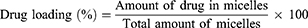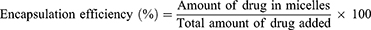Back to Journals » International Journal of Nanomedicine » Volume 17
Combined Shikonin-Loaded MPEG-PCL Micelles Inhibits Effective Transition of Endothelial-to-Mesenchymal Cells
Authors Li G, Shang C, Li Q, Chen L, Yue Z, Ren L, Yang J, Zhang J, Wang W
Received 18 May 2022
Accepted for publication 9 September 2022
Published 24 September 2022 Volume 2022:17 Pages 4497—4508
DOI https://doi.org/10.2147/IJN.S374895
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Guanglin Li,1,2 Chenxu Shang,3 Qingqing Li,4 Lifang Chen,1,2 Zejun Yue,1,2 Lingxuan Ren,3 Jianjun Yang,3 Jiye Zhang,4 Weirong Wang1,2
1Department of Medical Laboratory Animal Science, School of Basic Medical Sciences, Xi’an Jiaotong University Health Science Center, Xi’an, People’s Republic of China; 2Institute of Cardiovascular Science, Translational Medicine Institute, Xi’an Jiaotong University Health Science Center, Xi’an, People’s Republic of China; 3Department of Pharmacology, School of Basic Medical Sciences, Xi’an Jiaotong University Health Science Center, Xi’an, People’s Republic of China; 4School of Pharmacy, Xi’an Jiaotong University Health Science Center, Xi’an, People’s Republic of China
Correspondence: Weirong Wang, Xi’an Jiaotong University Health Science Center, No. 76 Yanta West Road, Xi’an, 710061, People’s Republic of China, Tel/Fax +86 29 82655362, Email [email protected]
Introduction: Shikonin is well known for its anti-inflammatory activity in cardiovascular diseases. However, the application of shikonin is limited by its low water solubility and poor bioavailability. Methoxy poly (ethylene glycol)-b-poly (ϵ-caprolactone) (MPEG-PCL) is considered a promising delivery system for hydrophobic drugs. Therefore, in this study, we prepared shikonin-loaded MPEG-PCL micelles and investigated their effect on endothelial-to-mesenchymal transition (EndMT) induced by inflammatory cytokines.
Methods: Shikonin was encapsulated in MPEG-PCL micelles using an anti-solvent method and the physiochemical characteristics of the micelles (particle size, zeta potential, morphology, critical micelle concentration (CMC), drug loading and encapsulation efficiency) were investigated. Cellular uptake of micelles in human umbilical vein endothelial cells (HUVECs) was evaluated using fluorescence microscopy. In vitro EndMT inhibition was explored in HUVECs by quantitative real-time PCR analysis.
Results: The average particle size of shikonin-loaded MPEG-PCL micelles was 54.57± 0.13 nm and 60 nm determined by dynamic light scattering and transmission electron microscopy, respectively. The zeta potential was − 6.23± 0.02 mV. The CMC of the micelles was 6.31× 10− 7mol/L. The drug loading and encapsulation efficiency were 0.88± 0.08% and 43.08± 3.77%, respectively. The MPEG-PCL micelles significantly improved the cellular uptake of cargo with low water solubility. Real-time PCR analysis showed that co-treatment with TNF-α and IL-1β successfully induced EndMT in HUVECs, whereas this process was significantly inhibited by shikonin and shikonin-loaded MPEG-PCL micelles, with greater inhibition mediated by the shikonin-loaded MPEG-PCL micelles.
Conclusion: Shikonin-loaded MPEG-PCL micelles significantly improved the EndMT-inhibiting effect of the free shikonin. MPEG-PCL is suitable for use more generally as a lipophilic drug carrier.
Keywords: shikonin, shikonin-loaded MPEG-PCL micelles, inflammation, EndMT
Introduction
Shikonin, a lipophilic naphthoquinone, is the major active component extracted from the root of Lithospermum erythrorhizon, also known as Zicao, a traditional Chinese herbal medicine.1 Shikonin possesses multiple pharmacological activities, including anti-inflammation,2,3 anti-tumor4,5 and anti-oxidation.6,7 Its prominent anti-inflammatory potential has gained considerable attention for the treatment of cardiovascular diseases. Shikonin was reported to diminish the release of inflammatory cytokines and macrophage infiltration in the heart of a lipopolysaccharide-induced cardiac dysfunction mouse model.8 Shikonin also attenuated the inflammatory response and fibrosis in an isoproterenol-induced myocardial damage mouse model.9 Moreover, shikonin decreased the levels of CD4+ T cells and proinflammatory macrophages in the inflammation-induced plaques and ameliorated the atherosclerosis induced by hyperhomocysteinemia.10
The water solubility of shikonin is relatively low (0.10 mg/mL), resulting in poor bioavailability and limited potential for clinical application.11 Several approaches have been adopted to improve its water solubility and bioavailability. In general, shikonin is formulated as an oil-based preparation, such as creams and ointments. When shikonin was encapsulated into cyclodextrin, the solubility, photostability and in vitro permeability of shikonin were successfully improved.11,12 Furthermore, the water solubility of shikonin was dramatically increased (up to 181-fold) bound with lactoglobulin.13,14 Methoxy poly (ethylene glycol)-b-poly (ε-caprolactone) (MPEG-PCL), a biocompatible and biodegradable amphipathic di-block copolymer, is well known to enhance solubility and facilitate controlled release of the encapsulated cargo.15 In aqueous environments, MPEG-PCL spontaneously forms a core-shell structure with the hydrophilic PEG as the outer shell and the lipophilic PCL as the inner core. During this process of micelle formation, a lipophilic drug can be encapsulated within the lipophilic PCL core, resulting in a marked improvement in its water solubility.16 When the lipophilic macrolide antibiotic azithromycin was loaded into MPEG-PCL micelles, its water solubility was greatly enhanced (>13-fold), and its antibacterial activity was comparable to that of the raw drug.17 Therefore, in this study, we explored the potential of MPEG-PCL di-block copolymers to improve the water solubility and bioavailability of shikonin.
Inflammation is one of the most important risk factors for endothelial dysfunction, which is considered to be an initiation factor in cardiovascular diseases.18–20 As the key link between inflammation and endothelial dysfunction, endothelial-to-mesenchymal transition (EndMT) is a complex biological process in which endothelial cells gradually transform into mesenchymal cells.21,22 During EndMT, endothelial cells lose their specific markers, such as CD31, Von Willebrand factor (vWF) and vascular endothelial-cadherin (VE-cadherin), accompanied by the emergence of mesenchymal markers, such as α-smooth muscle actin (α-SMA), fibroblast-specific protein 1 (FSP-1) and smooth muscle 22 α (SM22α). These changes are essential characteristics of EndMT. Inflammatory cytokines induce EndMT and lead to pathological changes including tissue fibrosis and atherosclerosis.23,24 Interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) have been reported to promote EndMT of cells,25 including human umbilical vein endothelial cells (HUVECs).26 However, the effect of shikonin and shikonin-loaded MPEG-PCL micelles on EndMT induced by inflammatory cytokines IL-1β and TNF-α remains elusive.
Therefore, in the present study, we prepared and characterized shikonin-loaded MPEG-PCL micelles and evaluated their ability to inhibit EndMT of HUVECs induced by TNF-α and IL-1β compared with raw shikonin.
Methods
Preparation of Shikonin-loaded Methoxy Poly (Ethylene Glycol)-b-Poly (ε-caprolactone) Micelles
Shikonin-loaded MPEG-PCL micelles were prepared using an anti-solvent method as previously described.27 Briefly, 5.0 mg shikonin (National Institutes for Food and Drug Control, China) was dissolved in 1 mL acetone, which was further diluted to obtain solution of different concentrations (0.625, 1.25, 2.50 and 5.00 mg/mL) before 12.0 mg MPEG-PCL (Daigang Biomaterial, China) was dissolved. The shikonin-MPEG-PCL mixtures were then added dropwise into 1.2 mL distilled water under magnetic stirring. After 4 h, the acetone was removed by rotary evaporation. The micelle solution was centrifuged at 15,000 rpm for 10 min and then filtered (0.22 μm membrane) to remove the unencapsulated drug. Coumarin 6-loaded MPEG-PCL micelles were prepared as a flourescent probe using the same method.
Characterization of Shikonin-Loaded Methoxy Poly (Ethylene Glycol)-b-Poly (ε-caprolactone) Micelles
Particle Size and Zeta Potential
After 10-fold dilution with distilled water, the average particle size, size distribution and zeta potential of the micelles were measured with a Zeta-sizer Nano (Malvern Instruments, UK).
Morphology
The morphology of the micelles was evaluated by transmission electron microscopy (TEM). A drop of micelle solution was placed on a copper grid for 10 min before the excess solution was removed with filter paper. Next, a drop of 2% phosphotungstic acid was placed on the copper grid for 30s and the excess solution was removed with filter paper. After air-drying at room temperature, the morphology of the micelles was evaluated by TEM (Hitachi H-7650, Japan).
Critical Micelle Concentration
The critical micelle concentration (CMC) of MPEG-PCL was determined as previously described.28,29 Briefly, 1.5 µL pyrene solution (0.2 mmol/L) in ethanol was added to 1 mL polymer solutions at different concentrations (1×10−9, 5×10−9, 1×10−8, 5×10−8, 1×10−7, 5×10−7, 1×10−6, 5×10−6, 1×10−5, 5×10−5, 1×10−4 and 1×10−3 mol/L). The fluorescence intensity at 372 nm (I1) and 383 nm (I3) was measured using a Flex Station 3 Multi-Mode Microplate Reader (Molecular Devices, USA). The CMC of MPEG-PCL was determined by plotting I3/I1 values versus log (polymer concentration), with the CMC represented by the lower inflection point of the plot.
Drug Loading and Encapsulation Efficiency
The amount of shikonin encapsulated in the MPEG-PCL micelles was examined by a HPLC under the conditions listed in Table 1. A 50-µL sample of shikonin-loaded MPEG-PCL micelles solution was mixed with 950 µL mobile phase (75% methanol and 15% H2O) and ultrasonicated for 30s. The sample was filtered (0.22 μm membrane) before injection into the HPLC.
 |
Table 1 HPLC Condition for Shikonin and Coumarin 6 Quantification |
The HPLC method was validated based on the guidelines provided by the US Food and Drug Administration (FDA). The specificity of the method was verified based on the chromatogram of shikonin-loaded micelles. In the linearity test, the calibration curves were generated by plotting the ratio of the peak area of freshly prepared shikonin samples versus the concentration. The coefficient of determination (r2) was then calculated. r2 > 0.99 typically is set as the threshold for acceptable linearity. The efficiency of the extraction procedures was determined as follows: blank MPEG-PCL was spiked with different concentrations of shikonin, which was then extracted and evaluated by HPLC.
The drug loading and encapsulation efficiency were calculated with the following equations:30–32
The precision of the assay was evaluated by analyzing the quality control samples at nominal concentrations (4, 6 and 8 μg/mL) with at least five replicates for each concentration within the same day. The threshold for acceptable assay precision was set at ±15% bias and 15% relative standard deviation (RSD).
In vitro Studies of Shikonin-Loaded Methoxy Poly (Ethylene Glycol)-b-Poly (ε-caprolactone) Micelles
HUVECs were obtained from the American Type Culture Collection (Manassas, Virginia). Briefly, HUVECs were cultured in tissue culture flasks in DMEM/F-12 supplemented with 10% FBS and 100 U/mL penicillin-streptomycin in a humidified atmosphere (Hyclone, USA). When the HUVECs reached confluence, cells were seeded in 12-well plates. After 24 h, the culture medium was replaced with serum-free medium and the cells were incubated for a further 12 h prior to experimental use.
Shikonin was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, USA) as a stock solution and diluted to the desired concentrations for use in experiments. The final concentration of DMSO was less than 0.05% in the culture medium and DMSO served as a control.
Cellular Uptake Study
The in vitro cellular uptake of MPEG-PCL micelles loaded with the fluorescent probe coumarin 6 (Tokyo Chemical Industry, Japan) was assessed according to a previously described method with some modifications.32,33 Briefly, a sample (100 μL) of the micelles was mixed with 400 μL distilled water and 500 μL acetonitrile and vortexed for 30s before the amount of coumarin 6 loaded in the MPEG-PCL micelles was determined by HPLC under the conditions listed in Table 1.
HUVECs were seeded in a 96-well plate at a density of 30,000 cells/well. After incubation for 24 h, the original medium was replaced with coumarin 6-loaded MPEG-PCL micelles dispersed in DMEM/F-12 solution, free coumarin 6 dissolved in DMSO-DMEM/F-12 solution at the same concentration as that of the micelles (DMSO content <0.1%) or saturated free coumarin 6 in DMEM/F-12. After incubation for 2, 8 and 24 h, the medium was discarded and the cells were washed three times with PBS to remove the un-internalized coumarin 6. Next, the cells were fixed with 4% paraformaldehyde before fluorescence images were taken and the fluorescence intensity in each group was evaluated under an inverted fluorescence microscope Axio Vert.A1 (ZEISS, Germany).
In vitro EndMT Inhibition Study by Quantitative Real-Time PCR
To determine the effects of shikonin and shikonin-loaded MPEG-PCL micelles on EndMT, HUVECs in 12-well plates were pretreated with shikonin or shikonin-loaded MPEG-PCL micelles at different concentrations (0.25, 0.5 and 1.0 μmol/L) for 1 h, followed by co-treatment with TNF-α (10 ng/mL) and IL-1β (10 ng/mL) (Pepro Tech, USA) for an additional 24 h. Total RNA was then extracted from cells using RNAiso Plus reagent (Takara, Japan). cDNAs were synthesized from total RNA using PrimeScript RT Master Mix according to the manufacturer’s instructions (Takara, Japan). The primers used in the real-time PCR analysis were displayed in Table 2. cDNAs were amplified using the SYBR Premix Ex Taq II kit (Takara, Japan). Data were normalized to the levels of GAPDH mRNA and the relative gene expression was analyzed quantitatively using the comparative Ct method (2−ΔΔCT).
 |
Table 2 Primer Sequences Used for Quantitative Real-Time PCR Analysis |
Statistical Analysis
Results were presented as mean ± standard deviations (SD). Differences among groups were evaluated by two-tailed Student’s t-tests and ANOVA using GraphPad Prism software. P < 0.05 was considered to be statistically significant.
Results
Particle Size and Zeta Potential of Shikonin-Loaded Methoxy Poly (Ethylene Glycol)-b-Poly (ε-caprolactone) Micelles
Dynamic light scattering (DLS) analysis is commonly used to analyze the size and surface charge of nanoparticles. As shown in Figure 1, the shikonin-loaded MPEG-PCL micelles prepared using the anti-solvent method showed a relatively homogenous distribution (polydispersity index (PDI) = 0.41±0.003) in terms of particle size (54.57±0.13 nm) and zeta potential (−6.23±0.02 mV). The average particle size and zeta potential of different formulations are listed in Figure S1 and Table S1. TEM analysis showed that the shikonin-loaded micelles exhibited a homogeneous spherical morphology with an average particle size of 60 nm (Figure 2), which was similar to that determined by DLS. As shown in Figure 3, the CMC of MPEG-PCL was approximately 6.31×10−7 mol/L.
 |
Figure 1 Characterization of shikonin-loaded MPEG-PCL micelles. (A) Particle size distribution of shikonin-loaded MPEG-PCL micelles. (B) Zeta potential of shikonin-loaded MPEG-PCL micelles. |
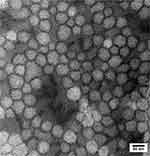 |
Figure 2 TEM image of shikonin-loaded MPEG-PCL micelles. |
 |
Figure 3 Critical micelle concentration of MPEG-PCL micelles. |
Drug Loading and Encapsulation Efficiency
After HPLC quantification, the drug loading and encapsulation efficiencies were calculated as described in the Methods section. First, the HPLC method for shikonin quantification was validated by examining the specificity, linearity and precision according to the guidelines provided by the US FDA.34 No interfering peak was observed at the retention time of shikonin (Figure 4A), suggesting that the method has good specificity. The shikonin calibration curve (Figure 4B) showed good linearity (r2 = 0.9985) in the range of 2 μg/mL to 10 μg/mL. The extraction recovery of shikonin from the micelles was 98.22–99.20% (Table 3). The precision of the assay (low-high shikonin concentrations) ranged from 0.90–1.43% RSD (Table 4), which was acceptable according to the US FDA regulatory requirement.34 The drug loading and encapsulation efficiency values for the micelles are shown in the Table 5. The formulation No.1 showed an acceptable drug loading of 0.88±0.08% and the highest encapsulation efficiency of 43.08±3.77% compared with other formulation, so it was used in the following research.
 |
Table 3 Extraction Recovery of Shikonin in Blank MPEG-PCL Micelles |
 |
Table 4 Precision Test Results of Shikonin by the HPLC Method |
 |
Table 5 Drug Loading and Encapsulation Efficiency of Shikonin-Loaded MPEG-PCL Micelles |
 |
Figure 4 Quantitative HPLC analysis of shikonin-loaded MPEG-PCL micelles. (A) HPLC spectrum of shikonin-loaded MPEG-PCL micelle demulsification solution. (B) Standard curve of shikonin. |
Cellular Uptake of Methoxy Poly (Ethylene Glycol)-b-Poly (ε-caprolactone) Micelles
To investigate the cellular uptake of MPEG-PCL micelles, we used the fluorescent molecule, coumarin 6, with low water solubility as a probe. Both coumarin 6 and shikonin are poorly soluble in water, and the average particle size and the zeta potential of coumarin 6-loaded MPEG-PCL micelles (58.64±0.27 nm and −3.31±0.22 mV, respectively) were similar to the corresponding characteristics of shikonin-loaded MPEG-PCL micelles (54.57±0.13 nm and −6.23±0.02 mV, respectively) (Figure S2). Therefore, in this uptake study, coumarin 6-loaded MPEG-PCL micelles were employed as a surrogate for evaluation of the cellular uptake of shikonin-loaded MPEG-PCL micelles.
As shown in Figure 5, the cellular uptake of coumarin 6 from a saturated aqueous solution was quite limited at all time-points and was not detectable under the microscope, possibly due to its extremely low solubility in water. After encapsulation in MPEG-PCL micelles, coumarin 6 uptake was significantly improved, which was consistent with a previous study.29 However, the uptake of coumarin 6-loaded MPEG-PCL micelles was still lower than that of free coumarin 6 at the same concentration solubilized with DMSO.
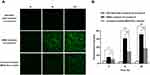
Effect of Shikonin-Loaded Methoxy Poly (Ethylene Glycol)-b-Poly (ε-Caprolactone) Micelles on Endothelial-to-Mesenchymal Transition
Our results showed that inflammatory cytokines TNF-α and IL-1β co-treatment induced EndMT in HUVECs (P < 0.01). Shikonin and shikonin-loaded MPEG-PCL micelles (0.25 μmol/L) had no significant effect on EndMT of HUVECs (Figure 6A, B, D and E). However, at 1.0 μmol/L, both shikonin and shikonin-loaded MPEG-PCL micelles upregulated expression of the endothelial cell markers CD31, vWF and VE-cadherin compared with the TNF-α and IL-1β co-treatment control group (Figure 6A–C), while expression of the mesenchymal cell markers α-SMA, FSP-1 and SM22α were downregulated (Figure 6D–F). Furthermore, shikonin-loaded MPEG-PCL micelles (1.0 μmol/L) showed significantly stronger EndMT inhibition than shikonin (1.0 μmol/L) (Figure 6A, C and F).
 |
Figure 7 Shikonin-loaded MPEG-PCL micelles exhibit a significant inhibitory effect on EndMT in HUVECs. |
Discussion
EndMT induced by inflammatory cytokines was reported to be a crucial process in vascular inflammation in cardiovascular diseases. Shikonin is well known for its anti-inflammatory activity, which may be a drug candidate for EndMT. However, the application of shikonin is limited by its low water solubility and poor bioavailability. Amphipathic di-block copolymer of MPEG-PCL exhibited good biocompatibility in various cytotoxicity studies35–38 and in vivo studies.39 It was considered as a promising drug delivery system for hydrophobic drugs.
In this study, shikonin was successfully encapsulated in MPEG-PCL micelles using an anti-solvent method and the micelles were characterized in terms of particle size, zeta potential, morphology, CMC, drug loading and encapsulation efficiency. The particle size of the shikonin-loaded MPEG-PCL micelles was in good accordance with that of the drug-loaded MPEG-PCL micelles reported previously.30 Typically, micelle sizes is proportional to the block length. Drug-loaded nanoparticles with a relatively small size (20–200 nm) can accumulate in pathological regions such as sites of inflammation and tumors via enhanced permeability and retention effect.16,40 Therefore, based on their size, our nanoparticles were expected to accumulate at sites of inflammation.41 The PDI demonstrated a narrow size distribution of the micelles generated on our study, which is preferable to a broad size distribution, which increases the uncertainty of the pharmacokinetic parameters of drug-loaded micelles.41 Furthermore, the slightly negative zeta potential of the shikonin-loaded MPEG-PCL micelles was similar with that reported previously.42,43 When the polymer concentration is lower than the CMC, the polymer distributes at the air-aqueous interface in the form of unimers. When the concentration is higher than its CMC, micelles are formed with molecules are incorporated into the hydrophobic core. The CMC of our MPEG-PCL micelles correlates well previous reports of CMCs of MPEG-PCL.29,44 In this study, the CMC was far below the concentration of MPEG-PCL used to generate shikonin-loaded micelles. Such low CMC values guaranteed self-assembly of these di-block copolymers into nano-scaled carriers with favorable thermodynamical stability.44 After confirming that the specificity, linearity, extraction recovery and precision of the HPLC method used to calculate the drug loading and encapsulation efficiencies of the shikonin-loaded MPEG-PCL micelles met the US FDA requirements, we showed that the drug loading of micelles increased along with the amount of shikonin added. However, the encapsulation efficiency decreased as the amount of shikonin added. Formulation No. 1 showed an acceptable drug loading and the highest encapsulation efficiency compared with other formulations and was therefore used in our subsequent research.
The cellular uptake of a saturated aqueous solution of coumarin 6 was undetectable by fluorescence microscopy at all time-points, possibly due to its extremely low water solubility. After encapsulation in MPEG-PCL micelles, the uptake of coumarin 6 was significantly improved, which was consistent with a previous study.29 However, the uptake of coumarin 6-loaded MPEG-PCL micelles was still lower than that of free coumarin 6 at the same concentration solubilized with DMSO. This limited uptake efficiency of MPEG-PCL micelles may stem from its energy-dependent, caveolae- and/or clathrin-mediated uptake, a mechanism which could be saturated.29 In contrast, free coumarin 6 solution was taken up by passive diffusion, which is an energy and receptor independent mechanism. Therefore, it can be hypothesized that the improved uptake of poorly water-soluble molecules by MPEG-PCL micelles is due mainly to the improved solubility through encapsulation in MPEG-PCL micelles.
EndMT is induced by inflammatory cytokines and is regarded as the key link between vascular inflammation and endothelial dysfunction, which leads to cardiovascular diseases. We showed that shikonin and shikonin-loaded MPEG-PCL micelles significantly inhibited EndMT. Therefore, we speculate that the inhibitory effect of shikonin on EndMT might be related with its anti-inflammatory activity. It has been reported that the anti-inflammatory effect of shikonin is mediated by inhibiting the activation of the nucleotide-binding oligomerization domain-like receptor inflammasome and NF-κB signaling pathway.45 More importantly, our results suggest that shikonin-loaded MPEG-PCL micelles mediate more efficient inhibition of EndMT than shikonin at the same concentration.
Conclusion
In summary, shikonin-loaded MPEG-PCL micelles were successfully prepared and shown to exert stronger inhibitory effect on EndMT than free shikonin (Figure 7). These findings provide the experimental basis for optimization of the dose of shikonin for micelle preparation and evaluation of the biological activity of shikonin-loaded MPEG-PCL micelles for clinical use. Furthermore, our study demonstrates that MPEG-PCL is suitable for use more generally as a lipophilic drug carrier.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81873520) and the Natural Science Foundation of Shaanxi Province (2019JM-394).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Andújar I, Ríos JL, Giner RM, Recio MC. Pharmacological properties of shikonin - a review of literature since 2002. Planta Med. 2013;79(18):1685–1697. doi:10.1055/s-0033-1350934
2. Tanaka S, Tajima M, Tsukada M, Tabata M. A comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkannin. J Nat Prod. 1986;49(3):466–469. doi:10.1021/np50045a014
3. Lu L, Qin A, Huang H, et al. Shikonin extracted from medicinal Chinese herbs exerts anti-inflammatory effect via proteasome inhibition. Eur J Pharmacol. 2011;658(2–3):242–247. doi:10.1016/j.ejphar.2011.02.043
4. Calonghi N, Pagnotta E, Parolin C, et al. A new EGFR inhibitor induces apoptosis in colon cancer cells. Biochem Biophys Res Commun. 2007;354(2):409–413. doi:10.1016/j.bbrc.2006.12.214
5. Xu J, Koizumi K, Liu M, et al. Shikonin induces an anti‑tumor effect on murine mammary cancer via p38‑dependent apoptosis. Oncol Rep. 2019;41(3):2020–2026. doi:10.3892/or.2019.6966
6. Han H, Weng X, Bi K. Antioxidants from a Chinese medicinal herb-Lithospermum erythrorhizon. Food Chem. 2008;106(1):2–10. doi:10.1016/j.foodchem.2007.01.031
7. Assimopoulu AN, Boskou D, Papageorgiou VP. Antioxidant activities of alkannin, shikonin and Alkanna tinctoria root extracts in oil substrates. Food Chem. 2004;87(3):433–438. doi:10.1016/j.foodchem.2003.12.017
8. Guo T, Jiang ZB, Tong ZY, Zhou Y, Chai XP, Xiao XZ. Shikonin ameliorates LPS-induced cardiac dysfunction by SIRT1-dependent inhibition of NLRP3 inflammasome. Front Physiol. 2020;11:570441. doi:10.3389/fphys.2020.570441
9. Yang J, Wang Z, Chen DL. Shikonin ameliorates isoproterenol (ISO)-induced myocardial damage through suppressing fibrosis, inflammation, apoptosis and ER stress. Biomed Pharmacother. 2017;93:1343–1357. doi:10.1016/j.biopha.2017.06.086
10. Lü SL, Dang GH, Deng JC, et al. Shikonin attenuates hyperhomocysteinemia-induced CD4(+) T cell inflammatory activation and atherosclerosis in ApoE-/-mice by metabolic suppression. Acta Pharmacol Sin. 2020;41(1):47–55. doi:10.1038/s41401-019-0308-7
11. Assimopoulou AN, Papageorgiou VP. Encapsulation of isohexenylnaphthazarins in cyclodextrins. Biomed Chromatogr. 2004;18(4):240–247. doi:10.1002/bmc.310
12. Chen CY, Chen FA, Wu AB, Hsu HC, Kang JJ, Cheng HW. Effect of hydroxypropyl-β-cyclodextrin on the solubility, photostability and in-vitro permeability of alkannin/shikonin enantiomers. Int J Pharm. 1996;141(1):171–178. doi:10.1016/0378-5173(96)04634-0
13. Albreht A, Vovk I, Simonovska B. Addition of β-lactoglobulin produces water-soluble shikonin. J Agric Food Chem. 2012;60(43):10834–10843. doi:10.1021/jf303153d
14. Yorulmaz Avsar S, Kyropoulou M, Di Leone S, Schoenenberger CA, Meier WP, Palivan CG. Biomolecules turn self-assembling amphiphilic block co-polymer platforms into biomimetic interfaces. Front Chem. 2019;6:645. doi:10.3389/fchem.2018.00645
15. Danafar H. MPEG–PCL copolymeric nanoparticles in drug delivery systems. Cogent Med. 2016;3(1):1142411. doi:10.1080/2331205X.2016.1142411
16. Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73(2–3):137–172. doi:10.1016/s0168-3659(01)00299-1
17. Wei W, Li S, Xu H, et al. MPEG-PCL copolymeric micelles for encapsulation of azithromycin. AAPS PharmSciTech. 2018;19(5):2041–2047. doi:10.1208/s12249-018-1009-0
18. Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15(2):117–129. doi:10.1038/nri3800
19. Gregersen I, Holm S, Dahl TB, Halvorsen B, Aukrust P. A focus on inflammation as a major risk factor for atherosclerotic cardiovascular diseases. Expert Rev Cardiovasc Ther. 2016;14(3):391–403. doi:10.1586/14779072.2016.1128828
20. Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115. doi:10.1016/0140-6736(92)93147-f
21. Cho JG, Lee A, Chang W, Lee MS, Kim J. Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front Immunol. 2018;9:294. doi:10.3389/fimmu.2018.00294
22. Bischoff J. Endothelial-to-mesenchymal transition. Circ Res. 2019;124(8):1163–1165. doi:10.1161/CIRCRESAHA.119.314813
23. Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MNM. The crucial roles of inflammatory mediators in inflammation: a review. Vet World. 2018;11(5):627–635. doi:10.14202/vetworld.2018.627-635
24. Chen PY, Qin L, Baeyens N, et al. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. 2015;125(12):4514–4528. doi:10.1172/JCI82719
25. Pérez L, Muñoz-Durango N, Riedel CA, et al. Endothelial-to-mesenchymal transition: cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor Rev. 2017;33:41–54. doi:10.1016/j.cytogfr.2016.09.002
26. Chen L, Shang C, Wang B, et al. HDAC3 inhibitor suppresses endothelial-to-mesenchymal transition via modulating inflammatory response in atherosclerosis. Biochem Pharmacol. 2021;192:114716. doi:10.1016/j.bcp.2021.114716
27. Sant VP, Smith D, Leroux JC. Novel pH-sensitive supramolecular assemblies for oral delivery of poorly water soluble drugs: preparation and characterization. J Control Release. 2004;97(2):301–312. doi:10.1016/j.jconrel.2004.03.026
28. Cai L, Gochin M, Liu K. A facile surfactant critical micelle concentration determination. Chem Commun (Camb). 2011;47(19):5527–5529. doi:10.1039/c1cc10605h
29. Li Q, Lai KL, Chan PS, et al. Micellar delivery of dasatinib for the inhibition of pathologic cellular processes of the retinal pigment epithelium. Colloids Surf B Biointerfaces. 2016;140:278–286. doi:10.1016/j.colsurfb.2015.12.053
30. Li Q, Yang X, Zhang P, et al. Dasatinib loaded nanostructured lipid carriers for effective treatment of corneal neovascularization. Biomater Sci. 2021;9(7):2571–2583. doi:10.1039/d0bm01599g
31. Wang S, Gou J, Wang Y, et al. Synergistic antitumor efficacy mediated by liposomal co-delivery of polymeric micelles of vinorelbine and cisplatin in non-small cell lung cancer. Int J Nanomedicine. 2021;16:2357–2372. doi:10.2147/IJN.S290263
32. Dimchevska S, Geskovski N, Koliqi R, et al. Efficacy assessment of self-assembled PLGA-PEG-PLGA nanoparticles: correlation of nano-bio interface interactions, biodistribution, internalization and gene expression studies. Int J Pharm. 2017;533(2):389–401. doi:10.1016/j.ijpharm.2017.05.054
33. Zhao Z, Wang Y, Han J, et al. Self-assembled micelles of amphiphilic poly(L-phenylalanine)-b-poly(L-serine) polypeptides for tumor-targeted delivery. Int J Nanomedicine. 2014;9:5849–5862. doi:10.2147/IJN.S73111
34. Brodie RR, Hill HM. Validation issues arising from the new FDA guidance for industry on bioanalytical method validation. Chromatographia. 2002;55(1):S91–S94. doi:10.1007/BF02493361
35. Li X, Wang Y, Xu F, et al. Artemisinin loaded mPEG-PCL nanoparticle based photosensitive gelatin methacrylate hydrogels for the treatment of gentamicin induced hearing loss. Int J Nanomedicine. 2020;15:4591–4606. doi:10.2147/IJN.S245188
36. Zhai Y, Zhou X, Zhang Z, et al. Design, synthesis, and characterization of Schiff base bond-linked pH-responsive doxorubicin prodrug based on functionalized mPEG-PCL for targeted cancer therapy. Polymers. 2018;10(10):1127. doi:10.3390/polym10101127
37. Kong D, Shi Y, Gao Y, Fu M, Kong S, Lin G. Preparation of BMP-2 loaded MPEG-PCL microspheres and evaluation of their bone repair properties. Biomed Pharmacother. 2020;130:110516. doi:10.1016/j.biopha.2020.110516
38. Bikiaris ND, Ainali NM, Christodoulou E, et al. Dissolution enhancement and controlled release of paclitaxel drug via a hybrid nanocarrier based on mPEG-PCL amphiphilic copolymer and Fe-BTC porous metal-organic framework. Nanomaterials. 2020;10(12):2490. doi:10.3390/nano10122490
39. Repp L, Rasoulianboroujeni M, Lee HJ, Kwon GS. Acyl and oligo(lactic acid) prodrugs for PEG-b-PLA and PEG-b-PCL nano-assemblies for injection. J Control Release. 2021;330:1004–1015. doi:10.1016/j.jconrel.2020.11.008
40. Deshantri AK, Varela Moreira A, Ecker V, et al. Nanomedicines for the treatment of hematological malignancies. J Control Release. 2018;287:194–215. doi:10.1016/j.jconrel.2018.08.034
41. Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf B Biointerfaces. 1999;16(1–4):3–27. doi:10.1016/S0927-7765(99)00058-2
42. Zamani M, Shirinzadeh A, Aghajanzadeh M, Andalib S, Danafar H. In vivo study of mPEG-PCL as a nanocarriers for anti-inflammatory drug delivery of simvastatin. Pharm Dev Technol. 2019;24(6):663–670. doi:10.1080/10837450.2018.1556689
43. Liu X, Li J, Huang L, et al. Preparation and evaluation of MPEG-PCL polymeric nanoparticles against gastric cancer. J Wuhan Univ Technol-Mater Sci Ed. 2020;35(6):1162–1168. doi:10.1007/s11595-020-2368-4
44. Forrest ML, Won CY, Malick AW, Kwon GS. In vitro release of the mTOR inhibitor rapamycin from poly (ethylene glycol)-b-poly (epsilon-caprolactone) micelles. J Control Release. 2006;110(2):370–377. doi:10.1016/j.jconrel.2005.10.008
45. Han H, Sun W, Feng L, et al. Differential relieving effects of shikonin and its derivatives on inflammation and mucosal barrier damage caused by ulcerative colitis. PeerJ. 2021;9:e10675. doi:10.7717/peerj.10675
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.