Back to Journals » Journal of Pain Research » Volume 9
Combining pain therapy with lifestyle: the role of personalized nutrition and nutritional supplements according to the SIMPAR Feed Your Destiny approach
Authors De Gregori M, Muscoli C, Schatman ME , Stallone T, Intelligente F, Rondanelli M, Franceschi F, Arranz LI, Lorente Cebrián S, Salamone M, Ilari S, Belfer I, Allegri M
Received 15 June 2016
Accepted for publication 4 August 2016
Published 8 December 2016 Volume 2016:9 Pages 1179—1189
DOI https://doi.org/10.2147/JPR.S115068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Robinson
Manuela De Gregori,1–3 Carolina Muscoli,2,4,5 Michael E Schatman,2,6 Tiziana Stallone,2,7 Fabio Intelligente,2,8 Mariangela Rondanelli,2,9 Francesco Franceschi,2,10 Laura Isabel Arranz,2,11 Silvia Lorente-Cebrián,2,12 Maurizio Salamone,2,13,14 Sara Ilari,2,5 Inna Belfer,2,15 Massimo Allegri2,16,17
1Pain Therapy Service, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 2Study in Multidisciplinary Pain Research Group, 3Young Against Pain Group, Parma, Italy; 4Department of Health Sciences, Institute of Research for Food Safety and Health, University “Magna Graecia” of Catanzaro, Parma, Italy; 5IRCCS San Raffaele Pisana, Roccelletta di Borgia, Catanzaro, Italy; 6US Pain Foundation, Bellevue, WA, USA; 7ENPAB, Rome, 8Chronic Pain Service Anestesia Day-Surgery, IRCCS Humanitas Research Hospital, Rozzano, 9Department of Public Health, Section of Human Nutrition and Dietetics, Azienda di Servizi alla Persona di Pavia, University of Pavia, Pavia, 10Institute of Internal Medicine, Catholic University of Rome, Rome, Italy; 11Department of Nutrition, Food Sciences and Gastronomy, University of Barcelona, Barcelona, 12Department of Nutrition, Food Science and Physiology, Center for Nutrition Research, University of Navarra, Pamplona, Spain; 13Metagenics Italia srl, Milano, 14Italian Lifestyle Medicine Association, Bari, Italy; 15Faculty of Dentistry, McGill University, Montreal, QC, Canada; 16Department of Surgical Sciences, University of Parma, 17Anesthesia, Intensive Care and Pain Therapy Service, Azienda Ospedaliero, Universitaria of Parma, Parma, Italy
Abstract: Recently, attention to the lifestyle of patients has been rapidly increasing in the field of pain therapy, particularly with regard to the role of nutrition in pain development and its management. In this review, we summarize the latest findings on the role of nutrition and nutraceuticals, microbiome, obesity, soy, omega-3 fatty acids, and curcumin supplementation as key elements in modulating the efficacy of analgesic treatments, including opioids. These main topics were addressed during the first edition of the Study In Multidisciplinary Pain Research workshop: “FYD (Feed Your Destiny): Fighting Pain”, held on April 7, 2016, in Rome, Italy, which was sponsored by a grant from the Italian Ministry of Instruction on “Nutraceuticals and Innovative Pharmacology”. The take-home message of this workshop was the recognition that patients with chronic pain should undergo nutritional assessment and counseling, which should be initiated at the onset of treatment. Some foods and supplements used in personalized treatment will likely improve clinical outcomes of analgesic therapy and result in considerable improvement of patient compliance and quality of life. From our current perspective, the potential benefit of including nutrition in personalizing pain medicine is formidable and highly promising.
Keywords: pain, personalized nutrition, nutritional supplements
Introduction
In addition to the severity of the underlying condition, interindividual variability in chronic pain depends on many factors, including its sociocultural context,1 patients’ genetic backgrounds,2,3 psychological factors,4 and pathophysiology,5 that can be modulated and monitored by altering nutritional habits.6 During the eighth annual international meeting of the Study In Multidisciplinary Pain Research (SIMPAR), we attempted to remedy the knowledge gap in this field by conducting a workshop, entitled “FYD (Feed Your Destiny): Fighting Pain”. The main focus of this workshop was on the association of nutrition and nutraceuticals with pain development and perception, appropriate nutrition for patients with cancer, the role of obesity in pain chronification, the microbiome involved in pain, and the specific role of soy, omega-3 fatty acids, curcumin, and polyphenols on inflammatory and degenerative painful diseases as well as on opioid tolerance (Figure 1). This workshop followed the philosophy of the SIMPAR group, which has strived to direct the international and multidisciplinary dissemination of the most innovative issues regarding pain, ranging from basic science to translational and clinical research. The workshop also integrated a diverse array of theoretical and practical views on the relationship between chronic pain and nutrition. Our ultimate goal was to identify a starting point for a study group aimed to define a new internationally accepted guideline that will seek the integration of nutrition with pharmacological approaches. To this end, we are designing a multicenter randomized placebo-controlled clinical trial evaluating the value of personalized nutrition and nutraceutical supplements as well as assessing the genetic backgrounds and epigenetic dynamicity to identify new objective biomarkers of efficacy for the management of chronic pain. This approach will further the efforts to define a precision medicine model in the treatment of pain. In particular, we will link personalized nutritional programs and nutraceutical supplements with clinical outcomes in terms of efficacy and safety of various therapies and analyze their correlation with the interindividual variability of genetic backgrounds and the modulation of epigenetic patterns (DNA methylation and microRNA expression). Our expectation is that this nutritional approach will further clarify the role of precision medicine in the treatment of pain, acting as an essential, integrated, non-pharmacological strategy and as an aspect of multidisciplinary treatment for chronic pain.
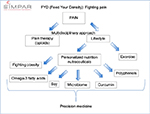  | Figure 1 Scheme of summary of FYD workshop. Abbreviation: FYD, Feed Your Destiny. |
Workshop description
The workshop was divided into two sessions. Doctor Stallone, who is the President of the Italian National Institution for Social Security and Assistance for Biologists (ENPAB) from Rome, described the state of the art of nutrition, stressing the importance of current evidence on obesity comorbidity in the development and progression of a variety of chronic pain conditions. Professor Muscoli from the University of Magna Graecia in Catanzaro presented the current data regarding nutraceuticals, with a focus on their roles in pain conditions. Then, Professor Rondanelli from the University of Pavia addressed topics of nutrition in patients receiving opioid therapy, emphasizing the ideal nutritional pyramid to be followed to optimize these patients’ pain management. Professor Franceschi from the Catholic University of Rome elucidated the importance of the gut microbiome in chronic pain. Professor Arranz from the University of Barcelona provided the final lecture of the first session, demonstrating the role of obesity in the function and quality of life (QoL) in patients with chronic pain conditions.
Professor Belfer from McGill University, who served as one of the Presidents of the SIMPAR meeting, described the role of soy in pain and analgesia. She was followed by Professor Lorente-Cebrián from the University of Navarra and Doctor Salamone from Metagenics, a privately held company in Italy that develops nutritional products to maximize genetic potentials, who provided an overview of the effects of omega-3 fatty acids and curcumin on chronic pain. Finally, Doctor Ilari from the Hospital IRCCS San Raffaele Pisana in Rome (a private rehabilitation hospital and research center in Rome) clarified the crucial role of natural derived antioxidants in inflammation and opioid tolerance.
Review of relevant research
The regulation of food intake involves a close relationship between homeostatic and non-homeostatic factors. In particular, homeostatic factors influence the hypothalamic arcuate nucleus and the solitary tract, acting on hunger satiety centers. Ghrelin, YY peptide, spinal nerves, and the vagus nerve provide short-term signals, while leptin, adiponectin, and insulin provide long-term signals of body weight and fat storage.7 The problem of poor nutrition also has neurochemical implications, as levels of dopamine, the “neurotransmitter of pleasure”, increase with consumption of sugars, fats, and salt.
Alonso-Alonso et al8 recently described “food addiction”, explaining that food, like any other rewarding stimulus, has the potential to cause addictive behavior. Drugs and foods share certain traits, yet differ qualitatively and quantitatively. Indeed, drugs directly influence brain dopamine circuits, while food influences dopamine in more indirect ways: from neuronal input from the taste buds to dopamine-secreting neurons in the brain, as well as from signals generated by digestion. Moreover, some people are less influenced than others by the reward system, due to a D2 receptor that is less responsive to dopamine due to interindividual variability of genetic backgrounds, resulting in variability in the amount of food required to reach the same level of pleasure.9 Behavioral drives for palatable food are also moderated by cognition; lateral and dorsomedial regions of the cortex (dorsomedial region and parietal cortex) can influence the reward system. Therefore, the combination of cognitive behavioral therapy, nutritional counseling, physical exercise, and human relationship counseling should be able to suppress the rewarding effects of food and provide reinforcement through the frontal cortex.
The ENPAB and Brain Research Foundation network is studying eating behavioral aspects of food addiction; Italian nutrition biologists have recently initiated an “observatory for the study of eating behavior”. The nutraceutical discipline has been coupled with nutritional counseling in the management and prevention of disease.6,10,11 The term “nutraceutical” was coined from the combination of “nutrition” and “pharmaceutical” in 1989 by Doctor Stephen DeFelice. The term refers to a food, or part of a food, providing health benefits, including the prevention and treatment of a disease.12 The global nutraceutical market has been valued at approximately $250 billion in 2014: the market is expected to reach $385 billion by 2020 at a compounded annual growth rate of 7.5% from 2014 to 2020. The nutraceutical market includes functional foods, beverages, and dietary supplements. The annual sales of nutraceutical ingredients market worldwide have been predicted to reach 29.5 billion USD, with nutraceutical product sales predicted to reach 204.8 billion USD in 2017.13,14 Nutraceuticals are products purified from foods generally sold in medicinal forms, such as powders, tablets, or capsules, which have been developed to provide protection against chronic disease. Nutraceuticals can be derived from plants, animals, and microorganisms (eg, essential fatty acids and enzymes) and from marine sources (eg, glucosamine, chitosan, and fish oils). A nutraceutical can also deliver bioactive agents in doses achievable through a healthy diet, although these compounds also provide specific features such as a formulation that enhances their absorption and/or physiological effect. Isolated compounds (although not extracts) from nonfood origin can be used in nutraceuticals only if these compounds are also usually present in foods. Other dietary supplements such as those containing vitamins, minerals, amino acids, and other nutrients or micronutrients with well-known roles in human nutrition and/or known recommended daily allowances are recognized as nutraceuticals if the dosage provided is clearly related to a beneficial effect beyond their recommended daily allowance values.
For the safe use of a new dietary ingredient (NDI), the following information about its chemical nature should be provided: the nature of the extract (raw, enriched, purified), fermentation, chemical synthesis, chemical name of the efficacious molecule, and its impurity profile.15 In the case of non-single compound NDI, it is necessary to standardize the dose/content of efficacious molecules and provide evidence regarding the role of other compounds with high prevalence in the mixture and their relevant effect; it is necessary to understand the stability of a compound in its galenic form and ascertain its bioavailability data in humans. With the assumption that the safe human use of an NDI is documented by food-based exposure data (human plasma levels) and by additional preclinical and clinical safety data, the dietary intake of populations with the highest safe and beneficial dietary intake of the compound is used as a basis to define the intended dose.16 The intended dose can be significantly higher (more than threefold) than the dietary intake, provided that it is supported by additional safety data. The recommended dose must be at least a small multiple below the safe upper limit of the dietary ingredient.17–19
Nutrition, opioids, and cancer
Despite their drawbacks, opioids are still considered by many as the most effective analgesic for chronic pain.20,21 One of the main concerns regarding their use is opioid-induced constipation (OIC), due to their binding to receptors in the gastrointestinal (GI) tract. In mild cases of OIC, symptoms can be mitigated by increasing physical activity together with dietary fiber and fluid intake. More severe cases require specialized medications that target the unique characteristics of OIC. Constipation has been reported to be one of the four factors that significantly and independently predict hospitalization in hospice care.22 Estimates of OIC have been found to vary widely, with investigations identifying rates of OIC among opioid users ranging from 15% to 90%.23–26 In addition, a series of studies conducted in a large hospice in Florida have evaluated the constipation induced by opioids and demonstrated that a considerable proportion of patients hospitalized with cancer (from 40% to 64%) experienced constipation. Interestingly, the number was found to be lower when the data were obtained from a screening of staff (40%) and higher when the researchers interviewed patients regarding their symptoms (63%–64%).27–29 In conclusion, mitigating constipation is an important aspect of the management of patients with opioid-related adverse drug events, as various studies have demonstrated that the presence of constipation is significantly negatively correlated with overall QoL.30,31 The ideal dietary approach for patients on opioids should include increased consumption of fiber and fluids (at least eight glasses daily unless contraindicated), in conjunction with physical activity,32 with abdominal exercises in bed or moving from bed to chair if the patient is not able to walk. Patients are encouraged to eat more high-fiber foods such as fruits (eg, raisins, prunes, peaches, and apples), vegetables (eg, squash, broccoli, carrots, and celery), and 100% whole-grain cereals, breads, and bran. Nevertheless, fiber intake should occur several hours prior to or following the drug administration to mitigate a reduction in the bioavailability of certain drugs.33 Rondanelli et al (unpublished, 2016) have recently designed a food pyramid for patients requiring opioid analgesia, based on anti-inflammatory and antioxidant agents (submitted article). In the presence of a constipation problem, a proper distribution of five meals during the day is suggested, based on breakfast, mid-morning snack, lunch, mid-afternoon snack, and dinner, to activate gastro-ileocecal reflection and allow the progression of food to the rectum. Moreover, it is beneficial to emphasize the intake of both soluble and insoluble fiber, in particular, to reduce constipation.34,35
Microbiome and pain
Mutualistic bacteria (~800–1,000 g) colonize the entire GI tract with different orders of magnitude, increasing from the esophagus to the anus. The so-called gut bacteriome involves >10,000 genes, more than nine phyla (of which ~95% of genes have been identified), >1,000 species (of which 99% of genes have been identified), and >17,000 strains (of which 100% of genes have been identified). The two major phyla are Firmicutes and Bacteroidetes (which constitute 80%–90% of microbiota).36–38 Gut microbiota have specific effects in each section of the GI tract: barrier effect, immunocompetence/tolerance, synthesis, metabolism, drug metabolism, and behavior conditioning. A strong balance between all microbiota species is modulated by several factors, including gastric acidity, biliary salts, mucus thickness, normal peristalsis, and normal anatomy, while a failure of their activity determines an imbalance of gut microbiota composition called dysbiosis, which, in turn, may cause GI and non-GI diseases, including metabolic syndromes. Small intestinal bacterial overgrowth is a specific kind of dysbiosis that is associated with various manifestations, including malabsorption and small bowel carbohydrate fermentation with gas production, abdominal pain, and diarrhea.
The most commonly used agents for preventing or treating nonsteroidal anti-inflammatory drugs (NSAIDs)-induced damage are proton pump inhibitors (PPIs). However, these medications do not offer protection to the lower small intestine and actually exacerbate NSAID-induced small intestinal lesions. A critical role of bacteria composing gut microbiota was recently reinforced by a study demonstrating that NSAID enteropathy in rats is exacerbated by concomitant treatment with a PPI through a dysbiotic mechanism. Specifically, the investigators identified a marked loss of Bifidobacterium and Lactobacilli following PPI treatment.39 NSAID-related gastroenteropathy is due to a decrease of Lactobacilli, with an impairment of the maintenance of luminal pH, mucosal permeability, enterocyte adhesion, mucus production, and immune system modulation. The concomitant decrease of Bifidobacterium exacerbates both intestinal motility and local immunity.40 These data allow us to conclude that NSAIDs induce mucosal injuries confined not only to the stomach but also to the small intestine.41 This provides an explanation for NSAID-related abdominal pain. In this regard, paracetamol administration may be safer than NSAIDs, as its utilization does not require concomitant treatment with PPI, impair gut permeability, and affect platelet function.42 Opioids may also be a valid option in treating abdominal pain. Based upon the Italian Intersociety Recommendations (SIAARTI, SIMEU, SIS 118, AISD, SIARED, SICUT, IRC) on pain management in the emergency setting, statement F, pain relief and the use of opioids in patients with acute abdominal pain do not increase the risk of error in diagnosis and therapeutic pathways in adults.43
Obesity and chronic pain
Obesity is categorized by using body mass index (BMI), which is calculated as the body weight divided by the square of body height (kg/m2). Depending on BMI values, people may be classified as underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9), or obese (≥30). Approximately 39% of adults (18 years and older) worldwide are overweight, with 13% obese (World Health Organization, 2015, http://www.who.int/mediacentre/factsheets/fs311/en/). Obesity is related to important metabolic diseases such as diabetes, hypertension, heart disease, and hypercholesterolemia. Nevertheless, ~80% of enrolled patients fail to complete weight loss programs (Federation of European Nutrition Societies data). It is estimated that by 2025, the global obesity prevalence will reach 18% among men and exceed 21% among women, while severe obesity will exceed 6% among men and 9% among women.44 Growing evidence suggests that there is a precise relationship between obesity and chronic pain; they coexist and adversely impact each other (reciprocal negative effects).45–47 Obesity and pain serve to further reduce functional capacity and QoL,48 causing patients to become less physically active and more depressed, with consequences for sleep, stress, lifestyle, and chronic inflammation status. Accordingly, a reduction from high to normal BMI may improve QoL.49 The effect of obesity in chronic pain conditions has been studied in fibromyalgia, osteoarthritis, rheumatoid arthritis, and low back pain. Thus, the management of obesity as well as chronic pain should be considered synergistic. Adipose tissue is not only an energy store but also an active endocrine organ involved, among other functions, in the regulation of inflammation.50 Obese individuals suffer more chronic pain than normal weight subjects;51 therefore, changes in lifestyle can help improve both obesity and chronic pain conditions.52,53 A study demonstrated that increasing BMI, specifically android distribution of fat mass, was strongly associated with foot pain and disability.54 In contrast, a beneficial effect of a gynoid distribution of fat was observed, suggesting that the mechanism of obesity’s effect on disability might be the result of both a mechanical effect (through increasing the load on the skeletal system) and a systemic effect related to metabolic factors and pro-inflammatory cytokine production.54
Soy and pain
Soybeans are a source of phytoestrogens (such as isoflavone genistein) with potent estrogenic activity. Studies suggest that soy consumption may result in several pain-related functions including increasing anti-nociception via GABAA receptor mechanisms55 inhibiting the protein kinase C,56 modulating cytokines57 and the immune response,58 and acting as an antioxidant.59 Interestingly, the effects of soy on human pain are gender based to a degree, with higher analgesia observed in men.60 In rats, the effects of soy on neuropathic pain have been demonstrated to be model specific. The US Food and Drug Administration in 1999 approved the claim of soy in lowering cholesterol, and soy immediately became part of a “healthy lifestyle”, with reported positive effects on reducing symptoms and signs of many diseases from diabetes and osteoporosis to diarrhea and kidney diseases.61 However, mixed results were found for soy’s analgesic effects, for example, on cyclical breast pain.62 Several animal and human studies have been performed, suggesting that the effects of soy depend on the kind of soy used; soy beverages seem not to have an impact on women with breast cancer,63 revival soy does not affect the symptoms of fibromyalgia (no improvements in pain, function, and depression),64 while whole soybean soymilk powder appears to reduce neuropathic pain when administered preemptively.57,65 The effects of soy on pain also depend upon the specific pain condition. It is protective against postsurgical (postmastectomy) pain66 and osteoarthritis67 and is an analgesic for menopausal68 and bone cancer pain,69 but it increases other types of pain such as migraines.70 Finally, soy protein has a gender-specific action, as it improves osteoarthritis pain in men although not in women.67 Currently, it remains to be determined when and how soy intervention is involved in obtaining major analgesic effects in the individual patient.
Omega-3 fatty acids and pain
Several diseases have an inflammatory component, such as obesity or neurodegenerative diseases of aging. Eicosapentaenoic acid (EPA) (20 carbons:5 double bonds) and docosahexaenoic acid (DHA) (22 carbons:6 double bonds) are long-chain polyunsaturated fatty acids (LC-PUFA), omega-3 (n-3) fatty acids, whose source is represented by fish oils and fish oil supplements. Both EPA and DHA derive from the α-linolenic acid (ALA 18:3 omega-3). They block inflammation through a variety of mechanisms of action, proresolving lipid mediators.71,72 In vivo studies demonstrated that omega-3 LC-PUFAs prevent inflammatory symptoms linked to obesity rather than adiposity in obese and metabolic syndrome conditions.73–93 Omega-3 LC-PUFAs also play a role in cardiovascular diseases.88,94–109 Various health associations including the American Heart Association and the American Diabetes Association recommend daily fish and/or n-3 LC-PUFAs (EPA/DHA) intake for the prevention of chronic heart disease and cardiovascular diseases. Regarding degenerative disorders, longitudinal studies have demonstrated negative correlations between fish intake and cognitive impairment in mental/cognitive decline, Alzheimer disease, and dementia, generally.110–119 Moreover, DHA is the precursor of the neuroprotectin D1, which reduces neuro-inflammation and protects neural cells against Alzheimer disease and photoreceptor renewal.5,120,121 Directions for further investigation include the analysis of the relationship between dietary EPA and DHA in regulating pain and the evaluation of the possible role of EPA and DHA as potential therapeutic agents in nociceptive and neuropathic pain.
Curcumin and pain
Curcumin represents a natural substance with anti-inflammatory activity; it is derived from the turmeric (Curcuma longa) plant, which is a rhizomatous, herbaceous, and perennial plant belonging to the Zingiberaceae family. It lives in mild temperatures (20°C–30°C in the tropical rainy regions), reaches 1 m in height and bears hermaphrodite flowers. The active substances of this plant are concentrated in the rhizomes. Turmeric powder from the rhizomes contains 3%–7% of three different curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin), essential oils, and fibers. The European Food Safety Authority, in a scientific evaluation of curcumin as food additive, declared that curcumin is not carcinogenic and there are no concerns regarding genotoxicity.122 The acceptable daily intake of curcumin corresponds to 3 mg/kg body weight (bw)/day based on the no observed adverse effect level of 250–320 mg/kg bw/day. Intake of curcumin from the normal diet amounts to <7% of the acceptable daily intake of 3 mg/kg bw/day. It might weakly interfere with anticoagulation, although that normally does not require dosage adjustments.
By using the keyword “curcumin”, we identified 8,394 publications in PubMed between January 1, 2000, and April 30, 2016. Aggarwal et al123 identified the inflammatory targets modulated by curcumin. A randomized, single-blind study on the efficacy and tolerability of curcumin in patients with rheumatoid polyarthritis demonstrated that curcumin extract produces a significant reduction of joint pain, joint swelling, and disease activity score.124 Ramadan and El-Menshawy125 demonstrated that the combination of curcumin and ginger is as effective as NSAIDs in a rat model of rheumatoid arthritis. Several animal model studies of curcumin have been performed in neuropathic pain.126–128 Hu et al129 recently determined that curcumin attenuates opioid-induced hyperalgesia by inhibiting Ca2+/calmodulin-dependent protein kinase II α activity, and recently, Hu et al130 found that a nanoformulation of poly(lactic-co-glycolide)-cucumin–curcumin may reverse opioid-induced hyperalgesia by inhibiting caMKIIa and downstream signaling. Curcumin is an antioxidant, as demonstrated by the 2,367 publications published in PubMed from 1988 to November 18, 2015. The antioxidant power is ten times that of vitamin E;131 it has a direct activity on reactive oxygen species, on induction of NRF2, and induces mitochondrial biogenesis.132–134 Curcumin also plays a role in regulating epigenetic patterns.135 Curcumin demonstrates synergistic activity with numerous drugs used to treat different painful conditions, and ongoing studies are evaluating the effect of 3-month treatment with curcumin on inflammation and QoL of patients with metabolic syndrome and osteoarthritis. Curcumin has low bioavailability, although bio-optimization techniques can improve it, thereby increasing the therapeutic and preventive potentials of the natural substance.136
Polyphenols and pain
The use of natural antioxidants can be considered a useful approach to control the development and progression of several diseases. Epidemiological studies have demonstrated a relationship between the Mediterranean diet and a reduced incidence of pathologies such as coronary disease and cancer. A central hallmark of this diet is the high consumption of virgin olive oil as its main source of fat. This oil contains antioxidant components in the non-saponifiable fraction, including phenolic compounds absent in seed oils. The olive is the fruit of Oleaeuropaea, a tree native to the coastal Mediterranean region, which produces 98% of the world’s total (~11 million tons) and lends important economic and dietetic benefits to the people of that region.137 Oleuropein and its hydrolysis product hydroxytyrosol are the main phenolic constituents of olive leaves and believed to be responsible for their pharmacological effects, as they are the most potent olive oil antioxidants.
In recent years, Citrus bergamia juice also has been raising interest and has been the subject of several studies considering its potential for health promotion. Bergamot is the common name for Citrus bergamia Risso et Poiteau, a plant belonging to the Rutaceae family (subfamily Esperidea). The beneficial effects of bergamot juice mainly derive from the bergamot polyphenol fraction. Currently, bergamot polyphenol fraction offers a viable alternative in the treatment of hypercholesterolemia in patients intolerant to treatment with statins. The mechanisms of action of bergamot-derived polyphenolic fraction include the reduction of cholesterol absorption and the inhibition of cholesterol biosynthesis, potentiating the effect of rosuvastatin.138–141 Muscoli et al142 recently demonstrated in a rodent model of opioid tolerance that the removal of free radicals with phenolic compounds of olive oil, such as hydroxytyrosol and oleuropein or bergamot polyphenolic fraction derivatives, reinstates the analgesic action of morphine.143 In particular, evidence exists that removal of nitric oxide, superoxide (SO), and peroxynitrite can prevent and reverse inflammatory pain, neuropathic pain, and morphine-induced hyperalgesia and tolerance.142–144 The identification of natural radical scavengers as novel non-narcotic agents is a viable therapeutic target for the development of non-narcotic analgesics in pain of various etiologies. Recent studies reinforce the importance of natural antioxidant products as a source of drugs to alleviate chronic pain.142,143,145 Chronic injection of morphine in mice led to the development of tolerance associated with increased nitrotyrosine and the marker of lipid peroxidation malondialdehyde formation together with nitration and deactivation of manganese SO dismutase (MnSOD) in the spinal cord. Removal of free radicals by hydroxytyrosol and oleuropein blocked morphine tolerance by inhibiting nitration and malondialdehyde formation and replacing MnSOD activity. Thus, the analgesic effect in vivo of phenolic fraction of virgin olive oil derives from its antioxidant activities.142 In addition, the development of anti-nociceptive tolerance to repeated doses of morphine in mice is consistently associated with increased tyrosine-nitrated proteins in the dorsal horn of the spinal cord as the enzyme glutamine synthase. Nitration of this protein is intimately linked to inactivation of its biological function and a resulting increase of glutamate levels in the spinal cord. Repeated administration of bergamot polyphenolic fraction as well as other substances with antioxidant properties such as N(G)-nitro-l-arginine methyl ester or manganese (III) tetrakis (4-benzoic acid) porphyrin significantly reduces the development of opioid-induced hyperalgesia. This effect was accompanied by a reduction of SO production, prevention of glutamine synthetase nitration, and reestablishment of its activity and of glutamate levels.145
Conclusion
The FYD workshop focused on the role of personalized nutrition and nutraceuticals, by considering how they might be helpful in the management of chronic pain, as well as their physiological features (such as body mass and microbiome) and pathological ones (such as cancer). We primarily focused on soy, curcumin, and omega-3 fatty acids, oleuropein and bergamot polyphenols. Although we have presented a considerable body of relevant literature in our review, additional investigations are needed to determine exactly which dietary recommendations and supplements are suitable in the clinical pain setting and how they might help patients by improving pain relief, functionality, and QoL according to a broader multidisciplinary therapeutic approach. New prospective, randomized clinical trials will need to be performed. A new edition of the FYD workshop will be organized to create a stable yet dynamic discussion within the scientific community to promote the employment of nutrition and nutraceuticals as viable tools in pain therapy and to produce innovative guidelines to better address the therapeutic needs of patients with chronic pain.
Acknowledgments
This work was supported by Grants from Italian Ministry of Instruction (PON03PE_00078_1, PON03PE_00078_2), European Commission (FP7 Collaborative Project Pain-OMICS; grant agreement number: 602736), and Italian Ministry of Health (project code: GR-2010-2318370).
Disclosure
The authors report no conflicts of interest in this work.
References
Di Tella M, Castelli L, Colonna F, et al. Theory of mind and emotional functioning in fibromyalgia syndrome: an investigation of the relationship between social cognition and executive function. PLoS One. 2015;10(1):e0116542. | ||
Burri A, Ogata S, Livshits G, Williams F. The association between chronic widespread musculoskeletal pain, depression and fatigue is genetically mediated. PLoS One. 2015;10(11):e0140289. | ||
Smith SB, Reenilä I, Männistö PT, et al. Epistasis between polymorphisms in COMT, ESR1, and GCH1 influences COMT enzyme activity and pain. Pain. 2014;155(11):2390–2399. | ||
Leonard BE. Pain, depression and inflammation: are interconnected causative factors involved? Mod Trends Pharmacopsychiatri. 2015;30:22–35. | ||
Lorente-Cebrián S, Costa AG, Navas-Carretero S, et al. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem. 2015;71(2):341–349. | ||
Tick H. Nutrition and pain. Phys Med Rehabil Clin N Am. 2015;26(2):309–320. | ||
Marx J. Cellular warriors at the Battle of the Bulge. Science. 2003;299:846–849. | ||
Alonso-Alonso M, Woods SC, Pelchat M, et al. Food reward system: current perspectives and future research needs. Nutr Rev. 2015;73(5):296–307. | ||
Davis C, Levitan RD, Yilmaz Z, Kaplan AS, Carter JC, Kennedy JL. Binge eating disorder and the dopamine D2 receptor: genotypes and sub-phenotypes. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):328–335. | ||
Houston M. The role of nutrition and nutraceutical supplements in the treatment of hypertension. World J Cardiol. 2014;6(2):38–66. | ||
Houston MC. The role of nutrition, nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Altern Ther Health Med. 2013;19(Suppl 1):32–49. | ||
Rajat S, Manisha S, Robin S, Kumar S. Nutraceuticals: a review. Int J Pharm. 2012;3(4):95–99. | ||
Kapoor A, Sharfstein JM. Breaking the gridlock: regulation of dietary supplements in the United States. Drug Test Anal. 2016;8(3–4):424–430. | ||
Shanahan CJ, de Lorimier R. From science to finance – a tool for deriving economic implications from the results of dietary supplement clinical studies. J Diet Suppl. 2016;13(1):16–34. | ||
Mister S, Hathcock J. Under the law, FDA must grant different standards for new dietary ingredients and food additives. Regul Toxicol Pharmacol. 2012;62(3):456–458. | ||
Umhau JC, Garg K, Woodward AM. Dietary supplements and their future in health care: commentary on draft guidelines proposed by the Food and Drug Administration. Antioxid Redox Signal. 2012;16(5):461–462. | ||
FDA (Food and Drug Administration) [webpage on the Internet]. Draft guidance for industry: dietary supplements: new dietary ingredient notifications and related issues; 2011. Available from: http://www.fda.gov/regulatoryinformation/guidances/ucm257563.htm. Accessed August 22, 2016. | ||
Institute of Medicine (US) Food Forum. The Human Microbiome, Diet, and Health: Workshop Summary. Washington, DC: National Academies Press (US); 2013. | ||
Talati AR. New dietary ingredient notifications: a comprehensive review and strategies for avoiding FDA objections. Food Drug Law J. 2007;62(2):387–398. | ||
Beal BR, Wallace MS. An overview of pharmacologic management of chronic pain. Med Clin North Am. 2016;100(1):65–79. | ||
Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7): | ||
Candrilli SD, Davis KL, Iyer S. Impact of constipation on opioid use patterns, health care resource utilization, and costs in cancer patients on opioid therapy. J Pain Palliat Care Pharmacother. 2009;23(3):231–241. | ||
Nelson AD, Camilleri M. Chronic opioid induced constipation in patients with nonmalignant pain: challenges and opportunities. Therap Adv Gastroenterol. 2015;8(4):206–220. | ||
Wan Y, Corman S, Gao X, Liu S, Patel H, Mody R. Economic burden of opioid-induced constipation among long-term opioid users with noncancer pain. Am Health Drug Benefits. 2015;8(2):93–102. | ||
Gaertner J, Siemens W, Camilleri M, et al. Definitions and outcome measures of clinical trials regarding opioid-induced constipation: a systematic review. J Clin Gastroenterol. 2015;49(1):9–16. | ||
Jones R, Prommer E, Backstedt D. Naloxegol: a novel therapy in the management of opioid-induced constipation. Am J Hosp Palliat Care. Epub 2015 Jul 6. | ||
Donnelly S, Walsh D, Rybicki L. The symptoms of advanced cancer: identification of clinical and research priorities by assessment of prevalence and severity. J Palliat Care. 1995;11(1):27–32. | ||
Weitzner MA, Moody LN, McMillan SC. Symptom management issues in hospice care. Am J Hosp Palliat Care. 1997;14:190–195. | ||
Vainio A, Auvinen A. Prevalence of symptoms among patients with advanced cancer: an international collaborative study. J Pain Symptom Manage. 1996;12:3–10. | ||
Hatswell AJ, Vegter S. Measuring quality of life in opioid-induced constipation: mapping EQ-5D-3 L and PAC-QOL. Health Econ Rev. 2015;6(1):14. | ||
Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and Wellness Survey. J Opioid Manag. 2009;5(3):137–144. | ||
Dorn S, Lembo A, Cremonini F. Opioid-induced bowel dysfunction: epidemiology, pathophysiology, diagnosis, and initial therapeutic approach. Am J Gastroenterol. 2014;2(1):31–37. | ||
González Canga A, Fernández Martínez N, Sahagún Prieto AM, et al. Dietary fiber and its interaction with drugs. Nutr Hosp. 2010;25(4): | ||
Stanghellini V, Bellacosa L, Cogliandro R. Fiber and macrogol in the therapy of chronic constipation. Minerva Gastroenetrol Dietol. 2013;59(2):217–230. | ||
Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2008;108(10):1716–1731. | ||
Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449(7164):811–818. | ||
Ley RE, Hamady M, Lozupone C, et al. Evolution of mammals and their gut microbes. Science. 2008;320(5883):1647–1651. | ||
Tap J, Mondot S, Levenez F, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11(10): | ||
Blackler RW, Gemici B, Manko A, Wallace JL. NSAID-gastroenteropathy: new aspects of pathogenesis and prevention. Curr Opin Pharmacol. 2014;19:11–16. | ||
Montenegro L, Losurdo G, Licinio R, et al. Non steroidal anti-inflammatory drug induced damage on lower gastro-intestinal tract: is there an involvement of microbiota? Curr Drug Saf. 2014;9(3):196–204. | ||
Matsui H, Shimokawa O, Kaneko T, Nagano Y, Rai K, Hyodo I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr. 2011;48(2):107–111. | ||
Remington-Hobbs J, Petts G, Harris T. Emergency department management of undifferentiated abdominal pain with hyoscine butylbromide and paracetamol: a randomised control trial. Emerg Med J. 2012;29(12):989–994. | ||
Savoia G, Coluzzi F, Di Maria C, et al. Italian intersociety recommendations on pain management in the emergency setting (SIAARTI, SIMEU, SIS 118, AISD, SIARED, SICUT, IRC). Minerva Anestesiol. 2015;81(2):205–225. | ||
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–1396. | ||
Narouze S, Souzdalnitski D. Obesity and chronic pain: opportunities for better patient care. Pain Manag. 2015;5(4):217–219. | ||
Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res. 2015;8:399–408. | ||
McVinnie DS. Obesity and pain. Br J Pain. 2013;7(4):163–170. | ||
Hassan MK, Joshi AV, Madhavan SS, Amonkar MM. Obesity and health-related quality of life: a cross-sectional analysis of the US population. Int J Obes. 2003;27(10):1227–1232. | ||
Arranz LI, Rafecas M, Alegre C. Effects of obesity on function and quality of life in chronic pain conditions. Curr Rheumatol Rep. 2014;16(1):390. | ||
Rodriguez A, Ezquerro S, Méndez-Giménez L, Becerril S, Frühbeck G. Revisiting the adipocyte: a model for integration of cytokine signaling in the regulation of energy metabolism. Am J Physiol Endocrinol Metab. 2015;309(8):E691–E714. | ||
Wright LJ, Schur E, Noonan C, Ahumada S, Buchwald D, Afari N. Chronic pain, overweight, and obesity: findings from a community-based twin registry. J Pain. 2010;11(7):628–635. | ||
Zdziarski LA, Wasser JG, Vincent HK. Chronic pain management in the obese patient: a focused review of key challenges and potential exercise solutions. J Pain Res. 2015;8:63–77. | ||
Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the idea randomized clinical trial. JAMA. 2013;310(12):1263–1273. | ||
Tanamas SK, Wluka AE, Berry P, et al. Relationship between obesity and foot pain and its association with fat mass, fat distribution, and muscle mass. Arthritis Care Res (Hoboken). 2012;64(2):262–268. | ||
Albertazzi P. Non-estrogenic approaches for the treatment of climacteric symptoms. Climacteric. 2007;10(Suppl 2):115–120. | ||
Luo S, Lan T, Liao W, Zhao M, Yang H. Genistein inhibits Aβ25–35-induced neurotoxicity in PC12 cells via PKC signaling pathway. Neurochem Res. 2012;37(12):2787–2794. | ||
Valsecchi AE, Franchi S, Panerai AE, Rossi A, Sacerdote P, Colleoni M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur J Pharmacol. 2011;650(2–3):694–702. | ||
Valsecchi AE, Franchi S, Panerai AE, Sacerdote P, Trovato AE, Colleoni M. Genistein, a natural phytoestrogen from soy, relieves neuropathic pain following chronic constriction sciatic nerve injury in mice: anti-inflammatory and antioxidant activity. J Neurochem. 2008; | ||
Shen CL, Smith BJ, Lo DF, et al. Dietary polyphenols and mechanisms of osteoarthritis. J Nutr Biochem. 2012;23(11):1367–1377. | ||
Tall JM, Raja SN. Dietary constituents as novel therapies for pain. Clin J Pain. 2004;20(1):19–26. | ||
Zaheer K, Akhtar MH. An updated review of dietary isoflavones: nutrition, processing, bioavailability and impacts on human health. Crit Rev Food Sci Nutr. Epub 2015 Nov 13. | ||
McFadyen IJ, Chetty U, Setchell KD, Zimmer-Nechemias L, Stanley E, Miller WR. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9(5):271–276. | ||
Van Patten CL, Olivotto IA, Chambers GK, et al. Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: a randomized, controlled clinical trial. J Clin Oncol. 2002;20(6):1449–1455. | ||
Wahner-Roedler DL, Thompson JM, Luedtke CA, et al. Dietary soy supplement on fibromyalgia symptoms: a randomized, double-blind, placebo-controlled, early phase trial. Evid Based Complement Alternat Med. 2011;2011:350697. | ||
Shir Y, Raja SN, Weissman CS, Campbell JN, Seltzer Z. Consumption of soy diet before nerve injury preempts the development of neuropathic pain in rats. Anesthesiology. 2001;95(5):1238–1244. | ||
Satija A, Ahmed SM, Gupta R, et al. Breast cancer pain management – a review of current & novel therapies. Indian J Med Res. 2014;139(2):216–225. | ||
Arjmandi BH, Khalil DA, Lucas EA, et al. Soy protein may alleviate osteoarthritis symptoms. Phytomedicine. 2004;11(7–8):567–575. | ||
Thomas AJ, Ismail R, Taylor-Swanson L, et al. Effects of isoflavones and amino acid therapies for hot flashes and co-occurring symptoms during the menopausal transition and early postmenopause: a systematic review. Maturitas. 2014;78(4):263–276. | ||
Zhao C, Wacnik PW, Tall JM, et al. Analgesic effects of a soy-containing diet in three murine bone cancer pain models. J Pain. 2004;5(2):104–110. | ||
Engel PA. New onset migraine associated with use of soy isoflavone supplements. Neurology. 2002;59(8):1289–1290. | ||
Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851(4):469–484. | ||
Lorente-Cebrián S, Costa AG, Navas-Carretero S, Zabala M, Martínez JA, Moreno-Aliaga MJ. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: a review of the evidence. J Physiol Biochem. 2013;69(3):633–651. | ||
Parra D, Ramel A, Bandarra N, Kiely M, Martinez JA, Thorsdottir I. A diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite. 2008;51(3):676–680. | ||
Thomas TR, Liu Y, Linden MA, Rector RS. Interaction of exercise training and n-3 fatty acid supplementation on postprandial lipemia. Appl Physiol Nutr Metab. 2007;32(3):473–480. | ||
Hill AM, Buckley JD, Murphy KJ, Howe PR. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am J Clin Nutr. 2007;85(5):1267–1274. | ||
Huerta AE, Prieto-Hontoria PL, Sáinz N, Martínez JA, Moreno-Aliaga MJ. Supplementation with α-lipoic acid alone or in combination with eicosapentaenoic acid modulates the inflammatory status of healthy overweight or obese women consuming an energy-restricted diet. J Nutr. Epub 2016 Mar 9. | ||
Kunesova M, Braunerova R, Hlavaty P, et al. The influence of n−3 polyunsaturated fatty acids and very low calorie diet during a short-term weight reducing regimen on weight loss and serum fatty acid composition in severely obese women. Physiol Res. 2006;55:63–72. | ||
Buckley JD, Howe PR. Long-chain omega-3 polyunsaturated fatty acids may be beneficial for reducing obesity-a review. Nutrients. 2010;2(12):1212–1230. | ||
Mori TA, Burke V, Puddey IB, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71(5):1085–1094. | ||
Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000;102(11):1264–1269. | ||
Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9(2):95–104. | ||
Tsitouras PD, Gucciardo F, Salbe AD, Heward C, Harman SM. High omega-3 fat intake improves insulin sensitivity and reduces CRP and IL6, but does not affect other endocrine axes in healthy older adults. Horm Metab Res. 2008;40(3):199–205. | ||
Derosa G, Cicero A, D’Angelo A, Borghi C, Maffioli P. Effects of n-3 pufas on fasting plasma glucose and insulin resistance in patients with impaired fasting glucose or impaired glucose tolerance. Biofactors. 2016;42(3):316–322. | ||
Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–1446. | ||
Ramel A, Martinez A, Kiely M, Morais G, Bandarra NM, Thorsdottir I. Beneficial effects of long-chain n−3 fatty acids included in an energy-restricted diet on insulin resistance in overweight and obese European young adults. Diabetologia. 2008;51:1261–1268. | ||
Abete I, Goyenechea E, Zulet MA, Martínez JA. Obesity and metabolic syndrome: potential benefit from specific nutritional components. Nutr Metab Cardiovasc Dis. 2011;21(Suppl 2):B1–B15. | ||
Lopez-Alvarenga JC, Ebbesson SO, Ebbesson LO, Tejero ME, Voruganti VS, Comuzzie AG. Polyunsaturated fatty acids effect on serum triglycerides concentration in the presence of metabolic syndrome components. The Alaska-Siberia Project. Metabolism. 2010;59(1):86–92. | ||
Nestel P, Shige H, Pomeroy S, Cehun M, Abbey M, Raederstorff D. The n−3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76:326–330. | ||
Kabir M, Skurnik G, Naour N, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86(6):1670–1679. | ||
Micallef MA, Garg ML. The lipid-lowering effects of phytosterols and (n−3) polyunsaturated fatty acids are synergistic and complementary in hyperlipidemic men and women. J Nutr. 2008;138:1086–1090. | ||
Micallef M, Munro I, Phang M, Garg M. Plasma n-3 polyunsaturated fatty acids are negatively associated with obesity. Br J Nutr. 2009;102:1370–1374. | ||
Jain AP, Aggarwal KK, Zhang PY. Omega-3 fatty acids and cardiovascular disease. Eur Rev Med Pharmacol Sci. 2015;19(3):441–445. | ||
Munro IA, Garg ML. Dietary supplementation with n–3 PUFA does not promote weight loss when combined with a very-low-energy diet. Br J Nutr. 2012;108(8):1466–1474. | ||
Burr ML, Ashfield-Watt PA, Dunstan FD, et al. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr. 2003;57(2):193–200. | ||
Thompkinson DK, Bhavana V, Kanika P. Dietary approaches for management of cardio-vascular health- a review. J Food Sci Technol. 2014;51(10):2318–2330. | ||
Del Gobbo LC, Imamura F, Aslibekyan S, et al; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe). ω-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med. 2016;176(8):1155–1166. | ||
Tavazzi L, Maggioni AP, Marchioli R, et al; Gissi-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–1230. | ||
Yokoyama M, Origasa H, Matsuzaki M, et al; Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098. | ||
Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–1290. | ||
Sala-Vila A, Guasch-Ferré M, Hu FB, et al. Dietary α-linolenic acid, marine ω-3 fatty acids, and mortality in a population with high fish consumption: findings from the PRevención con Dieta MEDiterránea (PREDIMED) Study. J Am Heart Assoc. 2016;5(1):e002543. | ||
Leaf A, Albert CM, Josephson M, et al. Prevention of fatal arrhythmias in high-risk subjects by fish oil n−3 fatty acid intake. Circulation. 2005;112:2762–2768. | ||
Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: current evidence and unanswered questions. Int J Environ Res Public Health. 2009;6(6):1894–1916. | ||
Calo L, Bianconi L, Colivicchi F, et al. n−3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–1728. | ||
Dallongeville J, Yarnell J, Ducimetiere P, et al. Fish consumption is associated with lower heart rates. Circulation. 2003;108(7): | ||
Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. | ||
Kris-Etherton PM, Innis S; American Dietetic Association, Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc. 2007;107(9):1599–1611. | ||
Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20(8):1493–1499. | ||
Hartweg J, Farmer AJ, Perera R, Holman RR, Neil HA. Meta-analysis of the effects of n−3 polyunsaturated fatty acids on lipoproteins and other emerging lipid cardiovascular risk markers in patients with type 2 diabetes. Diabetologia. 2007;50:1593–1602. | ||
Ueshima H, Stamler J, Elliott P, et al; INTERMAP Research Group. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure: INTERMAP study. Hypertension. 2007;50(2):313–319. | ||
Dullemeijer C, Durga J, Brouwer IA, et al. n 3 fatty acid proportions in plasma and cognitive performance in older adults. Am J Clin Nutr. 2007;86(5):1479–1485. | ||
van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly | ||
Nurk E, Drevon CA, Refsum H, et al. Cognitive performance among the elderly and dietary fish intake: the Hordaland Health Study. Am J Clin Nutr. 2007;86(5):1470–1478. | ||
Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2007;85(4):1103–1111. | ||
Freund Levi Y, Vedin I, Cederholm T, et al. Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer’s disease: the OmegAD study. J Intern Med. 2014;275:428–436. | ||
Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63(10):1402–1408. | ||
Freund-Levi Y, Vedin I, Hjorth E, et al. Effects of supplementation with omega-3 fatty acids on oxidative stress and inflammation in patients with Alzheimer’s disease: the OmegAD study. J Alzheimers Dis. 2014;42:823–831. | ||
Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304(17):1903–1911. | ||
Shinto L, Quinn J, Montine T, et al. A randomized placebo controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J Alzheimers Dis. 2014;38:111–120. | ||
Samieri C, Feart C, Letenneur L, et al. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am J Clin Nutr. 2008;88(3):714–721. | ||
Marcheselli VL, Mukherjee PK, Arita M, et al. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot Essent Fatty Acids. 2010;82(1):27–34. | ||
Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115(10):2774–2783. | ||
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific opinion on the re-evaluation of curcumin (E 100) as a food additive. EFSA Journal. 2010;8(9):1679. | ||
Aggarwal BB, Gupta SC, Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169(8):1672–1692. | ||
Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. 2012;26(11):1719–1725. | ||
Ramadan G, El-Menshawy O. Protective effects of ginger-turmeric rhizomes mixture on joint inflammation, atherogenesis, kidney dysfunction and other complications in a rat model of human rheumatoid arthritis. Int J Rheum Dis. 2013;16(2):219–229. | ||
Zhu X, Li Q, Chang R, et al. Curcumin alleviates neuropathic pain by inhibiting p300/CBP histone acetyltransferase activity-regulated expression of BDNF and cox-2 in a rat model. PLoS One. 2014;9(3):e91303. | ||
Zhao X, Xu Y, Zhao Q, Chen CR, Liu AM, Huang ZL. Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: descending monoamine system and opioid receptors are differentially involved. Neuropharmacology. 2012;62(2):843–854. | ||
Ji FT, Liang JJ, Liu L, Cao MH, Li F. Curcumin exerts antinociceptive effects by inhibiting the activation of astrocytes in spinal dorsal horn and the intracellular extracellular signal-regulated kinase signaling pathway in rat model of chronic constriction injury. Chin Med J (Engl). 2013;126(6):1125–1131. | ||
Hu X, Huang F, Szymusiak M, Liu Y, Wang ZJ. Curcumin attenuates opioid tolerance and dependence by inhibiting Ca2+/calmodulin-dependent protein kinase II α activity. J Pharmacol Exp Ther. 2015;352(3):420–428. | ||
Hu X, Huang F, Szymusiak M, Tian X, Liu Y, Wang ZJ. PLGA-curcumin attenuates opioid-induced hyperalgesia and inhibits spinal CaMKIIα. PLoS One. 2016;11(1):e0146393. | ||
Khopde SM, Priyadarsini KI, Venkatesan N, Rao MNA. Free radical scavenging ability and anti-oxidant efficiency of curcumin and its substituted analogue. Biophys Chem. 1999;80(2):83–89. | ||
Hosseinzadehdehkordi M, Adelinik A, Tashakor A. Dual effect of curcumin targets reactive oxygen species, adenosine triphosphate contents and intermediate steps of mitochondria-mediated apoptosis in lung cancer cell lines. Eur J Pharmacol. 2015;769:203–210. | ||
González-Reyes S, Guzmán-Beltrán S, Medina-Campos ON, Pedraza-Chaverri J. Curcumin pretreatment induces Nrf2 and an antioxidant response and prevents hemin-induced toxicity in primary cultures of cerebellar granule neurons of rats. Oxid Med Cell Longev. 2013;2013:8014–8018. | ||
Lone J, Choi JH, Kim SW, Yun JW. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J Nutr Biochem. 2016;27:193–202. | ||
Teiten MH, Dicato M, Diederich M. Curcumin as a regulator of epigenetic events. Mol Nutr Food Res. 2013;57(9):1619–1629. | ||
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. | ||
El SN, Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 2009;67(11): | ||
Mollace V, Ragusa S, Sacco I, et al. The protective effect of bergamot oil extract on lecitine-like oxyLDL receptor-1 expression in balloon injury-related neointima formation. J Cardiovasc Pharmacol Ther. 2008;13(2):120–129. | ||
Di Donna L, De Luca G, Mazzotti F, et al. Statin-like principles of bergamot fruit (Citrus bergamia): isolation of 3-hydroxymethylglutaryl flavonoid glycosides. J Nat Prod. 2009;72(7):1352–1354. | ||
Mollace V, Sacco I, Janda E, et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: from animal models to human studies. Fitoterapia. 2011;82(3):309–316. | ||
Gliozzi M, Walker R, Muscoli S, et al. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int J Cardiol. 2013;170(2):140–145. | ||
Muscoli C, Lauro F, Dagostino C, et al. Olea europaea-derived phenolic products attenuate antinociceptive morphine tolerance: an innovative strategic approach to treat cancer pain. J Biol Regul Homeost Agents. 2014;28(1):105–116. | ||
Lauro F, Ilari S, Giancotti LA, et al. The protective role of bergamot polyphenolic fraction on several animal models of pain. PharmaNutrition. In press 2016. | ||
Salvemini D, Little JW, Doyle T, Neumann WL. Roles of reactive oxygen and nitrogen species in pain. Free Radic Biol Med. 2011;51(5):951–966. | ||
Lauro F, Giancotti LA, Ilari S, et al. Inhibition of spinal oxidative stress by bergamot polyphenolic fraction attenuates the development of morphine induced tolerance and hyperalgesia in mice. PLoS One. 2016;11(5):e0156039. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
