Back to Journals » Psychology Research and Behavior Management » Volume 14
Combined Cognitive-Behavioral Therapy and Placebo Treatment for Patients with Depression: A Follow-Up Assessment
Authors Schienle A , Jurinec N
Received 1 December 2020
Accepted for publication 19 January 2021
Published 22 February 2021 Volume 2021:14 Pages 233—238
DOI https://doi.org/10.2147/PRBM.S294940
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Igor Elman
Anne Schienle,1 Nina Jurinec1,2
1 Instiute of Psychology, University of Graz, Graz, Austria; 2Community Health Center Gornja Radgona, Gornja Radgona, Slovenia
Correspondence: Anne Schienle Instiute of Psychology, University of Graz, Universitätsplatz 2, Graz, 8010, Austria
Email [email protected]
Introduction: A previous study revealed that patients with depression who received a combination of cognitive-behavioral therapy (CBT) and placebo treatment (CBT+placebo) showed greater symptom reduction than a CBT group without a placebo. Moreover, the CBT+placebo group practiced relaxation training more frequently. We conducted a 3-month follow-up assessment to investigate the temporal stability of the placebo effects.
Methods: Eighty-two outpatients with a diagnosis of major depressive disorder who had participated in a 4-week CBT course (CBT: n = 40; CBT with daily placebo treatment: n = 42) returned to a 3-month follow-up assessment. The participants of the CBT+placebo group had been debriefed directly after the course.
Results: Compared to the CBT group, the CBT+placebo group had lower scores on the Beck Depression Inventory-II (BDI-II) at follow-up and more participants were below the clinical cut-off score of the BDI-II. Additionally, the CBT+placebo group continued to practice relaxation more frequently.
Discussion: This study demonstrates that placebo effects are not short-lived and continue to be present after the debriefing.
Keywords: placebo effects, temporal stability, depression, cognitive-behavioral therapy
Plain Language Summary
A previous study found that patients with depression who received psychotherapy with added placebo treatment displayed greater improvement than a psychotherapy group without placebo. All patients were assessed again three months after the end of therapy. Upon follow-up, the placebo group still had a lower level of depression and used relaxation training more often for stress reduction. This study shows that placebo effects are not short-lived and continue to be present even when patients are informed that they had received a placebo.
Introduction
Major depressive disorder is a highly prevalent mental disorder that is associated with increased morbidity and mortality.1 Antidepressant medication and cognitive-behavioral therapy (CBT) are empirically-supported treatment options for this disorder.2–4 Furthermore, depression symptoms can be alleviated via placebo treatment.5–8 Estimated mean placebo response rates in antidepressant clinical trials varied between 30% and 45%.6,9 In his critical review, Kirsch argued that the benefits of antidepressants in the treatment of depression are largely due to the placebo effect and that the difference in improvement between drug and placebo is not clinically meaningful.7
Therapy-related improvement is typically assessed directly after the completion of the treatment period. Additionally, follow-up examinations are important to evaluate the durability of effects and possible relapse. The National Institute of Mental Health (NIMH) Treatment of Depression Collaborative Research Program reported relapse rates of 50% for antidepressant medication and only 33% for CBT and placebo treatment.10 More recent studies found lower relapse rates when the patients had been treated with antidepressants compared to placebos (for a review/meta-analysis see;11,12 however, patients with antidepressant treatment withdrew from the trials more often than those allocated to placebo.11
The present study focused on a follow-up evaluation of placebo effects. A previous study demonstrated that the combination of CBT and placebo treatment in patients with depression was superior (in terms of reduction of depression symptoms and increased practice of relaxation training) compared to CBT without placebo.13 The patients had been randomly assigned to either a standardized four-week CBT program (CBT group) or the same program with additional daily placebo treatment (‘natural medicine to mobilize the bodies’ natural healing powers’; CBT+placebo group). Directly after the completion of the therapy, both groups reported significantly reduced depression symptoms (as measured with the Beck Depression Inventory-II). The reduction was more pronounced in the CBT+placebo group compared to the CBT group. Moreover, the combination therapy was associated with improved homework compliance as indicated by a higher frequency of practicing a relaxation technique at home.
The participants of the mentioned study were invited to a three-month follow-up assessment to compare depression symptoms and the use of the relaxation technique between the two groups (CBT vs CBT+placebo).13
Method
Participants
A total of 82 participants with a diagnosis of major depressive disorder according to ICD-10 criteria with mild to moderate episodes participated in the follow-up study (for participants’ characteristics see Table 1).14 The diagnosis was made by a psychiatrist. Exclusion criteria for the study were a) severe psychiatric comorbidity (eg, bipolar disorder, psychotic disorder, substance dependence), b) acute suicide risk, c) severe somatic illness (eg, cancer), and d) current psychotherapy or counseling. Current antidepressant medication was accepted if the dose had been stable for at least 12 weeks before enrollment and remained unchanged during the study.
 |
Table 1 Group Characteristics |
The psychiatrist transferred the patients to an outpatient treatment program for depression in a community health center. The program has been implemented by the National Institute of Public Health (NIJZ) of Slovenia.
Procedure
The outpatient program (the brief version of the “Coping with Depression” course according to Lewinsohn) was conducted by an experienced therapist and consisted of four sessions (90 minutes, weekly, over four weeks; group setting with six patients).15 The course focused on psychoeducation about depression, cognitive skills, pleasant activities, and relaxation training to influence mood. The teaching was combined with daily homework assignments. The participants were asked to conduct a relaxation exercise at home every day (practicing period = 21 days).
Of the 84 participants who received treatment, 42 had been randomly assigned (with a random number table) to the CBT group, and 42 to the CBT+placebo group (“Coping with Depression” course with additional daily placebo treatment). (Another 42 participants had been assigned to the waiting-list group that was not assessed again at follow-up). Two participants of the CBT group did not show up to the follow-up assessment (see Figure 1 with a CONSORT flow diagram).
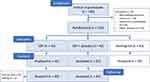 |
Figure 1 CONSORT flow diagram. |
The placebo group received 30 mL of sunflower oil provided in a blue glass bottle. The participants were instructed to take three drops (0.15 mL) orally per day. The oil was labeled “golden root oil” (Rhodiola Rosea), “natural medicine to help the patients focus on their inner strengths and to mobilize their bodies” natural healing powers’. The patients were instructed to take the oil 10 minutes before their daily relaxation exercise. At the end of the course, all participants were informed about the study design and the use of the placebo.
The study has been carried out following the Declaration of Helsinki and has been approved by the ethics committee of the University of Graz (Austria). All participants provided written informed consent.
Self-Report Measures
a) The Beck Depression Inventory-II (BDI-II) consists of 21 items rated on 4-point scales from 0 to 3, with higher scores indicating more severe depression symptoms (0–13: minimal depression, 14–19: mild depression, 20–28: moderate depression, 29–63: severe depression).16 Cronbach’s α of the BDI-II in the present study was 0.84. The participants completed this questionnaire before and directly after the CBT course, and at 3-month follow-up.
b) Frequency of relaxation exercises: The participants were asked at 3-month follow-up: “How often do you still practice relaxation?” (5-point Likert scale; 1 = rarely; 5 = almost every day).
Statistical Analysis
An analysis of variance (ANOVA) was computed to test the effects of Time (before, after course, follow-up) and Group (CBT, CBT+placebo) on reported depression symptoms (BDI-II scores). Partial eta squared is reported as a measure of effect size. Significant effects were followed up with t-tests with Holm-Bonferroni correction. To compare the number of participants in the two groups (CBT, CBT+placebo) who were below the clinical cut-off scores for the BDI-II (< 14) after the treatment, we computed a χ2 test. Moreover, χ2 tests were used to compare the groups concerning gender distribution, family status, education level, depression diagnosis (first episode vs recurrent depression), reported diagnoses of mental disorders in the family, and antidepressant medication. The frequency of relaxation exercises at follow-up was compared between the two groups via a t-test.
Results
Symptoms of Depression
The ANOVA revealed a significant main effect for Time (F(2,160) = 300.11, p < 0.001, partial eta2 = 0.79). The Group effect was not significant (p = 0.27). The depression scores of the total patient group were lower directly after the therapy (M = 15.16, SD = 8.22; t(80) = 15.07, p <0.001) and at follow-up (M = 13.01, SD = 7.54; t(80) = 17.56, p < 0.001) compared to the pre-therapy level (M = 23.27, SD = 8.25).
The interaction Group x Time (F(2,160) = 20.17, p < 0.001, partial eta2 = 0.20) was significant. Before the therapy, both groups did not differ in their BDI-II scores (MCBT = 22.58, SD = 7.69; MCBT+placebo = 23.93, SD = 8.78; t(80) = 0.74, p = 0.460). At the time of the follow-up assessment, the CBT+placebo group reported lower BDI-II scores (M = 11.38, SD = 7.16) than the CBT group (M = 14.73, SD = 7.64; t(80) = 2.05, p = 0.044). Thirty-one participants (74%) of the CBT+placebo group were below the clinical cut-off score for the BDI-II compared to 21 participants (53%) of the CBT group (χ2(1) = 4.01, p = 0.045). The assessment of remission was confirmed by the therapist.
Changes in BDI-II scores over time differed between the two groups (see Figure 2). The CBT+placebo group showed a greater reduction from the first to the second assessment (pre-therapy vs post-therapy; MCBT+placebo = −10.52, SD= 5.04; MCBT = −5.58; SD = 3.13; t(80) = 5.31, p < 0.001) and from the first to the third assessment (pre-therapy vs follow-up; MCBT+placebo = −12.55, SD = 5.64; MCBT = −7.85; SD = 3.62, t(80) = 4.47, p < 0.001). The changes in BDI-II scores from post-therapy to follow-up did not differ between the groups (p = 0.66).
Frequency of Relaxation Practice
The CBT+placebo group (M = 3.50, SD = 0.80) reported to practice more often than the CBT group (M = 2.68, SD = 0.86; t(80) = 4.49, p < 0.001).
Discussion
This study investigated the temporal stability of placebo effects in depression. Depression symptoms and frequency of relaxation practice were compared between patients who received CBT with or without placebo treatment. Compared to the standard therapy, CBT+placebo was associated with a greater reduction in BDI-II scores from pre-therapy to follow-up, and more participants of the CBT+placebo group were below the clinical cut-off score of the BDI-II at three-month follow-up (74% vs 53%). The latter finding reflects the clinical significance of the observed changes in depression symptoms. Additionally, the participants of the CBT+placebo group reported having continued with their relaxation exercises after therapy on a more regular basis than the CBT group.
The positive effects of the combination therapy occurred after the debriefing procedure. Directly after the end of the course, the participants had been informed that the “golden root oil” was a placebo. Nevertheless, the positive placebo effect continued to be present at follow-up.
The current findings suggest that it can be helpful to prescribe placebos during psychotherapeutic treatment.17 Placebos are safe and effective, with no side effects. The only critical aspect of prescribing placebos involves that they are administered deceptively as active treatment. Since honesty and transparency are two key components of the ethical framework for psychotherapy, open-label placebos should be considered for a subsequent investigation.18,19 Nonetheless, it needs to be pointed out that the procedure of using deceptive placebo treatment cannot be considered completely negative because the debriefing procedure was experienced as very positive by the patients. During the debriefing, the treated patients were informed that they accomplished all given tasks in the course by themselves. They learned that they were responsible for the achieved improvement and not the “medicine”. In this way, placebo treatment with subsequent debriefing supports the central aim of CBT as a self-management approach.20 The placebo helps the patients to recognize their self-management potential. Future research now needs to directly compare the long-term effects of deceptive and open-label placebos that are combined with CBT. Particularly, responses to the debriefing procedure need to be analyzed in more detail.
We also need to mention the following limitations of the present study. First, we conducted only one follow-up assessment. A study by Iovieno et al showed that an increased number of follow-up assessments predicted a greater active treatment–placebo separation in trials on major depressive disorder.21 Therefore, longer follow-up intervals with more frequent testing are recommended. Another approach to study long-term outcomes of depression treatments has been suggested by Jarrett et al.22,23 This approach is very suitable to disentangle the effects of placebo interventions, psychotherapy and antidepressants on relapse rates in depression. The authors tested the effects of different types of continuation therapy. After the acute treatment, the patients were either assigned to continued cognitive therapy (CT), antidepressant medication (fluoxetine), or placebo-pill treatment. The CT and fluoxetine groups were significantly less likely to relapse than the placebo group across eight months.
Second, the finding on the increased practicing frequency of relaxation training in the CBT+placebo group was based on self-report. Additional monitoring of the practicing should be realized in a future investigation. For example, a study on placebo effects on the compliance to practice relaxation training in healthy individuals used an app-assisted approach.24 The participants opened a smartphone application to listen to the relaxation instructions and to evaluate the relaxation exercise. This helps to gather reliable data on the frequency and effectiveness of practicing.
Third, psychophysiological measures could be used in a future study. The recording of neuroendocrine and neurophysiological data could help to elucidate the mechanisms behind the observed short-term and long-term placebo effect.25
In conclusion, this study demonstrated a very promising effect of CBT+placebo treatment in patients with depression at a 3-month follow-up. The positive outcome included reduced symptoms of depression and greater engagement in stress-reducing behavior (relaxation exercises) of patients that received the combination therapy.
Disclosure
The authors report no conflicts of interest in this work. The publication of this article has been supported by the University of Graz. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.
2. Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26:17–31. doi:10.1016/j.cpr.2005.07.003
3. Cuijpers P, Hollon SD, van Straten A, Bockting C, Berking M, Andersson G. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3:e002542. doi:10.1136/bmjopen-2012-002542
4. Hollon SD, Cohen ZD, Singla DR, Andrews PW. Recent developments in the treatment of depression. Behav Ther. 2019;50:257–269. doi:10.1016/j.beth.2019.01.002
5. Mayberg HS, Silva JA, Brannan SK. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi:10.1176/appi.ajp.159.5.728
6. Sonawalla SB, Rosenbaum JF. Placebo response in depression. Dialogues Clin Neurosci. 2002;4:105–113.
7. Kirsch I. Placebo effect in the treatment of depression and anxiety. Front Psychiatry. 2019;10:407. doi:10.3389/fpsyt.2019.00407
8. Peciña M, Bohnert ASB, Sikora M, et al. Association between placebo-activated neural systems and antidepressant responses: neurochemistry of placebo effects in major depression. JAMA Psychiatr. 2015;72:1087–1094. doi:10.1001/jamapsychiatry.2015.1335
9. Stolk P, Ten Berg MJ, Hemels ME, Einarson TR. Meta-analysis of placebo rates in major depressive disorder trials. Ann Pharmacother. 2003;37:1891–1899. doi:10.1345/aph.1D172
10. Shea MT, Elkin I, Imber SD, Sotsky SM, Watkins JT, Collins JF. Course of depressive symptoms over follow-up: findings from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Arch Gen Psychiatry. 1992;49:782–787. doi:10.1001/archpsyc.1992.01820100026006
11. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653–661. doi:10.1016/S0140-6736(03)12599-8
12. Sim K, Lau WK, Sim J, Sum MY, Baldessarini RJ. Prevention of relapse and recurrence in adults with major depressive disorder: systematic review and meta-analyses of controlled trials. Int J Neuropsychopharmacol. 2015;19:1–13. doi:10.1093/ijnp/pyv076
13. Jurinec N, Schienle A. Utilizing placebos to leverage effects of cognitive-behavioral therapy in patients with depression. J Affect Disord. 2020;277:779–784. doi:10.1016/j.jad.2020.08.087
14. World Health Organization. The ICD-10 classification of mental and behavioural disorders. Clinical descriptions and diagnostic guidelines; 2004. Geneva: WHO. Available from: https://apps.who.int/iris/handle/10665/37958.
15. Lewinsohn PM, Antonuccio DO, Breckenridge JS, Teri L. The Coping with Depression Course. Eugene: Castalia; 1984.
16. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
17. Enck P, Zipfel S. Placebo effects in psychotherapy: a framework. Front Psychiatry. 2019;10:1–12. doi:10.3389/fpsyt.2019.00456
18. Kelley JM, Kaptchuk TJ, Cusin C, Lipkin S, Fava M. Open-label placebo for major depressive disorder: a pilot randomized controlled trial. Psychother Psychosom. 2012;81:312–314. doi:10.1159/000337053
19. Charlesworth. JEG, Petkovic G, Kelley JM, et al. Effects of placebos without deception compared with no treatment: a systematic review and meta-analysis. Evid Based Med. 2017;10:97–107. doi:10.1111/jebm.12251
20. Kanfer FH, Schefft BK. Self-management therapy in clinical practice. In: Jacobson JS, editor. Psychotherapist in Clinical Practice: Cognitive and Behavioral Perspectives. New York: Guilford Press; 1987:10–77.
21. Iovieno N, Tedeschini E, Levkovitz Y, Ameral VE, Papakostas GI. Does the frequency of follow-up assessments affect clinical trial outcomes? A meta-analysis and meta-regression of placebo-controlled randomized trials. Int J Neuropsychopharm. 2012;15:289–296. doi:10.1017/S1461145711000666
22. Jarrett RB, Thase ME. Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: design of a double-blinded, fluoxetine- and pill placebo-controlled, randomized trial with 2-year follow-up. Contemp Clin Trials. 2010;31:355–377. doi:10.1016/j.cct.2010.04.004
23. Jarrett RB, Minhajuddin A, Gershenfeld H, Friedman ES, Thase ME. Preventing depressive relapse and recurrence in higher-risk cognitive therapy responders: a randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo. JAMA Psychiatr. 2013;70:11. doi:10.1001/jamapsychiatry.2013.1969
24. Höfler C, Schienle A. Placebo effects on the quantity and quality of relaxation training. J Health Psychol. 2020;1–8. doi:10.1177/1359105320954238
25. Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16:403–418. doi:10.1038/nrn3976
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

