Back to Journals » OncoTargets and Therapy » Volume 12
Combined silencing of VEGF-A and angiopoietin-2, a more effective way to inhibit the Ishikawa endometrial cancer cell line
Authors Xu X, Yan Y , Xun Q, Shi J, Kong X, Wu J, Zhou H
Received 9 November 2018
Accepted for publication 20 January 2019
Published 14 February 2019 Volume 2019:12 Pages 1215—1223
DOI https://doi.org/10.2147/OTT.S194064
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Xiaofeng Xu,1,* Yuhua Yan,1,2,* Qingying Xun,3 Jiayu Shi,1,4 Xiangyi Kong,1 Jun Wu,1 Huaijun Zhou1
1Department of Gynecology, Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing, Jiangsu 210008, China; 2Department of Gynecology, The People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi 530021, China; 3Department of Physiology, Medical College, Southeast University, Nanjing, Jiangsu 210009, China; 4Department of Obstetrics and Gynecology, The First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, Henan 450008, China
*These authors contributed equally to this work
Background: Angiogenesis is critical for the growth and metastasis of solid tumors and is, therefore, an important therapeutic target. Despite the great research advances in tumor therapies targeting vascular endothelial growth factor (VEGF), drug resistance frequently occurs, and further strategies targeting the tumor vasculature are of primary concern.
Purpose: The present study aimed to determine whether a combination of small interfering RNAs (siRNAs) targeting VEGF-A and angiopoietin-2 (Ang-2) inhibited the biologic mechanisms of endometrial cancer more effectively compared to either one alone, in vitro and in vivo.
Methods: VEGF-A and Ang-2 were silenced by siRNA in Ishikawa endometrial cancer cells. Cell growth, apoptosis, metastasis, and tumor angiogenesis were measured in vitro and in vivo.
Results: There was no difference observed in cell apoptosis rate; however, combined silencing of VEGF-A and Ang-2 resulted in a stronger inhibition of cell proliferation and invasion (P<0.05). Similarly, a greater reduction of tumor size and angiogenesis was seen with the concurrent administration of siRNAs targeting VEGF-A and Ang-2 in nude mice (P<0.05).
Conclusion: Our data indicated that simultaneous blockade of VEGF-A and Ang-2 may serve as a novel and effective therapeutic strategy in endometrial cancer.
Keywords: drug resistance, siRNA, Ang-2, angiogenesis
Introduction
Endometrial carcinoma is one of the most frequent gynecologic malignancies in developed countries, with an incidence of ~15–20 per 100,000 cases a year.1 It is estimated that 61,380 newly diagnosed cases and 10,920 mortalities occurred from endometrial cancer in the United States in 2017.2 Although adjuvant radiotherapy and chemotherapy may reduce local recurrence and systemic metastases, the associated toxicity and morbidity are of primary concern. In recent years, there has been considerable progress in molecular targeted therapy, particularly regarding antiangiogenesis; however, drug resistance remains an issue limiting therapeutic efficiency, and, therefore, further research in this area is required.
Angiogenesis is critical for the growth and metastasis of solid tumors and is, therefore, an important therapeutic target. Vascular endothelial growth factor (VEGF)-A and angiopoietin (Ang)-2 are key molecules involved in the process of angiogenesis. Overexpression of VEGF in tumor cells enhances tumor growth and metastasis in several malignancies, including endometrial cancer. VEGF-A is an independent predictor of poor prognosis in patients with endometrial cancer. Several approaches have been developed to block VEGF-A action and have achieved good clinical efficacy, including blocking antibodies, decoy receptors, and small interfering RNA (siRNA) against VEGF-A.3–5
However, tumors are usually inherently resistant or gradually develop adaptive resistance to VEGF pathway inhibition therapies. This may be mediated through numerous different pathways, with one established mechanism involving activation of alternative angiogenic pathways that promote tumor angiogenesis in a VEGF-independent manner, including the Ang/tyrosine-protein kinase receptor (Tie) signaling pathway.5–7 Several reports also indicate that the upregulation of Ang-1 and Ang-2 is part of “angiogenic rescue” when VEGF-A-VEGFR2 signaling is blocked during tumor progression.8,9 Therefore, the present study aimed to determine whether a combination of siRNAs targeting VEGF-A and Ang-2 could effectively inhibit the biologic mechanisms of endometrial cancer in vitro and in vivo.
Materials and methods
Cell culture
The human Ishikawa endometrial cancer cell line was kindly provided by Professor LiHui Wei (Peking University People’s Hospital, China), and all cell experiments were approved by the Ethics Committee of Drum Tower Hospital Affiliated to Nanjing University Medical School. Ishikawa cells were grown in DMEM (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cultures were supplemented with 10% FBS (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) and maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Plasmid construction and cell transfection
Target siRNA and mock siRNA were synthesized and generated for cloning into the vector pRNAT-CMV3.2-Ne (Genescript, Nanjing, China). The mock siRNA was used as a control to assure that the system worked properly. For the siRNA of VEGF-A, the siRNA oligo sequence was AUGUGAAUGCAGACCAAAGAA;10 and for the siRNA of Ang-2, the siRNA oligo was GGACAAACCTGTTGAACCAAA.11 The nonsilencing control siRNA sequence used was ACATTAATTAAGCAGGCGTG. Cell transfection was performed with Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol. After 24 hours, Ishikawa cells (1 × 105/well) were plated into a six-well plate and transfected with Lipofectamine reagent (8 μL/well) containing siRNA plasmid DNA (4 μg/well).
Reverse transcription-quantitative PCR (RT-qPCR)
RT-qPCR was performed as previously described.10 The sequence for the sense primer for VEGF-A was 5′-GGCCAGCACATAGGAGAGATG-3′ and for antisense primer was 5′-AGGCCCACAGGGATGTTCTT-3′;11 and for Ang-2, the sequence for sense primer was 5′-AACATCCCAGTCCACCTGAG-3′ and for antisense primer, 5′-GGTCTTGCTTTGGTCCGTTA-3′.12,13
Western blotting
Western blotting was performed as previously described.10 Primary antibodies’ dilutions were performed as the following: VEGF primary antibody (1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and Ang-2 primary antibodies (1:5,000; Abcam, Cambridge, UK). Each assay was repeated three times.
MTT assay
Transfected cells were plated at 8,000 cells per well in 96-well plates and incubated at 37°C. Following 1, 2, 3, and 4 days of incubation, 20 μL of MTT (5 mg/mL; Sigma-Aldrich; Merck KGaA) was added, and the cells were incubated for 4 hours. Plates were then centrifuged at 1,000 rpm for 1 minute, and the supernatant was removed. A total of 150 μL dimethyl sulfoxide was added to each well, and absorbance was measured at a wavelength of 570 nm. All experiments were conducted in quadruplicate.
Apoptosis assay
Apoptosis was detected by staining for Annexin V-PE and 7 aminoactinomycin (7-AAD) using an Annexin V-PE Apoptosis Detection Kit (BD Biosciences, Franklin Lakes, NJ, USA). At 24 hours after cell transfection (1 × 106 cells/mL), cells were washed in cold PBS buffer twice and then resuspended in 100 μL binding buffer (BD Biosciences). Annexin V-PE (5 μL) and 7-AAD (5 μL) were used for staining cells (1 × 105 cells/mL) by incubating for 15 minutes in the dark. Following this, results were acquired by flow cytometry (FACSCalibur; BD Biosciences) and analyzed using BD FACSDiva Software. Each assay was repeated three times.
Wound-healing assay
For a wound-healing assay, 2 × 105 cells were seeded into six-well plates. A wound was incised 24 hours later, and detached cells were washed away with PBS and fresh serum-free media (DMEM; Thermo Fisher Scientific, Inc.) was added. Images were obtained after 24 hours to monitor the migration of cells into the wounded area with an inverted microscope. All assays were performed in triplicate.
In vitro invasion assay
For a migration assay, transfected Ishikawa cells were cultured for 24 hours, then transferred onto the top of a matrigel-coated invasion chamber (24-well insert, 8 μm pore size; EMD Millipore) in serum-free DMEM at a density of 2 × 105 cells/mL. DMEM supplemented with 10% FBS was added as the chemoattractant to the lower chamber. After 24 hours of incubation, cells on the upper surface were gently removed from the inner part of the insert with a cotton swab. The cells that had invaded the matrigel and were attached to the lower surface were fixed and stained with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA). Cells were counted from five randomly selected high-power fields at 200× magnification in each well. The experiment was repeated three times.
In vivo tumor studies
The present study was carried out in strict accordance with the recommendations in the Eighth Edition Guide for the Care and Use of Laboratory Animals (NOT-OD-12-020). Tumorigenesis experiments in nude mice were approved by the Ethics Committee of Drum Tower Hospital Affiliated to Nanjing University Medical School. Ishikawa cells (1 × 107) were subcutaneously injected into the right back skin near the upper limb of female nude mice (18±2 g; 4–5 weeks; Comparative Medicine Center of Yangzhou University). Ten days later, the injected nude mice with tumor burden were randomly divided into five groups (n=6 for each group): mock (no siRNA), negative siRNA, VEGF-A siRNA, Ang-2 siRNA, or combined siRNAs, and then treated without (mock) or with the indicated siRNA complexes by intratumoral injection. SiRNA duplexes diluted in Lipofectamine 2000 (2 μL/μg; Invitrogen; Thermo Fisher Scientific, Inc.) were injected into the tumors at a concentration of 10 μg siRNA/50 mm3 tumor volume.14 These mice were treated every 3 days, eight times, and euthanized by CO2 asphyxiation 3 days following the final treatment. The tumor size was measured with calipers prior to each treatment or following sacrifice, and the tumor volume was obtained using the following equation: V (mm3) = (length (mm) × width (mm) × width (mm))/2.
Immunohistochemistry
The tumors were paraffin embedded and fixed with 10% neutral formalin. The samples were cut into 4–5 μm thick sections. Next, the sections were deparaffinized and the antigens were retrieved by steam treatment in a citrate buffer, then quenched for 15 minutes with 3% hydrogen peroxide at room temperature. Subsequently, the expression of Von Willebrand factor (vWF) was assessed by IHC using a vWF antibody (rabbit anti human, 1:1,500; Abcam). Then, the slides were rinsed with PBS and incubated with goat anti-rabbit serum polyclonal antibody (Origene Technologies, Inc., Beijing, China) for 30 minutes at room temperature. Following rinsing with PBS for 30 seconds, the slides were incubated for 15 minutes with 0.06% diaminobenzidine and counterstained with Harris modified hematoxylin (Beyotime Institute of Biotechnology, Jiangsu, China). Various sections were incubated with PBS instead of primary antibodies, as negative controls. The three most hypervascular areas were selected under low-power field. Any single endothelial cell or cluster of endothelial cells identified by positive vWF staining was counted as a single microvessel. Microvessel density (MVD) was counted as the number of vessels per high-power field (magnification, ×200). The mean value for the three fields was recorded as the MVD for each tumor sample.
Statistical analyses
Statistical analyses were performed using SPSS software, version 18.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean ± SD. Differences between groups were analyzed by unpaired Student’s t-test. A value of P<0.05 was considered statistically significant (P<0.05, P<0.01).
Results
Silencing of VEGF-A and Ang-2 expression by siRNA in Ishikawa cells
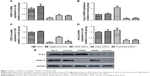
To investigate the potential application of VEGF-A and Ang-2 downregulation in endometrial carcinoma therapy, the present study used siRNA to deplete VEGF-A and Ang-2 expression in Ishikawa cells, to study the biologic effects. At 48 hours after transfection, the efficacy of downregulation of VEGF-A and Ang-2 expression was evaluated by RT-qPCR and western blotting. As presented in Figure 1A, VEGF-A mRNA levels were suppressed by up to 81.8%±4.1% in Ishikawa cells by siRNA VEGF-A alone. Ang-2 mRNA levels were upregulated 2.2±0.2-fold (Figure 1B). The treatment with combined siRNAs was equally effective compared with the treatment of siRNA VEGF-A alone, and Ang-2 mRNA levels were suppressed by 33.5%±4.4% (each P<0.01).
Further observation revealed that VEGF-A protein was eventually lowered by 87.7%±2.1% when compared with the negative siRNA, following siRNA VEGF-A transfection, and Ang-2 protein levels were upregulated 1.2±0.1-fold (each P<0.01). The treatment with combined siRNAs resulted in an 80.8%±6.3% decrease in VEGF-A protein levels, and Ang-2 protein was lowered by 68.3%±4.5% (Figure 1C–E; each P<0.01).
The siRNA results were highly consistent with the results of RT-qPCR and western blotting. It was demonstrated that the VEGF-A and Ang-2 siRNAs were able to knock down the target gene expression in Ishikawa cells with high specificity and potency, and thus may be useful in targeting VEGF-A and Ang-2 in endometrial carcinoma therapy.
Depletion of VEGF-A and Ang-2 inhibited Ishikawa cells more significantly
An MTT assay was used to detect the effect of combined siRNAs on cell proliferation. The MTT proliferation assay indicated that from the third day following plating, the cell survival rate of combined siRNAs-transfected Ishikawa cells was significantly less compared to the other groups. As presented in Figure 2A, Ishikawa cells treated with siRNA VEGF-A for 96 hours exhibited a 24.7%±3.0% decrease in cell viability when compared to the negative controls. A further suppression of cell viability of 44.6%±3.7% was observed in cells treated with combined siRNAs. These data suggested that combination therapy significantly affected cell viability. Meanwhile, the apoptosis rates and cell cycle distributions were not significantly different between the siRNA treatment and negative control (data not shown).
To evaluate the impact of siRNA therapy on cell migration, a wound-healing assay was performed. As shown in Figure 2B and C, the migration rate was significantly decreased in Ishikawa cells following combined siRNA therapy.
A Transwell assay is considered the most stringent assay in vitro for measuring malignant transformation abilities. To determine the effect of combined siRNAs on the transformation ability of Ishikawa cells, a Transwell assay was performed in vitro. The results demonstrated that migrated cell number in the combined siRNAs group was markedly lower than the numbers in the siRNA VEGF-A group and siRNA Ang-2 group (P<0.05, P<0.01; Figure 2D). Treatment of Ishikawa cells with negative siRNA did not reduce migrated cell numbers. Taken together, the results revealed that combined siRNA therapy inhibited the migration and invasion abilities of Ishikawa cells more effectively compared to singular treatments.
Simultaneous blockade of VEGF-A and Ang-2 inhibited the tumorigenicity and MVD more effectively in vivo
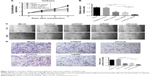
In vivo experiments were performed to evaluate the effect of VEGF-A and Ang-2 suppression in nude mice. As presented in Figure 3A and B, the tumor growth rate in the combined therapy group was observed to be decreased compared to that in the VEGF-A siRNA group. To provide evidence that the inhibition of tumor growth by combined therapy was due to its ability to downregulate VEGF-A and Ang-2 in vivo, protein expression levels of VEGF-A and Ang-2 in tumor tissues were also conducted by western blotting. As presented in Figure 3C–E, VEGF-A and Ang-2 protein expression levels were decreased compared to those following control treatment. This result suggested that simultaneous blockade of VEGF-A and Ang-2 inhibited the tumorigenicity of Ishikawa cells more effectively, compared to the singular administration, in vivo.
Synergistic inhibitory effects are achieved by combination therapy with VEGF-A siRNA and Ang-2 siRNA. MVD was estimated by counting the number of microvessels per high-power field in a section, with an antibody reactive to vWF (Figure 3F). The data indicated that the MVD of the combined therapy group was 13.3±1.5/vision (×200), whereas those of the groups siRNA VEGF-A and siRNA Ang-2 were 17.0±2.0/vision (×200) and 22.1±2.6/vision (×200), respectively. The MVD in the combined therapy group was significantly lower compared to the other groups (Figure 3G, P<0.01), whereas no statistical significance was observed between the siRNA VEGF-A and siRNA Ang-2 groups (P>0.05).
However, no obvious differences in morphology or tumor metastasis were histologically observed between vital organs from differently treated groups (data not shown).
Discussion
The importance of antiangiogenesis in tumor therapy has been widely explored and documented in previous decades. Angiogenesis in solid tumors has become a major target for cancer therapy, and various drugs including bevacizumab and sunitinib are currently used in patients. In the treatment of recurrent or persistent endometrial cancer, bevacizumab has good efficacy in Phase II clinical trials, with tumor progression-free survival significantly prolonged.15 However, the occurrence of drug resistance with angiogenesis inhibitors has directed research on the evaluation of alternative drugs, in order to overcome resistant pathways occurring in the presence of VEGF/VEGFR blockade.16,17
The specific success of VEGF blockade has prompted the use of agents that block VEGF in widely used clinical protocols. However, there are other signaling pathways that may shift vasculature to a less VEGF-dependent state.18,19 Several reports indicate that Ang-1 and Ang-2 are upregulated when VEGF-A/VEGFR2 signaling is blocked.9,20 In the present study, it was demonstrated that Ang-2 was overexpressed when VEGF-A was blocked in Ishikawa cells. Huang et al confirmed that Ang-1/Tie-2 is activated and contributes to vascular survival and tumor growth during VEGF blockade.9 Based on the analyzed data, it was hypothesized that the reduction of VEGF-A may inhibit proteolytic processing and shedding of the extracellular domain of Tie-2, and subsequently reduce the amount of Ang-2 competitively bound by soluble Tie, so that Ang-2 expression relatively increases. As such, it was hypothesized that combination therapy may overcome these limitations associated with treatment, including combination therapy of antiangiogenic drugs and combination of antiangiogenic drugs and radiotherapy or chemotherapy.18,21,22 Koh et al synthesized a dual decoy receptor for VEGF-A and angiopoietins namely double anti-angiogenic protein (DAAP) that effectively inhibited tumor angiogenesis and metastasis.23 The DAAP has been determined to be highly effective for reducing tumor angiogenesis, metastasis, and vascular leakage with greater effects in animal tumor models. The present study investigated whether the direct combination of siRNA VEGF-A and siRNA Ang-2 would inhibit the target gene expression in Ishikawa cells and enhance the antitumor effect on endometrial cancer cells. Based on the results that the single Ang-2 siRNA inhibited not only the Ang-2 mRNA level but also the VEGF-A mRNA level independently, it was hypothesized that the combined siRNAs would have greater and more efficient effects in reducing VEGF-A expression. However, we observed equal effectiveness between the combined siRNAs and siRNA VEGF-A alone. Besides angiogenesis, VEGF-A and ANG-2 also played important roles in cell biologic behavior. Bhattacharya et al24 revealed that RNAi-mediated depletion of VEGF decreased cell survival accompanied by reduction of phospho-AKT and phospho-ERK1/2 levels, which indicated the VEGF/AKT signaling pathway in cell survival. VEGF and ANG-2 could also promote endothelial cell migration and invasion through Notch signaling pathways.25 Our results that each of VEGF-A siRNA and ANG-2 siRNA significantly suppressed the proliferation, migration, and invasion abilities of Ishikawa cells in vitro also indicated the important roles they played in non-angiogenesis environment. Interestingly, a more effective inhibition of cell growth and invasion by the combination of VEGF-A and Ang-2 siRNAs were observed, which was in agreement with published results. Meanwhile, siRNA VEGF-A and siRNA Ang-2 did not induce cell cycle arrest or apoptosis, and thus further studies are required.
The in vitro results suggested that the combination treatment of endometrial cancer cells with VEGF-A and Ang-2 siRNAs may represent a novel approach for the treatment of endometrial cancer. We further aimed to extend the in vitro findings on the combined effects of siRNA VEGF-A and siRNA Ang-2 in inhibiting endometrial cancer cell growth, by conducting experiments in vivo. For this aim, an implantation tumor model was established. The animal experiments also clearly demonstrated that simultaneous blockade of VEGF-A and Ang-2 inhibited tumor growth and reduced intratumoral MVD more effectively.
Limitations
Tumor metastasis was not observed in vivo, which may have been associated with the limitations of the subcutaneous xenograft model and examination of H&E staining, or the short observation period. Ishikawa cells are estrogen receptor-positive and well-differentiated endometrial carcinoma cells, and thus many studies use the highly malignant cell line HEC to observe tumor metastasis.26–29 Furthermore, Takahashi et al overexpressed VEGF-C in a nude mice model to confirm lymph node metastasis.30 These methods should be considered for further experiments.
Conclusion
Targeting vasculature has been validated as an effective therapy for cancers, including endometrial cancer, with an increased number of clinical trials being conducted. However, reports of drug resistance are also increasing. The combined antiangiogenic therapy may be an effective treatment strategy for endometrial cancer exhibiting drug resistance, recurrence, or progression. The technology of siRNA delivery is rapidly developing, and nanotechnology is also being applied.31,32 The results of the present study reflect the possible beneficial effects of siRNA therapies. However, the adverse and long-term clinical effects require further clinical trials for verification.
Acknowledgments
The authors acknowledge Professors Lijun Ding, Guilun Yan, and Zhenyu Diao, Reproductive Medicine Center, Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing, China, for their critical experimental suggestions. This work was supported by the Natural Science Foundation of Jiangsu Province (BK20151096) and the Key Projects of National Health and Family Planning Commission of Nanjing City (ZKX17015) to HZ. Xiaofeng Xu and Yuhua Yan are co-first authors.
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. | ||
Waldner MJ, Neurath MF. Targeting the VEGF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):5–13. | ||
Eng L, Azad AK, Habbous S, et al. Vascular endothelial growth factor pathway polymorphisms as prognostic and pharmacogenetic factors in cancer: a systematic review and meta-analysis. Clin Cancer Res. 2012;18(17):4526–4537. | ||
Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis. 2017;20(2):185–204. | ||
van Beijnum JR, Nowak-Sliwinska P, Huijbers EJ, Thijssen VL, Griffioen AW. The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol Rev. 2015;67(2):441–461. | ||
Gotink KJ, Broxterman HJ, Labots M, et al. Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clin Cancer Res. 2011;17(23):7337–7346. | ||
Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. | ||
Huang J, Bae JO, Tsai JP, et al. Angiopoietin-1/Tie-2 activation contributes to vascular survival and tumor growth during VEGF blockade. Int J Oncol. 1992;34:79–87. | ||
Zhou H, Xu X, Xun Q. microRNA-30c negatively regulates endometrial cancer cells by targeting metastasis-associated gene-1. Oncol Rep. 2011;27:807–812. | ||
Narantuya D, Nagai A, Sheikh AM, et al. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS One. 2010;5(7):e11746. | ||
Loges S, Clausen H, Reichelt U, et al. Determination of microvessel density by quantitative real-time PCR in esophageal cancer: correlation with histologic methods, angiogenic growth factor expression, and lymph node metastasis. Clin Cancer Res. 2007;13(1):76–80. | ||
Daud II, Scott ME. Validation of reference genes in cervical cell samples from human papillomavirus-infected and -uninfected women for quantitative reverse transcription-PCR assays. Clin Vaccine Immunol. 2008;15(9):1369–1373. | ||
Singh A, Boldin-Adamsky S, Thimmulappa RK, et al. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68(19):7975–7984. | ||
Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29(16):2259–2265. | ||
Papa A, Zaccarelli E, Caruso D, Vici P, Benedetti Panici P, Tomao F. Targeting angiogenesis in endometrial cancer – new agents for tailored treatments. Expert Opin Investig Drugs. 2016;25(1):31–49. | ||
Wei XW, Zhang ZR, Wei YQ, Wei X, Zhang Z. Anti-angiogenic drugs currently in phase II clinical trials for gynecological cancer treatment. Expert Opin Investig Drugs. 2013;22(9):1181–1192. | ||
Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. | ||
Lupo G, Caporarello N, Olivieri M, et al. Anti-angiogenic therapy in cancer: downsides and new Pivots for precision medicine. Front Pharmacol. 2016;7(e10747):1–9. | ||
Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8(4):299–309. | ||
Griffioen AW, Weiss A, Berndsen RH, Abdul UK, Te Winkel MT, Nowak-Sliwinska P. The emerging quest for the optimal angiostatic combination therapy. Biochem Soc Trans. 2014;42(6):1608–1615. | ||
Kubota Y. Tumor angiogenesis and anti-angiogenic therapy. Keio J Med. 2012;61(2):47–56. | ||
Koh YJ, Kim HZ, Hwang SI, et al. Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cancer Cell. 2010;18(2):171–184. | ||
Bhattacharya R, Ye XC, Wang R, et al. Intracrine VEGF signaling mediates the activity of prosurvival pathways in human colorectal cancer cells. Cancer Res. 2016;76(10):3014–3024. | ||
Gao W, Sweeney C, Walsh C, et al. Notch signalling pathways mediate synovial angiogenesis in response to vascular endothelial growth factor and angiopoietin 2. Ann Rheum Dis. 2013;72(6):1080–1088. | ||
Doll A, Gonzalez M, Abal M, et al. An orthotopic endometrial cancer mouse model demonstrates a role for Runx1 in distant metastasis. Int J Cancer. 2009;125(2):257–263. | ||
Xiong S, Cheng JC, Klausen C, Zhao J, Leung PC. TGF-β1 stimulates migration of type II endometrial cancer cells by down-regulating PTEN via activation of Smad and ERK1/2 signaling pathways. Oncotarget. 2016;7(38):61262–61272. | ||
Chang CC, Ling XH, Hsu HF, et al. Siegesbeckia orientalis extract inhibits TGFβ1-induced migration and invasion of endometrial cancer cells. Molecules. 2016;21(8):1021. | ||
Takahashi K, Saga Y, Mizukami H, et al. Cetuximab inhibits growth, peritoneal Dissemination, and lymph node and lung metastasis of endometrial cancer, and prolongs host survival. Int J Oncol. 2009;35(4):725–729. | ||
Takahashi K, Saga Y, Mizukami H, et al. Development of a mouse model for lymph node metastasis with endometrial cancer. Cancer Sci. 2011;102(12):2272–2277. | ||
Shen H, Sun T, Ferrari M. Nanovector delivery of siRNA for cancer therapy. Cancer Gene Ther. 2012;19(6):367–373. | ||
Kala S, Mak AS, Liu X, et al. Combination of dendrimer-nanovector-mediated small interfering RNA delivery to target Akt with the clinical anticancer drug paclitaxel for effective and potent anticancer activity in treating ovarian cancer. J Med Chem. 2014;57(6):2634–2642. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.