Back to Journals » Cancer Management and Research » Volume 11
Combined ultrasound/computed tomography guidance in percutaneous radiofrequency ablation after transarterial chemoembolization for hepatocellular carcinoma in the hepatic dome
Authors Kan X, Wang Y , Han P, Yao Q, Qian K, Xiong B, Zheng C
Received 13 April 2019
Accepted for publication 28 July 2019
Published 15 August 2019 Volume 2019:11 Pages 7751—7757
DOI https://doi.org/10.2147/CMAR.S212127
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Xuefeng Kan,*,1,2 Yong Wang,*,1–3 Ping Han,1,2 Qi Yao,1,2 Kun Qian,1,2 Bin Xiong,*,1,2 Chuansheng Zheng*,1,2
1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China; 2Hubei Province Key Laboratory of Molecular Imaging, Wuhan 430022, China; 3Department of Vascular and Interventional Radiology, The Second Affiliated Hospital of Hainan Medical University, Haikou 570311, China
*These authors contributed equally to this work
Purpose: To assess the value of the combined ultrasound (US)/computed tomography (CT) guidance (US guidance was firstly used for puncture with the electrode needle to the site close to the tumor, and subsequently, CT guidance was used for precise positioning of the electrode tips) in percutaneous radiofrequency ablation (RFA) after transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC) in the hepatic dome.
Methods: From January 1, 2013 to June 30, 2017, medical records of 65 patients with HCCs in the hepatic dome who received TACE treatment before RFA procedure were retrospectively analyzed. Among them, 34 patients with 35 liver tumors underwent percutaneous RFA under combined US/CT guidance, and 31 patients with 35 liver tumors received percutaneous RFA under CT guidance alone. The efficacy of combined US/CT-guided RFA was analyzed, and the procedure time and safety between the two groups were compared.
Results: In the combined US/CT-guided RFA group, the 1-, 3-, and 5-year local recurrence rates were 3%, 6%, 9%, respectively, and the 1-, 3-, and 5-year overall survival rates were 100%, 97%, 94%, respectively. The mean procedure time in the CT-guided RFA group was significantly longer than that of the combined US/CT-guided RFA group (P<0.001). Although the overall complication rates between the two groups were not statistically significant, there were no occurrences of RFA-related complications in the combined US/CT-guided RFA group. The incidence of postoperative adverse reaction of right upper quadrant pain in the CT-guided RFA group was greater than that of the combined US/CT-guided RFA group (P=0.01).
Conclusion: Percutaneous RFA under the combined US/CT guidance was helpful for HCC in the hepatic dome.
Keywords: radiofrequency ablation, hepatocellular carcinoma, hepatic dome, combined US/CT guidance, CT guidance
Introduction
Percutaneous radiofrequency ablation (RFA) under image guidance, such as ultrasound (US) or computed tomography (CT), has become a frequent alternative treatment for unresectable hepatocellular carcinoma (HCC).1,2 As a minimally invasive procedure, the RFA efficacy for HCC is satisfactory.2–4 However, HCC in the hepatic dome is sometimes incompletely visible on US because of obstruction by the lung or ribs. US-guided RFA may lead to an incomplete ablation and damage to the surrounding organs during ablation for HCC in the hepatic dome. Therefore, CT-guided RFA is usually used for such HCC in clinical practice. Although previous studies5,6 reported that CT-guided RFA had excellent efficacy for HCC in the hepatic dome, CT-guided puncture is relatively time-consuming, and pneumothorax or injury to the major tissues or organs may occur because of non-real-time dynamic observation. Thus, percutaneous RFA for HCC in the hepatic dome is challenging.
In the present study, patients with HCC received transarterial chemoembolization (TACE) treatment before RFA to improve the efficacy and safety of percutaneous RFA for HCC. Then, percutaneous RFA under the combined US/CT guidance or under the CT guidance alone was performed for HCC, which was located in the hepatic dome and was incompletely visible on US. The clinical data of these patients were retrospectively reviewed. The efficacy of the combined US/CT-guided RFA was analyzed, and the procedure time and safety between the combined US/CT-guided RFA and the CT-guided RFA were compared. The purpose of the study is to assess the value of the combined US/CT guidance in percutaneous RFA after TACE for HCC in the hepatic dome.
Materials and methods
Study design and patient selection
From January 1, 2013, to June 30, 2017, medical records of 65 patients with HCCs in the hepatic dome were retrospectively analyzed. All the patients received TACE treatment before RFA procedure. Among them, 34 patients with 35 liver tumors underwent percutaneous RFA under combined US/CT guidance, and 31 patients with 35 liver tumors received percutaneous RFA under CT guidance alone. The efficacy of the combined US/CT-guided RFA was analyzed. The group of patients who underwent CT-guided RFA procedure was the control group, and the procedure time and safety between the combined US/CT-guided RFA and CT-guided RFA were compared. Approval for this retrospective study was obtained from Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. The informed consent was waived for this retrospective study. All data of patients were used confidentiality and anonymously. The research involved no more than minimal risk to the patients. Meanwhile, the waiver did not adversely affect the rights and welfare of the patients. The study protocol followed all appropriate guidelines according to the Declaration of Helsinki.
The inclusion criteria of the patients were as follows: (1) patients were diagnosed with HCC according to pathologic examination or noninvasive criteria in accordance with the European Association for the Study of the Liver/American Association for the study of Liver Disease guidelines;7 (2) HCC was unresectable or patients refused surgical treatment; (3) HCC was located in the hepatic dome, and was incompletely visible on US because of obstruction by the lung or ribs; (4) patients with less than or equal to three HCC lesions with a diameter smaller than or equal to 3 cm; (5) no vascular invasion; (6) no extrahepatic metastases; (7) liver function classified as Child-Pugh class A or B; (8) blood platelet count >50×109/L; (9) patients with normal prothrombin time and activated partial thromboplastin time, and fibrinogen activity was over 50%; (10) East Coast Oncology Group (ECOG) performance status value ≤2; and (11) no severe heart disease.
TACE procedure
All of the patients received TACE treatment before RFA. The operators (X.K., Y.W., S.S., B.X., C.Z.) of TACE had at least eight years of experience in performing TACE procedures. TACE was performed with a 5-French catheter (Cook, Bloomington, Indiana, USA) or a microcatheter (Pro great, Terumo, Tokyo, Japan) as selectively as possible through the lobar, segmental, or subsegmental arteries, depending on the tumor distribution and hepatic functional reserve. Initially, an emulsion of 5–10 mg doxorubicin hydrochloride (Hisun Pharmaceutical Co. LTD, Zhejiang, China) and 2–5 mL lipiodol (Lipiodol Ultrafluido, Guerbet, France) was administered into the tumor’s feeding arteries. The dosage of lipiodol and doxorubicin was determined according to the tumor’s size and vascularity, and the patient’s underlying liver function. Next, gelatin sponge particles (300–500 μM in size, Cook, Bloomington, Indiana, USA) were mixed with contrast material and then administered into the tumor-feeding arteries until stasis of the arterial flow was achieved.
Investigations before RFA
A series of panels including a complete blood count, liver, renal function tests, prothrombin time, and electrocardiogram were performed before RFA to investigate whether the patients fulfilled the RFA treatment criteria. US and CT were performed one day before RFA to observe the size, location of the HCC lesions, and lipiodol accumulation in the liver tumor.
Percutaneous RFA technique
The RFA operators of Bin Xiong had 10 years of experience, and Chuansheng Zheng had 15 years of experience in performing RFA procedures. Patients received RFA treatment within 5–10 days after TACE treatment. RFA was performed with a 460-kHz RF generator (Rita Medical Systems, Mountain View, California, USA), and a 14-gauge probe and 15-cm-long multiple electrode (Rita Medical Systems, Mountain View, California, USA). A color doppler ultrasound (Acuson X300, Siemens Medical Solutions, Muenchen, Germany) was used for guidance in the combined US/CT-guided RFA procedure. In the CT-guided or combined US/CT-guided RFA procedure, a 16-row spiral CT (somaton sensation, Siemens Medical Solutions, Muenchen, Germany) was used for guidance.
CT-guided percutaneous RFA
Patients lay in a supine or left lateral position to expose the operation site. A CT scan was conducted to determine the entry point of the puncture, path direction, puncture angle, and depth of the needle, which helped to avoid injury to the lung, major blood vessels, and bile duct. Analgesia was achieved by intravenous administration of 50–100 mg of a flurbiprofen axetil injection (Tide Pharmaceutical Co., Ltd, Beijing, China) and a local injection of 5–10 mL of 2% lidocaine. A skin incision that was approximately 0.5 cm in diameter was made in the point of puncture. The multiple electrode was inserted into the tumor center under the CT guidance and was spread out. The three-dimensional images of the CT scan were used to observe the position of the probe. Then, the ablation started. The ablation zone included at least a 0.5 cm rim of normal liver parenchyma. Needle track ablation was performed while withdrawing the electrode needle to reduce the risk of tumor cell seeding and hemorrhage.
Combined US/CT-guided percutaneous RFA
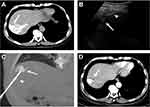
Patients lay in a supine or left lateral position to expose the operation site. For avoiding the lung, major blood vessels, and bile duct, an US and CT scan were firstly performed, respectively, to determine the entry point of the puncture, path direction, and puncture angle. Analgesia was achieved by intravenous administration of 50–100 mg of a flurbiprofen axetil injection and a local injection of 5–10 mL of 2% lidocaine. A skin incision that was approximately 0.5 cm in diameter was made in the point of puncture. In general, after TACE, HCC lesions with hypo- or iso-echoic on the images of US changed to hyper-echoic, and a part of the tumor was visible on US. HCC lesions with low-density on the images of CT changed to high-density because of lipiodol accumulation in the tumor. According to the combined information which was provided by the images of CT and US, we can detect the HCC lesions with incomplete visible on US. The electrode needle was inserted to the site close to the tumor under US guidance at first (Figure 1B). Then, the multiple electrode needle was introduced into the tumor center under the CT guidance and was spread out (Figure 1C). The precise positioning of the electrode tips was confirmed by a CT scan. The next ablation steps were the same as the CT-guided RFA.
Assessment of clinical outcomes
All of the patients underwent a follow-up, with the end of follow-up time being September 30, 2018. Patients were evaluated one month after the RFA procedure and then every three months post-RFA with laboratory and imaging examination, including α-fetoprotein, contrast-enhanced CT or contrast-enhanced magnetic resonance imaging. Local recurrence was defined as the appearance of a viable intrahepatic tumor within or at the periphery of the original ablated lesion.
A postoperative adverse reaction including low-grade fever (from 37.5°C to 38.5°C), right upper quadrant pain, and nausea and/or vomiting was observed and recorded after the procedure. Complications were evaluated according to the society of interventional radiology criteria.8 Any complication greater or equal to grade C was considered to be a major complication.
Statistical analysis
All analyses were performed with SPSS software (Version 17.0; IBM, Armonk, New York). The independent-samples t-test, Wilcoxon signed-rank test, Pearson’s x2 test, continuity correction x2 test, and Fisher’s exact test were used for comparison between the combined US/CT-guided RFA group and CT-guided RFA group. Kaplan-Meier method was used for calculating the overall survival rate in combined US/CT-guided RFA group. All statistical tests were two-sided. A value of P<0.05 was considered statistically significant.
Results
The baseline patient characteristics
All of the patients in this study successfully underwent TACE treatment before RFA. Thirty-one patients (29 males; aged from 38 to 80 years) with 35 liver tumors underwent 35 RFA procedures under CT guidance. Among them, four patients had two liver tumors in the hepatic dome, respectively. Thirty-four patients (29 males; aged from 32 to 73 years) with 35 liver tumors received 35 RFA procedures under combined US/CT guidance. Among them, one patient had two liver tumors in the hepatic dome. The mean tumor diameter was 2.3±0.6 cm (median, 2.5 cm; range, 1.1–3.0 cm) in the CT-guided RFA group, and 2.4±0.5 cm (median, 2.5 cm; range, 1.0–3.0 cm) in the combined US/CT-guided RFA group. There was no significant difference in the tumor diameter between the two groups (P=0.671). The detailed baseline patient characteristics in our study are presented in Table 1.
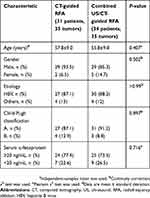 |
Table 1 The baseline patient characteristics |
The procedure time between the two groups and the efficacy in the combined US/CT-guided RFA group
All the patients successfully underwent CT-guided or combined US/CT-guided RFA after TACE treatment, and the primary technical success rate of CT-guided or combined US/CT-guided RFA procedure was 100%. One representative case of combined US/CT-guided RFA in the treatment of HCC in the hepatic dome is shown in Figure 1. The mean procedure time of the CT-guided RFA was significantly longer than that of the combined US/CT-guided RFA (81±11 vs 52±6 mins, P<0.001). The mean follow-up time in the combined US/CT-guided RFA group was 34±16 months (range, 13–67 months). During the follow-up, in the combined US/CT-guided RFA group, three tumors were diagnosed as local tumor recurrence. One patient died of liver failure, and one patient died of upper gastrointestinal hemorrhage. The 1-, 3-, and 5-year local recurrence rates were 3% (1/35), 6% (2/35), 9% (3/35), respectively, and the 1-, 3-, and 5-year overall survival rates were 100%, 97%, 94%, respectively.
Postoperative adverse reaction
A postoperative adverse reaction after the CT-guided RFA included: 21 cases (68%) of low-grade of fever, 13 cases (42%) of right upper quadrant pain, and 4 cases (13%) of nausea and/or vomiting. The postoperative adverse reaction after the combined US/CT-guided RFA included: 22 cases (65%) of low-grade of fever, 4 cases (12%) of right upper quadrant pain, and 5 cases (15%) of nausea and/or vomiting. The incidences of low-grade of fever, nausea, and/or vomiting between the two groups were not significantly different (P>0.99, P>0.99). However, the incidence of right upper quadrant pain in the CT-guided RFA group was greater than that of the combined US/CT-guided RFA group (42% vs 12%, P=0.01). These symptoms after the RFA procedure were easily controlled with symptomatic therapies and usually subsided within one week.
Complications
There was no occurrence of a major complication or RFA-related death in the two groups, and no minor complications occurred in the combined US/CT-guided RFA group. However, a small pneumothorax occurred in two patients (6.5%) in the CT-guided RFA group, and it was gradually absorbed within one week without pleural drainage. There were no significant differences in the overall complication rates between the combined US/CT-guided RFA group and the CT-guided RFA group (0% vs 6.5%, P=0.224).
Discussion
Percutaneous RFA has been widely used in clinical applications for the treatment of HCC.9–11 The majority of percutaneous RFA procedures are performed under US or CT guidance.1,2,12 US guidance allows real-time visualization of the electrode needle during puncture and enables a quick placement of the probe. Its disadvantages are a limited capability to monitor thermal effects and visualize tumor tissue resulting from air bubbles, which are produced by vaporization at the electrode tips during ablation. CT provides better edge detection of RFA lesions, conspicuity, and few artifacts in monitoring RFA compared with US,13 and it is not affected by the air in the lung and gastrointestinal tract.14 However, CT guidance also has some disadvantages: it increases the exposure time of X-ray radiation because repeated CT scans are needed to observe the location of the probe, and the accuracy of puncture is easily influenced by the patient’s respiratory motion. Additionally, since CT guidance cannot offer the visualization of the probe during the puncture, RFA under CT guidance may cause serious complications when the tumor is adjacent to major organs or vessels. In the present study, combined US/CT guidance was used in percutaneous RFA for HCC in the hepatic dome. This combination-guided strategy not only offered real-time images that could help to avoid the major organs or vessels during puncture, but also could provide high accuracy positioning of the electrode tips with the images of CT scan.
In combined US/CT-guided percutaneous RFA, the electrode needle placement was quickly performed under US guidance, and subsequently, CT guidance was used for precise positioning of the electrode tips. CT-guided percutaneous RFA required repeated adjustments of the puncture angle and path because of the non-consistent respiratory movement after each CT scan. Therefore, the CT-guided percutaneous RFA procedure took more time than the combined US/CT-guided percutaneous RFA procedure. In our study, the mean procedure time of the combined US/CT-guided RFA was significantly shorter than that of the CT-guided RFA. Although previous studies15,16 reported that the US-guided RFA with artificial pleural effusion was a good approach for HCC in the hepatic dome, this procedure was complicated and required a clinician with a high degree of experience. However, the combined US/CT-guided RFA for HCC in the hepatic dome did not require any infusion of the artificial pleural effusion. Therefore, the combined US/CT-guided RFA procedure was simple than the procedures of CT-guided RFA and US-guided RFA with artificial pleural effusion for HCC in the hepatic dome. Furthermore, percutaneous RFA under combined US/CT guidance reduced the number of CT scans and exposure time of X-ray radiation in comparison with CT guidance alone.
In the present study, a satisfactory efficacy was achieved in the combined US/CT-guided RFA group. The local recurrence rates at 1, 3, and 5 years were 3%, 6%, and 9%, respectively, and the overall survival rates at 1, 3, and 5 years were 100%, 97%, and 94%, respectively. Kagawa et al17 reported that the probabilities of overall survival at 1, 3, and 5 years in the surgical resection for early-stage HCC was 92.5%, 82.7%, and 76.9%, respectively. Meanwhile, a recent retrospective multicenter study18 reported that stereotactic body radiation therapy, as a salvage treatment, is also effective for inoperable HCC, and the overall survival rates at 1 and 3 years were 79.8% and 63.5%, respectively. These results demonstrated that the combined US/CT-guided percutaneous RFA was an ideal alternative treatment for unresectable HCC in the hepatic dome.
In our study, the complication of pneumothorax occurred in two patients in the CT-guided group. The reason for the pneumothorax was that the accuracy of the puncture path was influenced by the patients’ inconsistent respiratory motion, and that the puncture path passed through the lung. Fortunately, the pneumothorax in the two patients was gradually absorbed within one week without pleural drainage. Although previous studies6,19 reported that CT-guided transpulmonary RFA was useful for HCC in the hepatic dome, the relatively high rate of complications of pneumothorax requiring pleural drainage was a major drawback for the CT-guided transpulmonary RFA in the treatment of HCC in the hepatic dome. Additionally, the indication for CT-guided transpulmonary RFA was restricted to patients with lung emphysema. Although the overall complication incidences between the two groups were not statistically significant in our study, there were no occurrences of RFA-related complications in the combined US/CT-guided RFA group. Thus, percutaneous RFA under combined US/CT guidance for HCC in the hepatic dome is safe.
An interesting finding in our study was that the incidence of postoperative adverse reaction of right upper quadrant pain in the CT-guided RFA group was significantly greater than that of the combined US/CT-guided RFA group. Although the exact reason is not clear, it seems to be associated with the change of the guidance strategy from CT guidance to combined US/CT guidance.
In addition, all patients received TACE treatment before RFA in our study. There were several reasons for this. First, the accumulated lipiodol in the tumor, which was injected during TACE procedure, was a good marker to label the tumor. In general, after TACE, HCC lesions with hypo- or iso-echoic on the images of US changed to hyper-echoic, and HCC lesions with low-density on the images of CT changed to high-density, which significantly increased the visibility of HCC lesions on the images of US and CT. These can help to improve the accuracy and safety of percutaneous RFA. Second, occlusion of arterial flow by TACE may reduce heat-sink effects during RFA procedure. Thus, the combination treatment of TACE with RFA may achieve adequate ablation with wider necrotic areas. Third, the inclusion of TACE makes the evaluation of ablative margins easier, and enhances the control of satellite lesions.20,21 Lastly, the combined therapy of TACE with RFA is believed to provide better local tumor progression-free survival rate than RFA alone in the treatment of 2–3-cm sized HCCs.22
Our study had limitations. First, our study was retrospective, and the therapeutic options (combined US/CT-guided RFA or CT-guided RFA) for HCC in our study were individually determined on the basis of the preference of the RFA operators, which likely led to selection bias in our population. However, the bias was limited by choosing similar baseline characteristics and tumors between the two groups. Second, all patients enrolled in this study were from our one institution. A large-scale randomized controlled trial needs to be performed to validate our results.
Conclusion
Combined US/CT guidance was helpful in percutaneous RFA for HCC in the hepatic dome. This combination-guided strategy could shorten the procedure time and decrease the incidence of postoperative adverse reaction of right upper quadrant pain in comparison with CT guidance alone in percutaneous RFA for HCC in the hepatic dome.
Acknowledgment
This work was supported by grant from National Science Foundation of China (No.81601578, No.81873919, and No.81873917) and National Science Foundation of Hubei Province (No.2017CFB799).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wu J, Chen P, Xie YG, Gong NM, Sun LL, Sun CF. Comparison of the effectiveness and safety of ultrasound- and CT-guided percutaneous radiofrequency ablation of non-operation hepatocellular carcinoma. Pathol Oncol Res. 2015;21(3):637–642. doi:10.1007/s12253-014-9868-5
2. Kim JA, Shim JH, Kim MJ, et al. Radiofrequency ablation as an alternative to hepatic resection for single small hepatocellular carcinoma. Br J Surg. 2016;103(1):126–135. doi:10.1002/bjs.9960
3. Peng ZW, Zhang YJ, Chen MS, Lin XJ, Liang HH, Shi M. Radiofrequency ablation as first-line treatment for small solitary hepatocellular carcinoma: long-term results. Eur J Surg Oncol. 2010;36(11):1054–1060. doi:10.1016/j.ejso.2010.08.133
4. Zhang L, Yin X, Gan YH, et al. Radiofrequency ablation following first-line transarterial chemoembolization for patients with unresectable hepatocellular carcinoma beyond the Milan criteria. BMC Gastroenterol. 2014;14:11. doi:10.1186/1471-230X-14-11
5. Kim YK, Kim CS, Lee JM, Chung GH, Chon SB. Efficacy and safety of radiofrequency ablation of hepatocellular carcinoma in the hepatic dome with the CT-guided extrathoracic transhepatic approach. Eur J Radiol. 2006;60(1):100–107. doi:10.1016/j.ejrad.2006.05.002
6. Toyoda M, Kakizaki S, Horiuchi K, et al. Computed tomography-guided transpulmonary radiofrequency ablation for hepatocellular carcinoma located in hepatic dome. World J Gastroenterol. 2006;12(4):608–611. doi:10.3748/wjg.v12.i4.608
7. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi:10.1002/hep.20933
8. Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202.
9. Machi J, Bueno RS, Wong LL. Long-term follow-up outcome of patients undergoing radiofrequency ablation for unresectable hepatocellular carcinoma. World J Surg. 2005;29(11):1364–1373. doi:10.1007/s00268-005-7829-6
10. Dong J, Li W, Zheng Q, et al. CT-guided percutaneous step-by-step radiofrequency ablation for the treatment of carcinoma in the caudate lobe. Medicine (Baltimore). 2005;94(39):e1594. doi:10.1097/MD.0000000000001594
11. Guo Y, Zhang Y, Huang J, et al. Safety and efficacy of transarterial chemoembolization combined with CT-guided radiofrequency ablation for hepatocellular carcinoma adjacent to the hepatic hilum within milan criteria. J Vasc Interv Radiol. 2016;27(4):487–495. doi:10.1016/j.jvir.2016.01.002
12. Lee LH, Hwang JI, Cheng YC, et al. Comparable outcomes of ultrasound versus computed tomography in the guidance of radiofrequency ablation for hepatocellular carcinoma. PLoS One. 2017;12(1):e0169655. doi:10.1371/journal.pone.0169655
13. Cha CH, Lee FT
14. Widmann G, Schullian P, Haidu M, Bale R. Stereotactic radiofrequency ablation (SRFA) of liver lesions: technique effectiveness, safety, and interoperator performance. Cardiovasc Intervent Radiol. 2012;35(3):570–580. doi:10.1007/s00270-011-0200-4
15. Minami Y, Kudo M, Kawasaki T, et al. Percutaneous ultrasound-guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. J Gastroenterol. 2003;38(11):1066–1070. doi:10.1007/s00535-003-1197-5
16. Kondo Y, Yoshida H, Tateishi R, Shiina S, Kawabe T, Omata M. Percutaneous radiofrequency ablation of liver cancer in the hepatic dome using the intrapleural fluid infusion technique. Br J Surg. 2008;95(8):996–1004. doi:10.1002/bjs.6058
17. Kagawa T
18. Scher N, Janoray G, Riet FG, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: results from a retrospective multicentre study. Cancer Radiother. 2019;23(2):104–115. doi:10.1016/j.canrad.2018.07.138
19. Shibata T, Shibata T, Maetani Y, et al. Transthoracic percutaneous radiofrequency ablation for liver tumors in the hepatic dome. J Vasc Interv Radiol. 2004;15(11):1323–1327. doi:10.1097/01.RVI.0000132297.97113.C4
20. Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radio-frequency thermal ablation of sectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217(1):119–126. doi:10.1148/radiology.217.1.r00se02119
21. Kang SG, Yoon CJ, Jeong SH, et al. Single-session combined therapy with chemoembolization and radiofrequency ablation in hepatocellular carcinoma less than or equal to 5 cm: a preliminary study. J Vasc Interv Radiol. 2009;20(12):1570–1577. doi:10.1016/j.jvir.2009.09.003
22. Kim JW, Kim JH, Won HJ, et al. Hepatocellular carcinomas 2-3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs.radiofrequency ablation alone. Eur J Radiol. 2012;81(3):e189–e193. doi:10.1016/j.ejrad.2011.01.122
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.