Back to Journals » Cancer Management and Research » Volume 11
Combined Use of Mean Platelet Volume/Platelet Count Ratio and Platelet Distribution Width to Distinguish Between Patients with Nasopharyngeal Carcinoma, Those with Benign Tumors of the Nasopharynx, and Healthy Subjects
Authors Zhang X, Qin YY, Chen M , Wu Y, Lin F
Received 8 August 2019
Accepted for publication 20 November 2019
Published 11 December 2019 Volume 2019:11 Pages 10375—10382
DOI https://doi.org/10.2147/CMAR.S226050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Xuan Zhang, Yuan-yuan Qin, Min Chen, Yang-yang Wu, Fa-quan Lin
Department of Clinical Laboratory, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, People’s Republic of China
Correspondence: Fa-quan Lin
Department of Clinical Laboratory, The First Affiliated Hospital of Guangxi Medical University, No.6 Shuang Yong Road, Nanning 530021, Guangxi Zhuang Autonomous Region, People’s Republic of China
Tel +86 13877118318
Email [email protected]
Purpose: For the diagnosis of nasopharyngeal carcinoma (NPC), reliable early indicators with sensitivity and specificity should be sought. This study evaluated the effect of the combined use of mean platelet volume/platelet count ratio (MPV/PC ratio) and platelet distribution width (PDW) for differential diagnosis of NPC. In this study, MPV/PC ratio was used for the first time to diagnostically evaluate NPC.
Patients and methods: We retrospectively analyzed various hematological indices of three subject groups (208, 185, and 162 patients with NPC, benign tumors of the nasopharynx, and healthy subjects, respectively) and evaluated the value of combined use of MPV/PC ratio and PDW for differential diagnosis of the three groups using the one-way analysis of variance.
Results: Comparison of laboratory variables between the three groups showed a significant difference in MPV/PC ratio and PDW (P<0.001, all). The MPV/PC ratio in the NPC group was significantly lower than the other two groups (P<0.001); MPV/PC ratio also showed a statistically significant difference in different stages (P=0.034) and serosal invasions (P<0.001) of the NPC group. Receiver operating characteristic curve (ROC) analysis showed that areas under the curve (AUC) of either patients with benign tumors of the nasopharynx (AUCMPV/PCratio+PDW: 0.708) or healthy subjects (AUCMPV/PCratio+PDW: 0.909) were larger than those of MPV/PC ratio (AUCMPV/PCratio: 0.665, 0.869, respectively) and PDW (AUCPDW:0.614, 0.716, respectively) use alone (P<0.05, all).
Conclusion: MPV/PC ratio and PDW may be used as indexes of NPC. MPV/PC ratio combined with PDW could be considered as meaningful laboratory indexes for differential diagnosis of NPC, benign tumors of the nasopharynx, and healthy subjects. This finding could enhance the detection of NPC.
Keywords: mean platelet volume/platelet count ratio, platelet distribution width, nasopharyngeal carcinoma
Introduction
Although cancer-related mortality has declined since the 1990s, it still remains a significant cause of death.1 Nasopharyngeal carcinoma (NPC) is a common head and neck malignant tumor, and a poorly differentiated carcinoma of the nasopharyngeal mucosa. NPC has an incidence rate of approximately 1 in 100,000 individuals, with ethnic and geographical variations, and is relatively common in East and Southeast Asia.2 It also has a high incidence in southern China, accounting for 15–18% of malignant tumors.3 There are approximately 60,000 new cases, and more than half die every year in China.4 One of the causes of death is metastasis of the cancer.
It was reported that 90% of patients had lymph node metastasis when NPC was first diagnosed; of these, 5–10% already had distant metastasis.5 Therefore, most patients with NPC already show middle and advanced stages of the disease when NPC diagnosis was confirmed. As a result, it is essential to identify early diagnostic indicators with reliable sensitivity and specificity. Platelets are the smallest cells in the blood and are a key source of circulating angiogenesis-related proteins. Platelets accelerate the growth and metastasis of tumor cells by regulating tumor cell growth and angiogenesis.6 It is reported that thrombocytopenia is closely related to the progression of ovarian cancer7 and gastrointestinal cancer,8 which may be the mechanism by which platelets participate in the regulation of cancer-associated inflammation. Mean platelet volume/platelet count ratio (MPV/PC ratio) and platelet distribution width (PDW) as platelet-related index have the advantage of ease of collection, low cost, and high repeatability, and are associated with the prognosis of laryngeal cancer, colorectal cancer, and breast cancer.9–11 Therefore, we intended to explore the value of MPV/PC ratio and PDW to distinguish between patients with NPC, those with benign tumors of the nasopharynx, and healthy subjects.
Materials and Methods
Patients
The data of patients who were initially diagnosed with NPC by postoperative pathology from January 2012 to February 2019 in the First Affiliated Hospital of Guangxi Medical University, China, were retrospectively analyzed. The following were the exclusion criteria: patients who (1) underwent treatment for NPC; (2) had other malignancies; (3) had infection; (4) had cardiovascular disease, hypertension, and diabetes mellitus; (5) had blood disease and anemia; or (6) were using antiplatelet drugs. All patients were clinically staged in accordance with the seventh edition of the American Joint Committee on Cancer (AJCC)/TNM tumor stage. Patients initially pathological diagnosed with benign tumors of the nasopharynx in our hospital during the same period as well as healthy subjects were included. There was no statistically significant difference in sex and age between the three groups (P=0.999, P=0.402, respectively). The Ethics Committee of the first Affiliated Hospital of Guangxi Medical University approved the study.
Methods
Preoperative hematology was performed at the initial diagnosis of patients with NPC and those with benign tumors of the nasopharynx by postoperative pathology. Fasting venous blood was collected from healthy subjects, in ethylenediaminetetraacetic acid (EDTA) anticoagulant tubes in the morning. All the blood parameters were tested using Beckman Coulter LH780 hematology analyzer (Beckman Coulter, Brea, CA, USA), including white blood cells (WBC), platelets (PLT), mean platelet volume (MPV), neutrophils (N), lymphocytes (L), monocytes (M), PDW, and red blood cells (RBC). MPV/PC ratio was calculated from the mean platelet volume and the platelet count. The instrument has been quality controlled prior to sample testing. Quality control uses the Westgard multi-rule quality control method. When the two quality control values are within the limits of X±2S, they are judged to be in control.
There are 208, 185, and 162 patients with NPC, benign tumors of the nasopharynx, and healthy subjects, respectively. A total of 191 males and 17 females were included in the NPC group. According to the seventh edition of the AJCC/TNM tumor stage, there were 43 cases of stage I, 47 cases of stage II, 108 cases of stage III, and 10 cases of stage IV. According to the degree of serosal infiltration, there were 62 cases of T1+T2 and 146 cases of T3+T4. In accordance with the degree of lymph node metastasis, 90 cases were N0 and 118 cases were N1–N3. In the light of the situation of distant metastasis, M0 was 198 cases and M1 was 10 cases. The diagnosis of nasopharyngeal carcinoma and benign tumors of the nasopharynx was confirmed by postoperative pathology.
Statistical Analysis
In this study, data were analyzed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Hematological indices were presented as mean±standard deviation (SD), and categorical data were presented as number or rate. The Chi-square test was used to compare the rates. Graphs were drawn using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Differences in blood parameters between the three groups (patients with NPC, those with benign tumors of the nasopharynx, and healthy subjects) were analyzed using one-way analysis of variance (ANOVA). Differences in blood parameters were carried out to compare the three groups in pairs, using Tukey’s test. Mann–Whitney U-test and Kruskal–Wallis rank sum test were used to assess the difference in blood parameters between pathological stages. MedCalc version 15.0 (MedCalc Software, Mariakerke, Belgium) was used to draw the ROC curve, in order to assess the sensitivity, specificity, optimal cutoff values as well as the area under the ROC curves of MPV/PC ratio in combined with PDW. P<0.05 was considered statistically significant.
Results
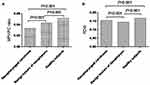
Comparison of laboratory variables between the three groups (patients with NPC, patients with benign tumors of the nasopharynx, and healthy subjects) showed a significant difference in MPV/PC ratio (P<0.001, Figure 1A) and PDW (P<0.001, Figure 1B). The MPV/PC ratio in the NPC group was significantly lower compared to those with benign tumors of the nasopharynx or healthy subjects. The PDW among patients with benign tumors of the nasopharynx was significantly lower than in the other two groups (NPC group vs. benign tumor group, P<0.001; NPC group vs. healthy subjects group, P<0.001; benign tumors of the nasopharynx group vs. healthy subjects group, P<0.001). The data from the three groups are summarized in Table 1.
 |
Table 1 Comparison of Demographics and Laboratory Variables Between Patients with Nasopharyngeal Carcinoma, Those with Benign Tumors of Nasopharynx, and Healthy Subjects |
The characteristics of the clinicopathologic staging of MPV/PC ratio and PDW in 208 patients with NPC are shown in Table 2. MPV/PC ratio showed statistically significant difference in different stages (P=0.034, Figure 2A), and serosal invasion between the T1+T2 and T3+T4 groups (P<0.001, Figure 2B), and in no difference was observed with PDW. Moreover, the value of MPV/PC ratio declined from early to late stages; on the contrary, the value of PDW increased in the late stage (Table 2).
 |
Table 2 MPV/PC Ratio and PDW in Serosal Invasion, Lymph Node Metastasis, and Distant metastasis of 208 Patients with Nasopharyngeal Carcinoma |
The data of correlation analysis of laboratory variables of 208 patients with nasopharyngeal carcinoma were presented in Table 3. Correlation analysis demonstrated that MPV/PC ratio was positively correlated with MPV (P<0.001, r=0.572), and negatively correlated with WBC (P<0.001, r=−0.317), M (P=0.005, r=−0.196), N (P<0.001, r=−0.345), PLT (P<0.001, r=−0.842), and serosal invasion (P<0.001, r=−0.288); PDW was negatively correlated with MPV (P<0.001, r=−0.505).
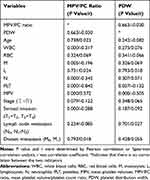 |
Table 3 Correlation Analysis of Laboratory Variables of 208 Patients with Nasopharyngeal Carcinoma |
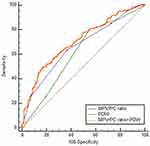
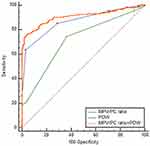
In ROC curve analysis, the AUC was between 0.5 and 1; and AUC between 0.5 and 0.7; between 0.7 and 0.9; and above 0.9, showed low, certain, and high accuracy, respectively. To distinguish between NPC and benign tumors of the nasopharynx, combined use of MPV/PC ratio and PDW (AUCMPV/PC ratio+PDW:0.708, 95% confidence interval [CI]: 0.660–0.752), showed a larger AUC than those of MPV/PC ratio (AUCMPV/PC ratio: 0.665, 95% CI: 0.616–0.712) or PDW (AUCPDW: 0.614, 95% CI: 0.564–0.663) use alone (P=0.0165, P=0.0019, respectively, Figure 3). The results showed increased specificity and accuracy (specificity: 83.24%, Table 4). To distinguish between patients with NPC and healthy subjects, the combined use of MPV/PC ratio and PDW (AUCMPV/PC ratio+PDW: 0.909, 95% CI: 0.875–0.936) showed a larger AUC than those of MPV/PC ratio (AUCMPV/PC ratio: 0.869, 95% CI: 0.830–0.902) or PDW (AUCPDW: 0.716, 95% CI: 0.667–0.761) use alone (P=0.0016, P<0.001, respectively, Figure 4). The results also showed increased sensitivity and high accuracy (specificity: 95.06%, Table 4).
 |
Table 4 Diagnostic Efficiency of MPV/PC Ratio and PDW |
Discussion
It has been reported that inflammation is closely related to the development of tumors.12,13 PLT serves as one of the inflammatory markers with great importance in thrombosis, a factor in tumor development.14,15 A previous study has found that an increased level of PLT was associated with a poor overall survival in NPC patients.16 However, PLT count does not accurately reflect abnormalities in PLT functional status. Under the effective compensation mechanism, hypercoagulability and inflammation may occur within the body even if PLT is normal.17 Thus, two PLT-related indicators, MPV (an indicator of platelet activation) and PDW (an indicator of reflecting variations in platelet volume), can be derived. MPV and PDW are often used in the preliminary diagnosis and prognostic assessment of malignant tumors. Studies have shown that an inverse relationship exists between PLT and MPV, suggesting the combination of the two variables as a ratio.18–20
MPV/PC ratio is used for the postmortem evaluation in patients with non-elevation myocardial infarction,21 as well as for prognosis and diagnosis in non-small cell gastric cancer cases and lung cancer.22,23 Low MPV/PC ratio serves as a reference index for cirrhosis with ascites, infection, and some immune diseases.24,25 Our study showed that MPV/PC ratio was the lowest in the NPC group compared with the other two groups (patients with benign tumors of the nasopharynx and healthy subjects), which is similar to the results of the study by Pietrzyk et al.22 Moreover, MPV/PC ratio also showed statistically significant differences in different stages and serosal invasions. The value of MPV/PC ratio decreased with increase in NPC pathological stages; all of which may suggest that MPV/PC ratio can be used to distinguish between patients with NPC and those with benign tumors of the nasopharynx or healthy subjects. Currently, it is thought that MPV/PC ratio may indicate the progression and metastasis of malignant tumors through an inflammatory tumor microenvironment. Low MPV/PC ratio is indicative of low MPV and high PLT. MPV is an indicator of PLTs’ early activation state, and MPV has significance in the diagnosis of some malignant tumors.26–28 As shown by our results, MPV and PLT showed the minimum and maximum levels in the NPC group, respectively, compared with the other two groups (patients with benign tumors of the nasopharynx and healthy subjects), which was similar to the findings by Bessman et al.19 Moreover, MPV/PC ratio was correlated with MPV, WBC, M, N, PLT, and serosal invasion. During the inflammatory phase, increased consumption of a large number of PLT leads to the reduction in MPV.29 Such reduction may account for high volume of platelets, which are more reactive and are likely to release chemicals with hemostasis and proinflammatory effects, thus participating in the inflammatory response.30,31 It aggravates endothelial damage, promotes cancer cell invasion, and lymph node metastasis; contributing to the development and metastasis of cancer.17,32 This better explains the involvement of the MPV/PC ratio in the progression of tumor-associated inflammation.
Changes in PDW level have been reported as a potential marker for the diagnosis and prognosis of laryngeal cancer, colorectal cancer, nasopharyngeal cancer, and liver cancer.9,28,33,34 Paulus35 showed in their study that the dysfunction of bone marrow cells could lead to changes in PDW. Bone marrow cell maturation and PLT size are regulated by some cytokines, including interleukin-6 granulocyte colony-stimulating factor and macrophage colony-stimulating factor,36 which promote tumor angiogenesis and metastasis.37 Studies have shown that PDW increases in melanoma and gallbladder cancer.38,39 In our results, PDW showed a statistically significant difference between the three groups (patients with NPC, patients with benign tumors of the nasopharynx, and healthy subjects), with statistically significant differences when the three groups are compared in pairs. Higher the NPC pathological staging the higher the PDW level, suggesting that the increase in PDW could be used to indicate the progression of NPC.
Ultimately, in the ROC curve analysis, the AUC value was from 0.5 to 1; the closer the AUC value was to 1, the better was the diagnostic effect. AUC of between 0.7 and 0.9 and that above 0.9 showed certain and high accuracy, respectively. In our study, the combined use of MPV/PC ratio and PDW to distinguish between patients with NPC and benign tumors of the nasopharynx had a large AUC, increased specificity, and certain accuracy. Furthermore, the combined use of MPV/PC ratio and PDW to distinguish between patients with NPC and healthy subjects showed a large AUC, increased specificity, and high accuracy.
Our study had some limitations. First, this is a retrospective study. Second, this was a single research center study, from China, in a certain population; hence, there is a need for larger prospective studies to clarify how MPV/PC ratio affects the progress and precise mechanism of NPC. Undoubtedly, this study is the first to explore the value of MPV/PC ratio in NPC, and to provide a reference for distinguishing between the diagnosis of NPC and others.
Conclusion
MPV/PC ratio and PDW may be used as indexes of NPC, MPV/PC ratio combined with PDW could be considered as significant laboratory indexes for differential diagnosis of NPC, benign tumors of the nasopharynx, and healthy subjects. This finding could enhance the detection of NPC. However, further large-scale prospective study is suggested.
Abbreviations
AUC, area under the curve; CI, confidence interval; L, lymphocytes; M, monocytes; MPV, mean platelet volumes; MPV/PC ratio, mean platelet volume/platelet count ratio; N, neutrophils; NPC, nasopharyngeal carcinoma; PDW, platelet distribution width; PLT, platelets; RBC, red blood cells; ROC, receiver operating characteristic; WBC, white blood cell.
Ethical Approval
The Ethics Committee of the first Affiliated Hospital of Guangxi Medical University approved the study. This article does not contain any studies with animals performed by any of the authors.
Acknowledgments
We would like to thank the staff at the Department of Clinical Laboratory, the First Affiliated Hospital of Guangxi Medical University, China.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi:10.3322/caac.21332
2. Tsao SW, Yip YL, Tsang CM, et al. Etiological factors of nasopharyngeal carcinoma. Oral Oncol. 2014;50:330–338. doi:10.1016/j.oraloncology.2014.02.006
3. Chong VF, Ong CK. Nasopharyngeal carcinoma. Eur J Radiol. 2008;66:437–447. doi:10.1016/j.ejrad.2008.03.029
4. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338
5. Kang H, Kiess A, Chung CH. Emerging biomarkers in head and neck cancer in the era of genomics. Nat Rev Clin Oncol. 2015;12:11–26. doi:10.1038/nrclinonc.2014.192
6. Sharma D, Brummel-Ziedins KE, Bouchard BA, et al. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. 2014;229:1005–1015. doi:10.1002/jcp.v229.8
7. Davis AN, Afshar-Kharghan V, Sood AK. Platelet effects on ovarian cancer. Semin Oncol. 2014;41:378–384. doi:10.1053/j.seminoncol.2014.04.004
8. Baranyai Z, Josa V, Toth A, et al. Paraneoplastic thrombocytosis in gastrointestinal cancer. Platelets. 2016;27:269–275. doi:10.3109/09537104.2016.1170112
9. Zhang H, Liu L, Fu S, et al. Higher platelet distribution width predicts poor prognosis in laryngeal cancer. Oncotarget. 2017;8:48138–48144. doi:10.18632/oncotarget.18306
10. Song X, Zhu H, Pei Q, et al. Significance of inflammation-based indices in the prognosis of patients with non-metastatic colorectal cancer. Oncotarget. 2017;8:45178–45189. doi:10.18632/oncotarget.16774
11. Gu M, Zhai Z, Huang L, et al. Pre-treatment mean platelet volume associates with worse clinicopathologic features and prognosis of patients with invasive breast cancer. Breast Cancer. 2016;23:752–760. doi:10.1007/s12282-015-0635-6
12. Maiuri AR, Li H, Stein BD, et al. Inflammation-induced DNA methylation of DNA polymerase gamma alters the metabolic profile of colon tumors. Cancer Metab. 2018;6:9. doi:10.1186/s40170-018-0182-7
13. Li C, Tian W, Zhao F, et al. Systemic immune-inflammation index, SII, for prognosis of elderly patients with newly diagnosed tumors. Oncotarget. 2018;9:35293–35299. doi:10.18632/oncotarget.24293
14. Elaskalani O, Berndt MC, Falasca M, et al. Targeting platelets for the treatment of cancer. Cancers (Basel). 2017;9:94. doi:10.3390/cancers9070094
15. Xu XR, Yousef GM, Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood. 2018;131:1777–1789. doi:10.1182/blood-2017-05-743187
16. Chen YP, Chen C, Mai ZY, et al. Pretreatment platelet count as a predictor for survival and distant metastasis in nasopharyngeal carcinoma patients. Oncol Lett. 2015;9:1458–1466. doi:10.3892/ol.2015.2872
17. Seretis C, Youssef H, Chapman M. Hypercoagulation in colorectal cancer: what can platelet indices tell us? Platelets. 2015;26:114–118. doi:10.3109/09537104.2014.894969
18. Lozano M, Narvaez J, Faundez A, et al. [Platelet count and mean platelet volume in the Spanish population]. Med Clin (Barc). 1998;110:774–777.
19. Bessman JD, Williams LJ, Gilmer PR
20. Biricik S, Narci H, Dundar GA, et al. Mean platelet volume and the ratio of mean platelet volume to platelet count in the diagnosis of acute appendicitis. Am J Emerg Med. 2018;37(3):411–414.
21. Norgaz T, Hobikoglu G, Aksu H, et al. The relationship between preprocedural platelet size and subsequent in-stent restenosis. Acta Cardiol. 2004;59:391–395. doi:10.2143/AC.59.4.2005204
22. Pietrzyk L, Plewa Z, Denisow-Pietrzyk M, et al. Diagnostic power of blood parameters as screening markers in gastric cancer patients. Asian Pac J Cancer Prev. 2016;17:4433–4437.
23. Inagaki N, Kibata K, Tamaki T, et al. Prognostic impact of the mean platelet volume/platelet count ratio in terms of survival in advanced non-small cell lung cancer. Lung Cancer. 2014;83:97–101. doi:10.1016/j.lungcan.2013.08.020
24. Suvak B, Torun S, Yildiz H, et al. Mean platelet volume is a useful indicator of systemic inflammation in cirrhotic patients with ascitic fluid infection. Ann Hepatol. 2013;12:294–300. doi:10.1016/S1665-2681(19)31368-7
25. Safak S, Uslu AU, Serdal K, et al. Association between mean platelet volume levels and inflammation in SLE patients presented with arthritis. Afr Health Sci. 2014;14:919–924. doi:10.4314/ahs.v14i4.21
26. Sun SY, Zhao BQ, Wang J, et al. The clinical implications of mean platelet volume and mean platelet volume/platelet count ratio in locally advanced esophageal squamous cell carcinoma. Dis Esophagus. 2018;31. doi:10.1093/dote/dox125
27. Gokcen K, Dundar G, Gulbahar H, et al. Can routine peripheral blood counts like neutrophil-to-lymphocyte ratio be beneficial in prediagnosis of testicular cancer and its stages? J Res Med Sci. 2018;23:64. doi:10.4103/jrms.JRMS_1009_16
28. Zhu X, Cao Y, Lu P, et al. Evaluation of platelet indices as diagnostic biomarkers for colorectal cancer. Sci Rep. 2018;8:11814. doi:10.1038/s41598-018-29293-x
29. Gasparyan AY, Ayvazyan L, Mikhailidis DP, et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17:47–58. doi:10.2174/138161211795049804
30. Thompson CB, Jakubowski JA, Quinn PG, et al. Platelet size and age determine platelet function independently. Blood. 1984;63:1372–1375. doi:10.1182/blood.V63.6.1372.1372
31. Budak YU, Polat M, Huysal K. The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: a systematic review. Biochem Med (Zagreb). 2016;26:178–193. doi:10.11613/issn.1846-7482
32. Matowicka-Karna J. Markers of inflammation, activation of blood platelets and coagulation disorders in inflammatory bowel diseases. Postepy Hig Med Dosw (Online). 2016;70:305–312. doi:10.5604/17322693.1199305
33. Xie X, Zeng X, Cao S, et al. Elevated pretreatment platelet distribution width and platelet count predict poor prognosis in nasopharyngeal carcinoma. Oncotarget. 2017;8:106089–106097. doi:10.18632/oncotarget.v8i62
34. Guo F, Zhu X, Qin X. Platelet distribution width in hepatocellular carcinoma. Med Sci Monit. 2018;24:2518–2523. doi:10.12659/MSM.909474
35. Paulus JM. Recent advances in the story of megakaryocyte physiology. Pathol Biol (Paris). 1981;29:133–135.
36. Kaushansky K. Growth factors and hematopoietic cell fate. A new feature: controversies in hematology. Blood. 1998;92:345–354. doi:10.1182/blood.V92.2.345
37. Kowanetz M, Wu X, Lee J, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107:21248–21255. doi:10.1073/pnas.1015855107
38. Li N, Diao Z, Huang X, et al. Increased platelet distribution width predicts poor prognosis in melanoma patients. Sci Rep. 2017;7:2970. doi:10.1038/s41598-017-03212-y
39. Zhang X, Niu Y, Wang X, et al. Mean platelet volume and platelet distribution width are associated with gallbladder cancer. Asian Pac J Cancer Prev. 2018;19:351–355. doi:10.22034/APJCP.2018.19.2.351
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.