancer Management and Research » Volume 12
Combining PD-1 Inhibitor Nivolumab with Radiotherapy Successfully Treated a Patient with Refractory Primary Mediastinal Large B-Cell Lymphoma: A Case Report and Literature Review
Authors Yan Z , Yao ZH, Yao SN, Xia QX, Wang HY, Chu JF, Song M, Zhao S, Liu YY
Received 15 March 2020
Accepted for publication 8 July 2020
Published 27 July 2020 Volume 2020:12 Pages 6311—6316
DOI https://doi.org/10.2147/CMAR.S254007
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Xueqiong Zhu
Zheng Yan,1 Zhi-Hua Yao,1 Shu-Na Yao,1 Qing-Xin Xia,2 Hai-Ying Wang,1 Jun-Feng Chu,1 Ming Song,1 Shuang Zhao,1 Yan-Yan Liu1
1Department of Internal Medicine, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China; 2Department of Pathology, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China
Correspondence: Yan-Yan Liu
Department of Internal Medicine, Affiliated Cancer Hospital of Zhengzhou University, No. 127 Dongming Road, Zhengzhou, Henan 450008, People’s Republic of China
Tel/ Fax +86 371-65587791
Email [email protected]
Abstract: Primary mediastinal large B-cell lymphoma (PMBCL) is relatively infrequent and generally has a good prognosis with standard immunochemotherapy. However, treatment options are limited for patients with relapsed/refractory PMBCL who are ineligible for stem cell transplantation. In this report, we treated a refractory PMBCL patient, who did not respond to salvage chemotherapy, with combined nivolumab and radiotherapy. The patient achieved a complete remission with mild adverse reactions and has survived without relapse 2 years after treatment.
Keywords: primary mediastinal large B-cell lymphoma, immunotherapy, radiotherapy, nivolumab
Introduction
Primary mediastinal large B-cell lymphoma (PMBCL) is an unusually aggressive lymphoma, presumably originating from transformed thymic B cells.1 According to the current World Health Organization classification,2 PMBCL is a distinct entity from diffuse large B-cell lymphoma (DLBCL) with specific clinical, immunohistochemical, and genetic features.3 In most cases, this disease has an extremely aggressive behavior. By definition, PMBCL is present in anterior mediastinal lymphatic tissues, with a rapid growth capacity, usually leading to bulky masses (in at least 70% of patients) and compression of nearby vessels and airways. However, distant lymph nodes are rarely involved, so an early stage at presentation is the rule. Despite remarkable advances in first-line treatment for PMBCL patients in the rituximab era, 10–30% of patients still experienced disease progression or relapse.4 Moreover, the rarity of relapsed/refractory (rr) PMBCL has limited the ability to conduct clinical trials, and no standard treatment has been identified. Patients with chemosensitive rrPMBCL are often treated with high-dose chemotherapy followed by autologous or allogeneic stem cell transplantation (SCT). Long-term survival has been observed in about 50% of chemosensitive patients after SCT.5–7 However, for patients with chemoresistant rrPMBCL, they have limited treatment options and their prognosis is extremely poor.1,7
Recently, immunotherapy represented by PD-1 inhibitors has shown promising activity in treating rrPMBCL. Yet, immunotherapy is not a cure since over 50% of patients are not responding to it, with a median progression-free survival (PFS) of only 5.5 to 10.4 months.8,9 On the other hand, increasing evidence has documented a synergistic effect of immunotherapy and radiotherapy in solid cancers.10 Therefore, we speculated that combining radiotherapy and immunotherapy is a reasonable treatment choice for localized chemoresistant rrPMBCL. Here, we reported a successful treatment of a refractory PMBCL patient using combined nivolumab and radiotherapy.
Case Report
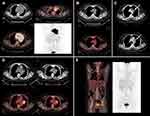
A 33-year female was admitted in November 2016 with complaints of dry cough for 3 months. She denied dyspnea, fever, night sweats, and weight loss. Computerized tomography (CT) revealed a big mass in the anterior mediastinum. CT-guided percutaneous core needle biopsy of the mass showed a diffuse proliferation of medium to large sized B cells with sclerosis and compartmentalization. The cells displayed varied morphology with polymorphic nuclei and abundant clear cytoplasm. The tumor cells were positive for CD20, Pax-5, CD30, Bcl-2, and Bcl-6, and negative for CD3, CD15, CD23, CD10, and Mum-1. Positron emission tomography-computed tomography (PET/CT) showed a huge hypermetabolic mass (123 mm × 93 mm) in the mediastinum, with a fluorodeoxyglucose (FDG)-avid lymph node in the left supraclavicular fossa. The SUVmax was as high as 30 (Figure 1A). Laboratory examination revealed an elevated lactate dehydrogenase (LDH, 414 U/L) (Table 1), and bone marrow examination was negative. Based on these clinical and pathological features, the patient was diagnosed with PMBCL, stage IIX, according to the Lugano classification.11 The patient received 6 cycles of R-DA-EPOCH (rituximab, dose-adjusted etoposide, prednisone, vincristine, and doxorubicin) chemotherapy from January 2017 to April 2017. A complete response (CR) was achieved at the end of treatment based on the PET/CT evaluation (Figure 1B) and according to the Lugano classification. The LDH reduced to 192 U/L. Three months after the treatment, recurrence occurred at the primary tumor site indicated by CT scan (Figure 1C). Then, LDH was 185 U/L. Two cycles of salvage chemotherapy with R-DHAP (rituximab, dexamethasone, cytarabine, and cisplatin) were administered. However, the disease remained stable (Figure 1D), with LDH rising to 779 U/L.
 |
Table 1 LDH Values During Treatment Course |
Radiotherapy might be a reasonable choice for this localized chemoresistant tumor; however, systemic failure is a major concern for its use. Immunohistochemical (IHC) staining of PD-L1 in previous biopsy specimen demonstrated positive (approximately 80% positive cells; Ventana PD-L1 antibody, cat. SP263) (Figure 2). Given the highly chemoresistant nature of the disease, autologous stem cell transplantation was not recommended while a suitable allogeneic donor was not found. After multidisciplinary consultation, we gave the patient concurrent radiation therapy and immunotherapy with nivolumab. A total dose of 5000 cGy was delivered to the tumor site in 25 fractions, 200 cGy per fraction, over 5 weeks. Nivolumab was given intravenously a total dose of 200 mg every two weeks, and treatment continued after radiotherapy. A PET/CT-assessed CR was obtained three months after radiation in March 2018 (Figure 1E), when LDH was 181 U/L. Nivolumab was administered continuously until September 2019.
 |
Figure 2 PD-L1 immunohistochemistry. Original magnification 20 x. |
A grade 1 radiation pneumonitis occurred two months after radiotherapy, which spontaneously resolved one month later. During the maintenance immunotherapy, no other adverse events were observed except for a grade 1 hypothyroidism. The patient was still in CR by the submission date of this manuscript.
Discussion and Literature Review
Treatment options for patients with chemoresistant rrPMBCL are limited. Treatment guidelines, including NCCN guidelines in the United States, ESMO Clinical Practice Guidelines in Europe, and South Wales Cancer Network in the United Kingdom, currently recommend clinical trial, palliative chemotherapy, and palliative radiation for rrPMBCL patients who are not candidates for high-dose therapy and SCT.1
Several new drugs have shown some antitumor activity in the treatment of rrPMBCL in recent years. As CD30 antigen is heterogeneously present in 80% of cases with PMBCL,12 brentuximab vedotin (BV) has been introduced for patients with rrPMBCL. In two Phase 2 trials with limited sample sizes, the ORRs of BV-treated rrPMBCL patients were only 17% (1/6) and 13.3% (2/15).13,14 Although the efficacy of BV monotherapy was disappointing, the addition of BV to the PD-1 inhibitor pembrolizumab showed impressive activity in a phase 2 trial. In the trial of 30 patients with rrPMBCL, the CR and ORR were 37% and 73%, respectively (Table 2).15 So far, BV is not available in China.
 |
Table 2 Clinical Trials Associated with Immunotherapy for rrPMBCL |
Like classical Hodgkin lymphoma, PMBCL frequently exhibits chromosomal aberrations at 9p24.1, a region that contains the genes CD274 and PDCD1LG2 encoding PD-L1 and PD-L2, respectively, leading to the elevated levels of PD-L1 and PD-L2 in tumor cells.16–19 Aside from amplification, chromosome breaks at 9p are frequently observed as well,18 which result in the fusion of CD274 or PDCD1LG2 with other genes, such as CIITA or IgH, leading to overexpression of PD-L1 and/or PD-L2.20,21 Published data of chromosomal aberrations associated with PD-L1/PD-L2 checkpoints are summarized in Table 3. PD-L1 overexpression in PMBCL rationalizes the attempt of using a PD-1 inhibitor to treat this disease. PD-1 inhibitor pembrolizumab has been evaluated in two trials in patients with rrPMBCL. The ORR was 48% among 21 patients in the KEYNOTE-013 trial and 45% among 53 patients in the KEYNOTE-170 trial. However, the median PFS was only 10.4 and 5.5 months, respectively, which were far from satisfactory (Table 2).8,9 Clinical trials using various cancer therapies in combination with immunotherapy are under investigation, including CAR-T cell therapy, histone deacetylase inhibitor, PI3K inhibitor, anti-CD27 antibody, demethylation agent, and cytotoxic drugs (Table 4).
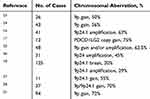 |
Table 3 Chromosomal Aberrations Involving PD-L1/PD-L2 Checkpoints in PMBCL |
 |
Table 4 Ongoing Clinical Trials Utilizing Immune Checkpoint Blockade in rrPMBCL (by 25-Apr-2020) |
The combination of radiotherapy and immunotherapy is under intensive investigation in patients with solid cancers. It is believed that radiation has immunomodulatory effects. Radiation-induced DNA damage can induce the release of neoantigens from tumor cells for immune recognition. Radiation may stimulate the expression of MHC molecules on tumor cells to facilitate the presentation of tumor antigens to cytotoxic T cells. Radiation is also capable of promoting the phagocytosis of damaged tumor cells by antigen-presenting cells, leading to increased priming and activation of tumor-specific T cells. Moreover, radiation activates antitumor immune responses by triggering the stimulator of interferon genes (STING)-mediated DNA-sensing pathway, increasing infiltration of CD8+ T cells while reducing myeloid-derived suppressor cell accumulation, and upregulating the surface expression of PD-L1 on tumor cells.29 On the other hand, immunotherapy may also sensitize tumors to radiation by promoting tumor blood vessel normalization, improving tissue perfusion, and decreasing intratumoral hypoxia.30,31 It has been shown that the combination of immunotherapy and radiotherapy is well tolerated by most patients with solid cancers.10 For patients with thoracic cancer, the most safety concern of radiation and immunotherapy is pneumonitis, as either one can cause pneumonitis. In the ETOP NICOLAS trial evaluating the safety of concurrent nivolumab and radiotherapy in stage III non-small-cell lung cancer, the overall incidence of pneumonia was 42.5% and that of grade 3 pneumonitis 10%, which seem acceptable.32 Reflecting evidence from these clinical studies, we gave our patient nivolumab in combination with radiotherapy.
The patient in this report was not a candidate for high-dose therapy and SCT. Consistent with previous reports, we found that PD-L1 as a generally recognized response predictor was overexpressed in the patient’s tumor tissue. Based on the aforementioned theories and evidences, we give our patient radiation to the tumor site to obtain the best local control, and nivolumab to synergize with radiation and prevent systemic failure. As we expected, the patient achieved a durable CR with mild toxicities. According to the characteristics of immunotherapy, this patient was possibly cured, as the response has lasted for 2 years. To our knowledge, this is the first case with rrPMBCL to be successfully treated by immunotherapy and radiotherapy. Immunotherapy in combination with radiotherapy might be a feasible option for patients with localized rrPMBCL. This treatment strategy warrants confirmation in future clinical trials.
Ethics Statement
This study was approved by the Research Ethics Committee of affiliated Cancer Hospital of Zhengzhou University, and written informed consent has been provided by the patient to have the case details and any accompanying images published.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Addis BJ, Isaacson PG. Large cell lymphoma of the mediastinum: a B-cell tumour of probable thymic origin. Histopathology. 1986;10(4):379–390. doi:10.1111/j.1365-2559.1986.tb02491.x
2. Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83–87.
3. Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102(12):3871–3879. doi:10.1182/blood-2003-06-1841
4. Takyar J, Raut M, Borse R, Balakumaran A, Sehgal M. Relapsed/refractory primary mediastinal large B-cell lymphoma: a structured review of epidemiology, treatment guidelines and real-world treatment practices. Expert Rev Hematol. 2020;1–13.
5. Aoki T, Shimada K, Suzuki R, et al. High-dose chemotherapy followed by autologous stem cell transplantation for relapsed/refractory primary mediastinal large B-cell lymphoma. Blood Cancer J. 2015;5(12):e372. doi:10.1038/bcj.2015.101
6. Vardhana S, Hamlin PA, Yang J, et al. Outcomes of relapsed and refractory primary mediastinal (Thymic) Large B cell lymphoma treated with second-line therapy and intent to transplant. Biol Blood Marrow Transplant. 2018;24(10):2133–2138. doi:10.1016/j.bbmt.2018.06.009
7. Herrera AF, Chen L, Khajavian S, et al. Allogeneic stem cell transplantation provides durable remission in patients with primary mediastinal large B cell lymphoma. Biol Blood Marrow Transplant. 2019;25(12):2383–2387. doi:10.1016/j.bbmt.2019.07.041
8. Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130(3):267–270. doi:10.1182/blood-2016-12-758383
9. Armand P, Rodig S, Melnichenko V, et al. Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma. J Clin Oncol. 2019;37(34):3291–3299. doi:10.1200/JCO.19.01389
10. Meng X, Feng R, Yang L, Xing L, Yu J. The role of radiation oncology in immuno-oncology. The Oncologist. 2019;24(Suppl S1):S42–S52. doi:10.1634/theoncologist.2019-IO-S1-s04
11. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi:10.1200/JCO.2013.54.8800
12. Steidl C, Gascoyne RD. The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood. 2011;118(10):2659–2669. doi:10.1182/blood-2011-05-326538
13. Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125(9):1394–1402. doi:10.1182/blood-2014-09-598763
14. Zinzani PL, Pellegrini C, Chiappella A, et al. Brentuximab vedotin in relapsed primary mediastinal large B-cell lymphoma: results from a phase 2 clinical trial. Blood. 2017;129(16):2328–2330. doi:10.1182/blood-2017-01-764258
15. Zinzani PL, Santoro A, Gritti G, et al. Nivolumab combined with brentuximab vedotin for relapsed/refractory primary mediastinal large B-cell lymphoma: efficacy and safety from the Phase II CheckMate 436 study. J Clin Oncol. 2019;37(33):3081–3089. doi:10.1200/JCO.19.01492
16. Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–3277. doi:10.1182/blood-2010-05-282780
17. Shi M, Roemer MG, Chapuy B, et al. Expression of programmed cell death 1 ligand 2 (PD-L2) is a distinguishing feature of primary mediastinal (thymic) large B-cell lymphoma and associated with PDCD1LG2 copy gain. Am J Surg Pathol. 2014;38(12):1715–1723. doi:10.1097/PAS.0000000000000297
18. Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123(13):2062–2065. doi:10.1182/blood-2013-10-535443
19. Van Roosbroeck K, Ferreiro JF, Tousseyn T, et al. Genomic alterations of the JAK2 and PDL loci occur in a broad spectrum of lymphoid malignancies. Genes Chromosomes Cancer. 2016;55(5):428–441. doi:10.1002/gcc.22345
20. Steidl C, Shah SP, Woolcock BW, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471(7338):377–381. doi:10.1038/nature09754
21. Mottok A, Hung SS, Chavez EA, et al. Integrative genomic analysis identifies key pathogenic mechanisms in primary mediastinal large B-cell lymphoma. Blood. 2019;134(10):802–813. doi:10.1182/blood.2019001126
22. Armand P, Rodig S, Melnichenko V, Thieblemont C. Pembrolizumab in patients with relapsed or refractory primary mediastinal large B-Cell Lymphoma (PMBCL): data from the Keynote-013 and Keynote-170 studies. Blood. 2018;132(Supplement 1):228. doi:10.1182/blood-2018-99-110220
23. Joos S, Otano-Joos MI, Ziegler S, et al. Primary mediastinal (thymic) B-cell lymphoma is characterized by gains of chromosomal material including 9p and amplification of the REL gene. Blood. 1996;87(4):1571–1578. doi:10.1182/blood.V87.4.1571.bloodjournal8741571
24. Bentz M, Barth TF, Bruderlein S, et al. Gain of chromosome arm 9p is characteristic of primary mediastinal B-cell lymphoma (MBL): comprehensive molecular cytogenetic analysis and presentation of a novel MBL cell line. Genes Chromosomes Cancer. 2001;30(4):393–401. doi:10.1002/1098-2264(2001)9999:9999<::AID-GCC1105>3.0.CO;2-I
25. Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198(6):851–862. doi:10.1084/jem.20031074
26. Lenz G, Wright GW, Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A. 2008;105(36):13520–13525. doi:10.1073/pnas.0804295105
27. Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869–881. doi:10.1182/blood-2015-10-673236
28. Chapuy B, Stewart C, Dunford AJ, et al. Genomic analyses of PMBL reveal new drivers and mechanisms of sensitivity to PD-1 blockade. Blood. 2019;134(26):2369–2382. doi:10.1182/blood.2019002067
29. Wang Y, Liu ZG, Yuan H, et al. The reciprocity between radiotherapy and cancer immunotherapy. Clin Cancer Res. 2019;25(6):1709–1717. doi:10.1158/1078-0432.CCR-18-2581
30. Tian L, Goldstein A, Wang H, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544(7649):250–254. doi:10.1038/nature21724
31. Zheng X, Fang Z, Liu X, et al. Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J Clin Invest. 2018;128(5):2104–2115. doi:10.1172/JCI96582
32. Peters S, Felip E, Dafni U, et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer – The ETOP NICOLAS trial. Lung Cancer. 2019;133:83–87. doi:10.1016/j.lungcan.2019.05.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.