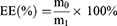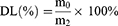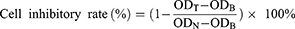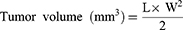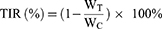Back to Journals » International Journal of Nanomedicine » Volume 18
Combined Thermosensitive Gel Co-Loaded with Dermaseptin-PP and PTX Liposomes for Effective Local Chemotherapy
Authors Dong Z, Zhang Q, Wang C, Hu W, Yu X, Guo M, Zhang X, Sun M, Du S, Lu Y
Received 8 August 2022
Accepted for publication 13 December 2022
Published 21 January 2023 Volume 2023:18 Pages 413—424
DOI https://doi.org/10.2147/IJN.S385470
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Ziyi Dong,* Qing Zhang,* Changhai Wang, Wenjun Hu, Xianglong Yu, Mingxue Guo, Xinyu Zhang, Meng Sun, Shouying Du, Yang Lu
School of Chinese Pharmacy, Beijing University of Chinese Medicine, Beijing, 102488, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shouying Du, School of Chinese Pharmacy, Beijing University of Chinese Medicine, Beijing, 102488, People’s Republic of China, Email [email protected] Yang Lu, School of Chinese Pharmacy, Beijing University of Chinese Medicine, Beijing, 102488, People’s Republic of China, Email [email protected]
Introduction: Chemotherapeutic drugs are often ineffective due to the delivery. Local chemotherapy, which has high drug concentration, low systemic toxicity, and long duration, has shown excellent potential. Cationic antimicrobial peptides have been proved to enhance the tumor cells’ uptake of chemotherapeutic drugs through the membrane-breaking effect. In this study, we designed and developed a thermosensitive gel co-loaded with Dermaseptin-PP and paclitaxel liposomes to increase local chemotherapy.
Methods: The paclitaxel liposomes were prepared. Then, it was co-loaded with Dermaseptin-PP in a poloxamer-based thermosensitive gel to obtain Dermaseptin-PP/paclitaxel liposomes gel. The thermosensitivity of gels was investigated by test tube inversion method. The rheology was tested by rheometer. The in vitro cytotoxicity and the permeation in tumor of gels were examined by H157 cells and the 3D cell model, respectively. The retention in tumor and antitumor activity of gels were evaluated by H157 tumor-bearing nude mice.
Results: The particle size of paclitaxel liposomes was 148.97 ± 0.21 nm. The encapsulation rate was 86.1%, and the drug loading capacity was 19.4%. The gels had slow-release and temperature-sensitive properties. The porous 3D network structure of the gels could ensure that the drug was fixed into the tumor. In vitro and in vivo distribution studies showed that Dermaseptin-PP promoted the permeation of the gels in H157 multicellular tumor spheres and achieved longer retention in tumor. In vitro and in vivo antitumor studies demonstrated that Dermaseptin-PP/paclitaxel liposomes gel significantly inhibited the growth of tumors for local chemotherapy with good biosafety.
Conclusion: This study provided a promising nanomedicine platform for combining antimicrobial peptides and chemotherapeutic drugs for local chemotherapy.
Keywords: cationic antimicrobial peptides, nanoparticles, temperature-sensitive, membrane-breaking, combination therapy
Introduction
Cancer is one of the most threatening diseases to human health. Lung cancer is the leading cause of cancer deaths, and its death rate exceeds that of breast, prostate and pancreatic cancers combined.1 Chemotherapy is still one of the most commonly used treatments in clinical practice.2,3 However, chemotherapeutic drugs usually have poor selectivity, low bioavailability, and systemic toxic side effects. All these disadvantages result in poor clinical efficacy.4,5 To overcome the limitations of chemotherapeutic drugs, antitumor drugs with novel mechanisms and drug delivery systems have been extensively researched and developed.
To ensure sufficient drug concentration and duration in tumor, local chemotherapy is often used clinically to achieve a high therapeutic index.6–8 The thermosensitive in situ gels has unique advantages in drug delivery.9,10 Therefore, loading chemotherapeutic drugs into gels has been chosen. The drug reservoir in tumor is formed to ensure the drug concentration in tumor and prolong the drug action time.11,12 Poloxamer, a triblock copolymer composed of poly (ethylene oxide) and poly (propylene oxide), is a promising gel carrier material with excellent biocompatibility, biodegradability, non-toxicity.13,14 Notably, a certain concentration of poloxamer has the temperature-sensitive property of being liquid at low temperatures and becoming into gels at high temperatures.15
Antimicrobial peptides are a class of short peptides consisting of 10–100 amino acids, which are positively charged and amphiphilic.16,17 Currently, more than 250 antimicrobial peptides in Antimicrobial Peptide Database (APD) have been found to have antitumor effects. The membrane-breaking effect is considered as the primary antitumor mechanism of antimicrobial peptides. Specifically, the peptides bind to the negatively charged tumor cell membranes by electrostatic force, disrupt the cell membrane integrity, and thus kill tumor cells.18,19 In short, antimicrobial peptides are one of the most promising new antitumor drugs.20,21
Dermaseptin-PP is a cationic antimicrobial peptide that can be classified into Dermaseptin family. It was first identified and characterized from the skin secretion of Phyllomedusa palliata. The antitumor activity of antimicrobial peptides in Dermaseptin family has been reported.22,23 Based on this, the in vitro and in vivo antitumor activity and mechanism of Dermaseptin-PP have been investigated previously,24 and the results showed that Dermaseptin-PP exhibited strong in vitro antitumor activity through membrane disruption and apoptosis activation. However, there are some limitations of its individual application in antitumor therapy: the amount of natural antimicrobial peptides is small and the cost of synthesis is high; the hemolytic toxicity of Dermaseptin-PP at high concentrations is not to be ignored; multiple doses are required and the efficacy is not satisfactory. These problems are also common to the other antimicrobial peptides.25–27 It has been shown that antimicrobial peptides have synergistic antitumor activity in combination with chemotherapeutic drugs and can even reverse the chemotherapeutic drug resistance of tumor cells.
Nanocarriers have significant advantages in altering the drug delivery route, improving drug targeting, and enhancing the deep penetration in tumor.28–30 Among them, liposomes are commonly used drug carriers. Liposomes can be simply prepared, prolong the blood circulation time of drugs, and have good biocompatibility. The advantages of liposomes in improving the efficacy and reducing the toxicity of chemotherapeutic drugs are unparalleled.31–33 Paclitaxel (PTX) is one of the most common chemotherapeutic drugs, but its low bioavailability leads to minimal accumulation in tumor and significantly limits the application. An increasing number of studies have used nanocarriers loaded with PTX to improve its bioavailability and antitumor efficacy.34–36 Currently, several liposomes, such as PTX liposomes for injection, have been marketed.
Based on this, a thermosensitive gel co-loaded with Dermaseptin-PP and paclitaxel liposomes (PTXL) was designed and developed in this study. Through the characterization, in vitro evaluation, tumor retention observation, and in vivo efficacy evaluation of the drug-loaded gels, it was hoped to clarify whether Dermaseptin-PP could be efficiently combined with PTXL through its membrane-breaking permeability. Collectively, the design presented here not only provided a promising delivery system for local cancer therapy, but also a novel idea for the combination of antimicrobial peptides and chemotherapeutic drugs.
Material and Methods
Dermaseptin-PP (Sequence: ALWKDMLKGIGKLAGKAALGAVKTLV-NH2) was purchased from GL Biochem Co., Ltd. (Shanghai, China). PTX was purchased from Meilun Biotechnology Co., Ltd. (Dalian, China). mPEG2000, soy lecithin (SPC) and cholesterol (CHO) were purchased from Ruixi Biotechnology Co., Ltd. (Xi’an, China). Poloxamer 407 was purchased from Feng Li Jing Qiu Trading Co., Ltd. (Beijing, China). All other reagents (AR grade) were used without further purification.
Human non-small cell lung adenocarcinoma cell line (H157) was purchased from BeNa Culture Collection (Beijing, China). H157 cells were cultured in RPMI medium containing fetal bovine serum (FBS, 10%), penicillin (100 U/mL), and streptomycin (100 mg/mL). They were incubated under a 5% CO2 atmosphere at 37°C. All cell culture reagents were purchased from Gibco (USA).
Male Balb/C nude mice (20 ± 2 g) were purchased from SPF Biotechnology Co., Ltd. (Beijing, China). Animal care was performed in compliance with the guidelines of the Ministry of Science and Technology of China (2006) and the related ethical regulations of Beijing University of Chinese Medicine. The protocol of the current study was approved by the Ethical Committee (27-5-2018) for Laboratory Animals of Beijing University of Chinese Medicine (No.: BUCM-4-2021070501-3007). All experimental procedures were designed to minimize animal suffering and the number of animals used.
Preparation and Characterization of Paclitaxel Liposomes
Specifically, 8 mg SPC, 1.15 mg CHO, 4.5 mg DSPE-mPEG2000 (65:25:10, mol:mol:mol), and 3.2 mg PTX were dissolved in 3 mL dichloromethane. Then the solution was rotary evaporated at 37°C under reduced pressure to remove dichloromethane. 1 mL PBS buffer (pH 7.4) was added into the eggplant-shaped flask, and then whirled to make the lipid film completely fall off the flask wall. PTXL was sonicated by ultrasonic cell crusher (SCIENTZ-IID, Ningbo Xinzhi Biotechnology Co., Ltd., China) for 5 min. Three samples were set up in parallel.
100 μL PTXL was taken and diluted 10 times with deionized water. 1 mL diluted solution was taken to test its particle size, PDI and zeta potential.
Since PTX was insoluble in water, the encapsulation rate (EE) and drug loading (DL) capacity of PTX were determined by low-speed centrifugation.37 1 mL PTXL was centrifuged at 582 g for 10 min. 100 μL supernatant was added to 900 μL methanol. Then the solution was sonicated for 5 min. The PTX content in the liposomes was determined by high-performance liquid chromatography (HPLC, Agilent 1100, USA). Before centrifugation, 100 μL PTXL was centrifuged at 1618 g for 5 min. Then, 900 μL methanol was added. The PTX content in the PTXL was determined by HPLC.
The content of PTX was quantified by HPLC equipped with a InertsilR ODS-3 column (250 mm × 4.6 mm, 5 μm) with 60:40 mixture of acetonitrile and water as mobile phase for isocratic elution, the flow rate was 1.0 mL/min and detection wavelength was 227 nm.
The EE and DL were calculated according to the following formulas. m0 is mass of PTX in PTXL. m1 is mass of the feeding PTX. m2 is mass of PTXL.
Preparation of Dermaseptin-PP/Paclitaxel Liposomes@Gel
According to the results of the previous study,24 the gels were prepared in the ratio of PTX: Dermaseptin-PP 5:1 (Figures S1, S2, Table S1). To prepare Der/PTXL@gel, 200 mg poloxamer 407 and 0.68 mg Dermaseptin-PP were mixed in 1 mL PTXL. The solution was stirred to dissolve them fully. Similarly, the blank gel was prepared using the above technique without adding Dermaseptin-PP or PTXL. PTXL@gel was prepared without adding Dermaseptin-PP and Der@gel without adding PTXL.
Characterization of Dermaseptin-PP/Paclitaxel Liposomes@Gel
Morphology
The blank gel and Der/PTXL@gel were freeze-dried and then observed by scanning electron microscopy (SEM, JEM-2100F, Electron Corporation, Japan).
Thermosensitive Properties
The phase transition process of the gels was observed using the test tube inversion method.38 When the temperature of Der/PTXL@gel increased 1°C, it was inverted to detect whether it flowed. If it did not flow within 30s, this temperature was considered to be the sol-gel transition temperature (Tgel). The states of the blank gel, Der/PTXL@gel at different temperatures were photographed.
Rheological Properties
Rheology analysis of Der/PTXL@gel was carried out by a rheometer system (MARS60, HAAKE, Germany). Der/PTXL@gel was heated at a rate of 2°C/min, and the heating temperature range was 15–45°C. The variation of the storage modulus (G′) and loss modulus (G″) with temperature was detected at a frequency of 1 Hz and a strain of 1%. The temperature of the intersection of the G′ and G″ curves was the Tgel.
In vitro Drug Release
200 μL samples were taken at 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, 16 h and 24 h and used for the determination of Dermaseptin-PP and PTX by HPLC. Three samples were set up in parallel at each time point.
The chromatographic conditions for PTX were as before. The chromatographic conditions for PTX were as before. The content of Dermaseptin-PP was quantified by HPLC equipped with a InertsilR ODS-3 column (250 mm × 4.6 mm, 5 μm) with 60:40 mixture of 0.1% trifluoroacetic acid (TFA) aqueous solution and 0.1% TFA acetonitrile solution as mobile phase for isocratic elution, the flow rate was 1.0 mL/min and detection wavelength was 280 nm.
In vitro Cytotoxicity
Specifically, H157 cells (7 × 103 /well) were seeded into 96-well plate and incubated overnight at 5% CO2, 37°C. The cells were then incubated with PTXL@gel, Der/PTXL@gel and blank gel (PTX: 0.01–10 µg/mL) for 24 h. The cell inhibition rate after the incubation was analyzed using CCK-8 assays and calculated according to the following formula.
ODT is the OD of the administration group, ODB is the OD of the blank control group and ODN is the OD of the negative control group.
Tumor Permeability in a 3D Cell Model
A 3D cell model was constructed to examine whether Dermaseptin-PP could increase the fluorescence penetration of the gels in the tumor. For observation, IR780 was used instead of PTX to prepare IR780 liposome gel (IRL@gel) and Dermaseptin-PP-IR780 liposome gel (Der/IRL@gel).
0.5 g agarose was added into 25 mL deionized water and heated for 3 min to dissolve completely. 200 μL agarose solution was added dropwise to the bottom of 24-well plate. H157 cells (2 × 103/well) were seeded into pre-coated 24-well plate and cultured for 5–7 days to form H157 multicellular tumor spheroids (MCTS). 200 μL of IRL@gel or Der/IRL@gel (IR780: 40 μg) was co-incubated with MCTS for 4 h. Fluorescence penetration of gels at different depths in tumor was observed by confocal laser scanning microscope (FV1000, Olympus, Japan).
In vivo Behavior
One of the advantages of gels is the ability to achieve prolonged retention for slow release. Therefore, the retention of the gels should be investigated.
To construct the H157 tumor-bearing nude mice, the cells (8 × 106) in 100 μL PBS were inoculated into the nude mice. The drug administration was started until the tumors reached the required volumes for the experiment. For observation, IRL@gel and Der/IR@gel were still used for the following experiment. The groups were free IR780, IRL@gel and Der/IR@gel (IR780: 200 μg/mL). 50 μL of each group was injected slowly into tumor site when the tumor volumes were approximately 300–400 mm3. Fluorescence of IR780 was captured using an IVIS Spectrum system (MetaMorpH-MIIS, USA) at different intervals (24 h, 48 h, 96 h, 120 h, 144 h).
In vivo Antitumor Effect and Biosafety
The conditions for tumor-bearing mice model construction were the same as before. The groups included control group, blank gel group, Der@gel group, PTXL@gel group, and Der/PTXL@gel group. The nude mice with a tumor volume of 150 mm3 were randomly divided into 6 groups of 5 mice each. The mice in each group were administered 50 μL (PTX: 3.40 mg/mL; Dermaseptin-PP: 0.68 mg/mL) every 2 days for three doses. The weight, length and width of the tumors were recorded until the end of the experiment. Tumor volumes were calculated according to the following formula. L is the length of the tumor and W is the width.
The mice in each group were executed on day 15. The tumor tissues were stripped, photographed and weighed. The tumor inhibition rate (TIR) was calculated according to the following formula. WT is the tumor weight of the administration group, and WC is the average tumor weight of the control group.
Meanwhile, the major organs (heart, liver, spleen, lungs, and kidneys) were collected and sectioned. The sections were stained with H&E kit and then photographed by a fluorescent microscope (NIKON ECLIPSE C1, Nikon, Japan) to observe the damage of the organs.
Statistical Analysis
All the data were given as mean ± SD and plotted with GraphPad Prism 8.0. Meanwhile, student’s test was used to analyze and compare the differences between the groups.
Results
Preparation and Characterization of Paclitaxel Liposomes
PTXL had a particle size of 148.97 ± 0.21 nm, a PDI of 0.21 ± 0.01, and a zeta potential of −1.01 ± 0.31 mV, indicating that the liposomes were uniformly dispersed and stable (Figure 1A, Table S2).
The standard curve of PTX content was established according to the aforementioned HPLC conditions. The standard curve was: y = 37885x + 374939, R2 = 0.9993 (Figure 1B). The concentration of PTX could be calculated from the standard curve. The EE of PTXL on PTX was 86.1% and the DL capacity was 19.4%, indicating that PTXL had an appropriate EE and DL capacity to be loaded into the gels.
Preparation and Characterization of Dermaseptin-PP/Paclitaxel Liposomes@Gel
SEM Images
The freeze-dried blank gel and Der/PTXL@gel were observed by SEM. The blank gel showed a 3D network structure with a loose structure and a smooth surface (Figure 1C). After being loaded with drugs, the structure of Der/PTXL@gel was denser and the 3D network structure was more apparent. PTXL was clearly observed on the surface of the gels, demonstrating that the gels were successfully loaded with PTXL (Figure 1C).
Thermosensitive Behaviors
As shown in Figure 1D, the drugs did not change the temperature-sensitive property of the gels, and the phase transition temperature of Der/PTXL@gel was consistent with that of the blank gel. At a low temperature, the gels were flowing. When the temperature raised to 27°C, the gels changed to a colloidal state that could not flow.
Rheological Behaviors
The storage modulus (G′) and loss modulus (G″) are the two important indicators to measure the kinetics of gel formation. When G″ is greater than G′, the sample is in a gel state and a network-like structure is formed. The curves of G′ and G″ with temperature for Der/PTXL@gel were consistent with the results of the test tube inversion experiment (Figure 1E). The intersection temperature of G′ and G″ for Der/PTXL@gel was about 27°C. It could be used to form gels under body temperature to prolong drug retention time.
The in vitro Studies of Drug Release by Gels
The results of PTX release in Der/PTXL@gel are shown in Figure 1F. The release of PTX in the gels was slow in PBS buffer, and only 32.83 ± 2.71% of PTX was released in 24 h. This indicated that Der/PTXL@gel had slow release characteristic. The release results of Dermaseptin-PP in Der/PTXL@gel are shown in Figure 1G. Since Dermaseptin-PP was a water-soluble peptide, it was more soluble in PBS buffer and could be released more easily. Its cumulative release rate within 24 h was 84.53 ± 3.90%. This release behavior allowed Dermaseptin-PP to be released rapidly from Der/PTXL@gel and to enhance the tumor permeability of PTXL.
The Cytotoxicity of Drug-Loaded Gels
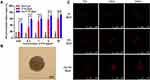
The inhibition ratio of the blank gel was less than 15% at all doses, indicating that the blank gel had no effect on cell growth (Figure 2A). Both Der/PTXL@gel and PTXL@gel exhibited concentration-dependent cytotoxicity, and the cytotoxicity of Der/PTXL@gel was significantly higher than that of PTXL@gel at all doses. This indicated that the addition of Dermaseptin-PP could significantly improve the antitumor activity of PTXL@gel. Notably, the difference in the inhibition ratios between Der/PTXL@gel and PTXL@gel was greater when the concentration of PTX was low. When the concentration of PTX was 0.01 μg/mL, the inhibition ratio of Der/PTXL@gel was approximately 20% higher than that of PTXL@gel (Figure 2A). The results suggested that the addition of Dermaseptin-PP could significantly increase the antitumor activity of PTXL@gel.
Tumor Permeation Behavior in a 3D Cell Model
A 3D cell model similar to the solid tumor was used to examine the membrane-breaking penetration ability of the drug-loaded gels. After 7 days of incubation as described previously, H157 MCTS were successfully constructed (Figure 2B). After co-incubation of IRL@gel or Der/IRL@gel with MCTS for 4 h, Der/IRL@gel group showed more vital membrane-breaking penetration ability, ie, significantly stronger red fluorescence signals. At the same penetration depth, Der/IRL@gel had a more distinct red fluorescent signal in the center of the tumor spheres, indicating that Dermaseptin-PP was able to promote drug through membrane-breaking penetration into the tumor (Figure 2C).
Tumor Retention and Distribution of Drug-Loaded Gels in vivo
The retention of free IR780, IRL@gel and Der/IRL@gel at the tumors was examined. The retention time of free IR780 group at the tumors was short, and its fluorescence intensity decreased from 24 h. Both IRL@gel and Der/IRL@gel groups were able to stay at the tumors for a long time after intratumoral administration, and the fluorescence intensity remained high until 96 h. This indicated that the gels had a slow-release property and could remain in vivo for a long time. In addition, Der/IRL@gel group had higher fluorescence intensity and longer fluorescence retention time than IRL@gel group. This might be due to the ability of Dermaseptin-PP to help IR780 penetrate deeper into the tumors. This was consistent with the results of in vitro 3D cell model permeability assay (Figures 3 and S3).
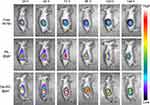 |
Figure 3 Residence time of different gels in tumor after intratumoral injection. Abbreviations: IRL@gel, IR780 liposomes@gel. Der/IRL@gel, Dermaseptin-PP/IR780 liposomes@gel. |
In vivo Antitumor Activity and Biosafety
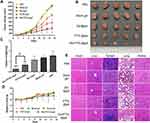
The results of tumor growth trend showed (Figure 4A) that the tumors in the control group (without administration) and blank gel group had aggressive growth characteristics with tumor volumes more than 1500 mm3 on day 15. The growth of tumors of Der/PTXL@gel group was significantly restrained. As seen from the photographs of the tumors (Figure 4B), the control group had the largest tumor volume and Der/PTXL@gel group had the smallest tumor volume. The results of tumor weight showed (Figure 4C) that the control group and blank gel group had similar tumor weight which was the heaviest, and Der@gel group had a slightly lighter tumor weight. The tumor weights of both PTXL@gel and Der/PTXL@gel groups were significantly lower than the control group, and Der/PTXL@gel group also had a significantly lighter tumor weight than PTXL@gel group. The TIR of blank gel, Der@gel, PTXL@gel and Der/PTXL@gel were 12.0%, 26.4%, 56.3% and 81.7%, respectively. This indicated that single-loaded gels’ in vivo antitumor efficacy was weak, while co-loaded gels had stronger in vivo antitumor efficacy.
The changes in body weight of mice could reflect their health status to some extent. During the experiment, the body weight of mice in all groups showed a slowly increasing trend (Figure 4D). The H&E staining results of the main organs of mice (Figure 4E) showed that there were no noticeable inflammatory reactions and injuries in the heart, liver, spleen, lung and kidneys of each administration group, and no evident tumor metastasis was found. The above results indicated that Der/PTXL@gel had a good in vivo safety.
Discussion
This study designed and developed a thermosensitive gel co-loaded with Dermaseptin-PP, which was discovered and characterized by us for the first time, and PTXL. In this drug delivery system, the peptides played a role in membrane-breaking penetration and PTXL achieved local chemotherapy.
Liposomes are commonly used as carriers of chemotherapeutic drugs, which have significant advantages in improving therapeutic efficiency and reducing toxicity.31,32 To overcome the low solubility of PTX, PTXL with a particle size of 148.97 ± 0.21 nm, a PDI of 0.21 ± 0.01, and a zeta potential of −1.01 ± 0.31 mV was firstly prepared. In general, nanocarriers of 20–200 nm have the EPR effect to passively target to the tumors. The gels showed a 3D network structure. With the addition of drugs, the 3D network structure was denser and more apparent. Fan et al39 developed a PTX loaded thermosensitive gel with a gel network structure that extended drug release. So this structure provided structural basis for the slow release of drugs. For local chemotherapy, the slow release of drugs is essential to the improvement of efficacy and reduction of adverse effects. In the study of Abdeltawab et al,40 a certain concentration of poloxamer had the temperature-sensitive property which was liquid at low temperature and turned into gel at high temperature. Consistently, the drug-loaded gels we prepared also had a significant temperature sensitivity with the variable temperature of about 27°C.
The cytotoxicity of Der/PTXL@gel was more significant than that of PTXL@gel. This was attributed to the fact that Dermaseptin-PP could produce a membrane-breaking effect and increase the uptake of PTX by tumor cells.24 The main anti-tumor mechanism of Dermaseptin antimicrobial peptide family is to disrupt tumor cell membranes. They could bind to tumor cells through electrostatic effect, insert into cell membranes and create holes. The holes caused the leakage of tumor cell contents, thus helping the peptides quickly and effectively kill tumor cells.41,42 The in vitro 3D cell model was used to simulate in vivo tumor cells to investigate the membrane-breaking penetration of drug-loaded gels.43,44 Consistent with the expected results, Dermaseptin-PP resulted in more membrane-breaking penetration and distribution of drug-loaded gels.
Thermosensitive gels had a potential to achieve proper dispersion and in situ persistence.9,45 In the study of Geng et al,45 a facile one-step method was first developed to fabricate a novel injectable in situ forming photothermal modulated hydrogel drug delivery platform (DPPy@PNAs), and it could promise a high drug loading capacity as well as precise synchronization between the controllable release of chemotherapeutics and the duration of near-infrared PTT. Correspondingly, in our study, in vivo distribution results showed that the drug-loaded gels could achieve prolonged retention in tumor and act as a drug reservoir with slow-release effect. Moreover, Dermaseptin-PP was further demonstrated to improve the membrane-breaking penetration of drug-loaded gels, as evidenced by the stronger fluorescence signals and longer fluorescence retention time. The addition of Dermaseptin-PP resulted in a stronger in vivo antitumor effect of the drug-loaded gels and enhanced the chemotherapy effect of PTXL. In addition, the biocompatibility and toxicity of the drug delivery systems are also important to be investigated.9,10,43,45 During the experimental cycle, the weight of mice administered intratumorally in all groups showed slow increase, and no significant damage was observed in any of the major organs. Therefore, the drug-loaded gels could be considered to have good biosafety.
Similar drug delivery systems have been reported.9,10,46,47 Gao et al10 designed an in situ drug-loaded injectable thermosensitive gel system for the simultaneous delivery of norcantharidin-loaded nanoparticles (NCTDNPs) and doxorubicin (Dox) via intratumoral administration to HCC tumors. Drug-loaded gels had good thermal sensitivity and both in vivo and in vitro studies showed that drug-loaded gels significantly inhibited tumor growth. This study showed that loading the combined drugs into the thermosensitive gels was a promising strategy for the local-regional treatment via intratumoral administration. Zhang et al9 developed a temperature-sensitive injectable hydrogel loaded with garcinia cambogia nanoparticles and the tumor-penetrating peptide iRGD made of hydroxypropyl cellulose, silk protein and glycerol, which exhibited a short gelation time, good compatibility and slow-release properties. Similar to our results, the retention effect, local administration and sustained slow release of iRGD facilitated the penetration of nanoparticles into the tumor deep. It could be clarified that iRGD played the same role as Dermaseptin-PP. The temperature-sensitive gels established in this study achieved local chemotherapy by loading PTXL and membrane-breaking penetration by loading Dermaseptin-PP. Using the gels as carriers, its characteristics of good biocompatibility, low toxic side effects, strong drug retention and temperature sensitivity could be fully utilized to achieve good drug release from the drug delivery systems. In addition, the proposed strategy of combining cationic antimicrobial peptides with chemotherapeutic drugs could be considered as a new idea. This strategy was applied to enhance the cell uptake of chemotherapeutic drugs through the membrane-breaking effect of antimicrobial peptides and improve the penetration and accumulation of chemotherapeutic drugs in tumor. However, intratumoral injection is only suitable for superficial tumors, while intravenous systemic chemotherapy should be used for deep tumors prone to metastasis in clinical practice.
Conclusion
In this study, a localized codelivery system of Dermaseptin-PP and PTXL was developed for regional chemotherapy with slow-release, thermosensitive properties and a porous 3D network-like structure. Through the unique membrane-breaking effect of Dermaseptin-PP, local chemotherapy was realized efficiently. Moreover, in vivo experiments demonstrated that intratumoral administration of the drug-loaded gels significantly inhibited tumor growth with good biosafety. Collectively, the drug-loaded gels represent a promising drug delivery system for local chemotherapy. In addition, the synergistic form of combination of Dermaseptin-PP and chemotherapeutic drugs is broadened and the new idea of cationic antimicrobial peptides for antitumor research is also provided.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (Nos. 82173989).
Disclosure
All authors have no conflicts of interest for this work to disclose.
References
1. Siegel RL, Miller KD, Fuchs HE., et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi:10.3322/caac.21708
2. Gaspar D, Veiga AS, Castanho MARB. From antimicrobial to anticancer peptides. A review. Front Microbiol. 2013;4:294. doi:10.3389/fmicb.2013.00294
3. Woodman C, Vundu G, George A, et al. Applications and strategies in nanodiagnosis and nanotherapy in lung cancer. Semin Cancer Biol. 2021;69:349–364. doi:10.1016/j.semcancer.2020.02.009
4. Chatterjee S. Mechanisms of resistance against cancer therapeutic drugs. Curr Pharm Biotechnol. 2014;15(12):1105–1112. doi:10.2174/1389201015666141126123952
5. Munker S, Gerken M, Fest P, et al. Chemotherapy for metastatic colon cancer: no effect on survival when the dose is reduced due to side effects. Bmc Cancer. 2018;18(1):455. doi:10.1186/s12885-018-4380-z
6. Ta HT, Dass CR, Dunstan DE. Injectable chitosan hydrogels for localised cancer therapy. J Controlled Release. 2008;126(3):205–216. doi:10.1016/j.jconrel.2007.11.018
7. Tang RZ, Liu ZZ, Gu SS, et al. Multiple local therapeutics based on nano-hydrogel composites in breast cancer treatment. J Materials Chem B. 2021;9(6):1521–1535. doi:10.1039/D0TB02737E
8. Sun S, Tang Q, Wang Y, et al. In situ micro–nano conversion augmented tumor-localized immunochemotherapy. ACS Appl Mater Interfaces. 2022;14:27013–27027. doi:10.1021/acsami.2c02490
9. Zhang D, Chu Y, Qian H, et al. Antitumor activity of thermosensitive hydrogels packaging gambogic acid nanoparticles and tumor-penetrating peptide iRGD against gastric cancer. Int J Nanomedicine. 2020;15:735–747. doi:10.2147/IJN.S231448
10. Gao B, Luo J, Liu Y, et al. Intratumoral administration of thermosensitive hydrogel co-loaded with norcantharidin nanoparticles and doxorubicin for the treatment of hepatocellular carcinoma. Int J Nanomedicine. 2021;16:4073–4085. doi:10.2147/IJN.S308057
11. Sanjana A, Ahmed MG, Bh JG. Preparation and evaluation of in-situ gels containing hydrocortisone for the treatment of aphthous ulcer. J Oral Biol Craniofacial Res. 2021;11(2):269–276. doi:10.1016/j.jobcr.2021.02.001
12. Cao D, Zhang X, Akabar MD, et al. Liposomal doxorubicin loaded PLGA-PEG-PLGA based thermogel for sustained local drug delivery for the treatment of breast cancer. Artif Cells, Nanomed Biotechnol. 2019;47(1):181–191. doi:10.1080/21691401.2018.1548470
13. Jung Y, Park W, Park H, et al. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr Polym. 2017;156:403–408. doi:10.1016/j.carbpol.2016.08.068
14. Liu Y, Lu WL, Wang JC, et al. Controlled delivery of recombinant hirudin based on thermo-sensitive Pluronic® F127 hydrogel for subcutaneous administration: in vitro and in vivo characterization. J Controlled Release. 2007;117(3):387–395. doi:10.1016/j.jconrel.2006.11.024
15. Goo YT, Yang HM, Kim CH, et al. Optimization of a floating poloxamer 407-based hydrogel using the Box-Behnken design: in vitro characterization and in vivo buoyancy evaluation for intravesical instillation. Eur J Pharmaceutical Sci. 2021;163:105885. doi:10.1016/j.ejps.2021.105885
16. Ageitos JM, Sánchez-Pérez A, Calo-Mata P, et al. Antimicrobial peptides (AMPs): ancient compounds that represent novel weapons in the fight against bacteria. Biochem Pharmacol. 2017;133:117–138. doi:10.1016/j.bcp.2016.09.018
17. Andreu D, Rivas L. Animal antimicrobial peptides: an overview. Peptide Sci. 1998;47(6):415–433. doi:10.1002/(SICI)1097-0282(1998)47:6<415::AID-BIP2>3.0.CO;2-D
18. Deslouches B, Di YP. Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget. 2017;8(28):46635–46651. doi:10.18632/oncotarget.16743
19. Shi D, Hou X, Wang L, et al. Two novel dermaseptin-like antimicrobial peptides with anticancer activities from the skin secretion of Pachymedusa dacnicolor. Toxins. 2016;8(5):144. doi:10.3390/toxins8050144
20. Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24(12):1551–1557. doi:10.1038/nbt1267
21. Riedl S, Zweytick D, Lohner K. Membrane-active host defense peptides–challenges and perspectives for the development of novel anticancer drugs. Chem Phys Lipids. 2011;164(8):766–781. doi:10.1016/j.chemphyslip.2011.09.004
22. Long Q, Li L, Wang H, et al. Novel peptide dermaseptin‐PS 1 exhibits anticancer activity via induction of intrinsic apoptosis signalling. J Cell Mol Med. 2019;23(2):1300–1312. doi:10.1111/jcmm.14032
23. Tan Y, Chen X, Ma C, et al. Biological activities of cationicity-enhanced and hydrophobicity-optimized analogues of an antimicrobial peptide, dermaseptin-PS3, from the Skin Secretion of Phyllomedusa sauvagii. Toxins. 2018;10(8):320. doi:10.3390/toxins10080320
24. Dong Z, Hu H, Yu X, et al. Novel frog skin-derived peptide dermaseptin-PP for lung cancer treatment: in vitro/vivo evaluation and anti-tumor mechanisms study. Front Chem. 2020;8:476. doi:10.3389/fchem.2020.00476
25. Moret F, Gobbo M, Reddi E. Conjugation of photosensitisers to antimicrobial peptides increases the efficiency of photodynamic therapy in cancer cells. Photochem Photobiol Sci. 2015;14(7):1238–1250. doi:10.1039/c5pp00038f
26. Polyansky AA, Vassilevski AA, Volynsky PE, et al. N-terminal amphipathic helix as a trigger of hemolytic activity in antimicrobial peptides: a case study in latarcins. FEBS Lett. 2009;583(14):2425–2428. doi:10.1016/j.febslet.2009.06.044
27. Ahmad A, Yadav SP, Asthana N, et al. Utilization of an amphipathic leucine zipper sequence to design antibacterial peptides with simultaneous modulation of toxic activity against human red blood cells. J Biol Chem. 2006;281(31):22029–22038. doi:10.1074/jbc.M602378200
28. Bai S, Zhang Y, Li D, et al. Gain an advantage from both sides: smart size-shrinkable drug delivery nanosystems for high accumulation and deep penetration. Nano Today. 2021;36:101038. doi:10.1016/j.nantod.2020.101038
29. Li B, Shao H, Gao L, et al. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. 2022;29(1):2130–2161. doi:10.1080/10717544.2022.2094498
30. Mu W, Chu Q, Liu Y, et al. A review on nano-based drug delivery system for cancer chemoimmunotherapy. Nano-Micro Letters. 2020;12(1):1–24. doi:10.1007/s40820-020-00482-6
31. Yang T, Choi MK, Cui FD, et al. Antitumor effect of paclitaxel-loaded PEGylated immunoliposomes against human breast cancer cells. Pharm Res. 2007;24(12):2402–2411. doi:10.1007/s11095-007-9425-y
32. Rahman M, Beg S, Verma A, et al. Liposomes as Anticancer Therapeutic Drug Carrier’s Systems: more than a Tour de Force. Curr Nanomed. 2020;10(2):178–185. doi:10.2174/2468187309666190618171332
33. Filipczak N, Pan J, Yalamarty SSK, et al. Recent advancements in liposome technology. Adv Drug Deliv Rev. 2020;156:4–22. doi:10.1016/j.addr.2020.06.022
34. Ashrafizadeh M, Ahmadi Z, Mohamadi N, et al. Chitosan-based advanced materials for docetaxel and paclitaxel delivery: recent advances and future directions in cancer theranostics. Int J Biol Macromol. 2020;145:282–300. doi:10.1016/j.ijbiomac.2019.12.145
35. Das T, Anand U, Pandey SK, et al. Therapeutic strategies to overcome taxane resistance in cancer. Drug Resistance Updates. 2021;55:100754. doi:10.1016/j.drup.2021.100754
36. Chen Q, Xu S, Liu S, et al. Emerging nanomedicines of paclitaxel for cancer treatment. J Controlled Release. 2022;342:280–294. doi:10.1016/j.jconrel.2022.01.010
37. Luo LM, Huang Y, Zhao BX, et al. Anti-tumor and anti-angiogenic effect of metronomic cyclic NGR-modified liposomes containing paclitaxel. Biomaterials. 2013;34(4):1102–1114. doi:10.1016/j.biomaterials.2012.10.029
38. Zhao S, Zhu H, Chen Z, et al. Preparation and properties of a temperature-and pH-responsive polypeptide hydrogel. Mater Res Express. 2019;6(8):085711. doi:10.1088/2053-1591/ab253e
39. Fan R, Sun W, Zhang T, et al. Paclitaxel-nanocrystals-loaded network thermosensitive hydrogel for localised postsurgical recurrent of breast cancer after surgical resection. Biomed Pharmacother. 2022;150:113017. doi:10.1016/j.biopha.2022.113017
40. Abdeltawab H, Svirskis D, Sharma M. Formulation strategies to modulate drug release from poloxamer based in situ gelling systems. Expert Opin Drug Deliv. 2020;17(4):495–509. doi:10.1080/17425247.2020.1731469
41. Couty M, Dusaud M, Miro-Padovani M, et al. Antitumor activity and mechanism of action of hormonotoxin, an LHRH analog conjugated to Dermaseptin-B2, a multifunctional antimicrobial peptide. Int J Mol Sci. 2021;22(21):11303. doi:10.3390/ijms222111303
42. Pouny Y, Rapaport D, Mor A, et al. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogs with phospholipid membranes. Biochemistry. 1992;31(49):12416–12423. doi:10.1021/bi00164a017
43. Shen M, Xu YY, Sun Y, et al. Preparation of a thermosensitive gel composed of a mPEG-PLGA-PLL-cRGD nanodrug delivery system for pancreatic tumor therapy. ACS Appl Mater Interfaces. 2015;7(37):20530–20537. doi:10.1021/acsami.5b06043
44. Mohammad-Hadi L, MacRobert AJ, Loizidou M, et al. Photodynamic therapy in 3D cancer models and the utilisation of nanodelivery systems. Nanoscale. 2018;10(4):1570–1581.
45. Geng S, Zhao H, Zhan G, et al. Injectable in situ forming hydrogels of thermosensitive polypyrrole nanoplatforms for precisely synergistic photothermo-chemotherapy. ACS Appl Mater Interfaces. 2020;12(7):7995–8005. doi:10.1021/acsami.9b22654
46. Wang X, Gao J, Li C, et al. In situ gelatinase-responsive and thermosensitive nanocomplex for local therapy of gastric cancer with peritoneal metastasis. Materials Today Bio. 2022;2022:100305. doi:10.1016/j.mtbio.2022.100305
47. Chen H, Sun R, Zheng J, et al. Doxorubicin-encapsulated thermosensitive liposome-functionalized photothermal composite scaffolds for synergistic photothermal therapy and chemotherapy. J Materials Chem B. 2022;10:4771–4782. doi:10.1039/D2TB00993E
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.