Back to Journals » Lung Cancer: Targets and Therapy » Volume 10
Combination pembrolizumab plus chemotherapy: a new standard of care for patients with advanced non-small-cell lung cancer
Authors Weinberg F , Gadgeel S
Received 8 January 2019
Accepted for publication 9 April 2019
Published 4 June 2019 Volume 2019:10 Pages 47—56
DOI https://doi.org/10.2147/LCTT.S176391
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sai-Hong Ignatius Ou
Frank Weinberg, Shirish Gadgeel
Division of Hematology and Oncology, Department of Medicine, University of Michigan, Ann Arbor, MI, USA
Abstract: Until recently, the treatment of patients with advanced non-small-cell lung cancer (NSCLC) whose tumors did not have a targetable genetic alteration was cytotoxic chemotherapy alone. This treatment provided only modest survival benefit. The introduction of immune checkpoint inhibitors targeting programmed cell death 1 protein (PD-1) signaling pathway in the treatment of patients with NSCLC has had significant effect on patient survival. Atezolizumab, nivolumab and pembrolizumab have been shown to be superior to chemotherapy in patients with recurrent NSCLC. Recently, pembrolizumab has been combined with chemotherapy in the front-line setting and has demonstrated an improvement in overall survival in NSCLC patients as compared to chemotherapy alone. In this review we will focus on the clinical trials that led to approval of combination pembrolizumab and chemotherapy as first-line treatment for patients with advanced NSCLC as well as discuss other combinations of immunotherapy and chemotherapy that have also been evaluated.
Keywords: NSCLC, immunotherapy, chemotherapy, clinical trials
Introduction
It has become increasingly more evident that the host immune system is integral to tumor survival as the ability to avoid host immune destruction as well as promote inflammation are now recognized as “hallmarks” of cancer.1 In the past decade, much research has been undertaken to understand host immunity and tumor interaction and has led to multiple treatments designed to enhance host immunity against tumor cells.2–4 These treatments were largely based on immune checkpoints, which exist to decrease the immune response to protect the host against damaging inflammation and autoimmunity.4–6 These same immune checkpoints are used by cancer cells in order to evade host immune response. Therefore, agents were formulated against these immune checkpoints in order to relieve the inhibition placed on the host immune system thus allowing for immune-mediated destruction of the tumor.
In advanced non-small-cell lung cancer (NSCLC) there are currently three FDA-approved checkpoint inhibitors based on randomized trials that demonstrated survival advantage with these agents in recurrent NSCLC (Figure 1). Nivolumab and pembrolizumab are monoclonal antibodies targeting the programmed death receptor 1 (PD-1) receptor, atezolizumab, a monoclonal antibody, targets programmed death ligand 1 (PD-L1). Furthermore, certain patients with advanced NSCLC treated with checkpoint inhibitors have experienced durable, long-term responses adding to the excitement of these drugs. These results prompted evaluation of these agents in the front-line management of advanced NSCLC. In this review, we will examine the clinical trial data that evaluated the combination of chemotherapy with immune checkpoint inhibitors for the front-line treatment of advanced NSCLC patients.
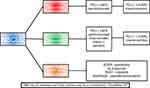 | Figure 1 Front-line therapy for advanced NSCLC.Abbreviation: NSCLC, non-small-cell lung cancer. |
Immunotherapy
Pembrolizumab
Pembrolizumab is an immunoglobulin G (IgG)4 monoclonal antagonist antibody to PD-1 that is approved for firstline treatment of patients with advanced EGFR/anaplastic lymphoma kinase (ALK) wild-type NSCLC whose tumors have ≥50% PD-L1 expression based on the 22C3 pharmDx test. Pembrolizumab was first approved in 2015 for use as second-line treatment for patients with lung cancer. This was based on the KEYNOTE 001 and KEYNOTE 010 trials. KEYNOTE 001 was a Phase I trial assessing efficacy of pembrolizumab in paitents with advanced NSCLC. Objective response rate (ORR) was 19.4% (95%CI: 16–23) with median overall survival (OS) of 12 months (95%CI: 9.3–14.7). Interestingly, patients with at least 50% PD-L1 tumor expression had ORR of 45.2% (95%CI: 33.5–57.3) and median OS was not reached.7 The KEYNOTE 010 trial was a Phase II/III trial that compared pembrolizumab to docetaxel in patients with advanced NSCLC who previously received treatment. Compared to docetaxel, improved median OS was observed with pembrolizumab at 2 mg/kg and 10 mg/kg—10.4 months and 12.7 months vs 8.5 months for docetaxel treated group (HR: 0.71; 95%CI: 0.58–0.88 and 0.61; 95%CI: 0.49–0.75, respectively). However, progression-free survival (PFS) was no different in any of the treatment arms. It should be noted that in patients with at least 50% tumor PD-L1 expression (442 patients) response rates were ~30% with pembrolizumab treatment vs 8% in docetaxel-treated patients (P<0.0001 and P<0.0001, respectively). These patients also had improved OS and PFS—pembrolizumab 2 mg/kg and 10 mg/kg, median OS was 14.9 months and 17.3 months vs 8.2 months for docetaxel-treated group (HR: 0.54; 95%CI: 0.38–0.77 and 0.50, 95%CI: 0.36–0.70, respectively).8
Given the benefit of pembrolizumab in advanced NSCLC with tumors expressing at least 50% PD-L1, trials were designed to determine the benefit of pembrolizumab as first-line therapy. The KEYNOTE-024 trial, was a Phase III study comparing pembrolizumab monotherapy (200 mg intravenous (IV) every 3 weeks) to standard platinum-doublet chemotherapy in 305 patients with advanced, untreated, EGFR/ALK wild-type NSCLC with PD-L1 tumor expression of at least 50%.9 Of the 1,653 screened patients with tumor tissue available, 30% were found to have tumors with at least 50% PD-L1 expression, of which only 305 patients were enrolled. At median follow-up (11.2 months) the primary outcome, PFS, was increased compared to platinum-doublet chemotherapy (10.3 vs 6 months; HR: 0.50; 95%CI: 0.37–0.68). OS was also prolonged with pembrolizumab compared to platinum-doublet chemotherapy (HR: 0.60; 95%CI: 0.41–0.89). ORR (based on RECIST) was 45% with pembrolizumab compared to 28% with chemotherapy with median duration of response 12.1 vs 5.7 months. Updated results showed that the median survival with pembrolizumab was 30 months vs 14.2 months (HR: 0.63; 95%CI: 0.47–0.86; 12 month OS rate 70% vs 55% (43.7% of patients in chemotherapy group crossed over to receive pembrolizumab after disease progression).10
To understand if benefits with front-line pembrolizumab extended to advanced stage NSCLC patients with tumor PD-L1 tumor expression of at least 1%, the KEYNOTE-042 study was initiated. This Phase III trial, comparing single agent pembrolizumab with standard, histology-appropriate, platinum-doublet chemotherapy enrolled 1,274 patients.11 Patients were stratified by tumor levels (>50% vs 1–49%) and OS was the primary endpoint. At median follow-up of 12.8 months, median OS with pembrolizumab was 16.7 months and with chemotherapy was 12.1 months (HR: 0.81, P=0.0018). In patients with at least 50% PD-L1 expression (599 patients) was 20 months vs 12 months in patients treated with chemotherapy (HR: 0.69; 95%CI: 0.56–0.85). In exploratory analysis, OS of patients with PD-L1 expression between 1 and 49% was 13.4 months vs 12.1 months (HR: 0.92; 95%CI: 0.77–1.11). This suggests that the survival benefit with pembrolizumab in patients with advanced stage NSCLC is most profound for those patients with tumor PD-L1 expression of at least 50%. Taken together, these studies show a significant survival benefit with the use of pembrolizumab in patients with advanced NSCLC whose tumors express at least 50% PD-L1. However, in patients with tumor PD-L1 expression ≤50% there does not appear to be a survival advantage with pembrolizumab. Understanding, how to improve response and outcomes to immunotherapy in those patients with advanced NSCLC with less than 50% PD-L1 expression is extremely important.
Nivolumab
Nivolumab is another IgG4 monoclonal antibody antagonist to PD-1. Similar to pembrolizumab, nivolumab was evaluated in recurrent NSCLC patients in two separate trials. CheckMate 017 and CheckMate 057 compared nivolumab to docetaxel in patients with recurrent squamous cell lung cancer and nonsquamous NSCLC, respectively. Nivolumab was also evaluated as first-line therapy. CheckMate 026 was a Phase III randomized trial of 541 patients with advanced NSCLC who were PD-L1 positive (at least 1% of tumor cells expressing PD-L1) and had not received previous treatment. Patients were randomized to nivolumab (3 mg/kg IV every 2 weeks) or standard first-line, histology-based, platinum doublet chemotherapy. The co-primary endpoints, PFS and OS were not improved with nivolumab (HR for disease progression and death in patients with >5% PD-L1 tumor expression: 1.15; 95%CI: 0.91–1.45; HR for death: 1.02; 95%CI: 0.80–1.30), this included patients with PD-L1 expression of >50% (HR: 1.07; 95%CI: 0.77–1.49).12 Further exploratory analysis was performed to determine the effect of tumor mutational burden (TMB) on outcomes. Among patients with high TMB who received nivolumab, the response rate was 47% compared to 28% in patients treated with chemotherapy. PFS was also increased, 9.7 months vs 5.8 months (HR: 0.62; 95%CI: 0.38–1).12 OS was similar between the groups. The exact reasons for lack of survival benefit with nivolumab in CheckMate 026, even in patients with high tumor PD-L1 expression are unclear. Currently nivolumab is not approved for use as front-line therapy for the management of advanced NSCLC patients.
Atezolizumab
Atezolizumab is an IgG1 antagonist antibody to PD-L1 engineered to avoid antibody-dependent, cell-mediated cytotoxicity of activated T cells that may express PD-L1. Atezolizumab is FDA approved for treatment of patients with metastatic NSCLC whose disease has progressed following platinum-containing chemotherapy. In the Phase III OAK trial, 1,225 patients with advanced NSCLC, PD-L1 unselected, treated with one or more platinum-based combination therapies were randomized to either single agent atezolizumab (1,200 mg IV every 3 weeks) or docetaxel. The results of this study were comparable to those observed with pembrolizumab and nivolumab.13,14
First-line atezolizumab monotherapy was recently studied in patients with locally advanced or metastatic NSCLC, PD-L1-unselected (B-F1RST trial). In this trial, 152 patients received atezolizumab 1,200 mg every 3 weeks until disease progression or loss of clinical benefit. The authors were interested in assessing whether blood-based TMB can be used as a biomarker to predict benefit from atezolizumab. High TMB was defined as ≥16 (mutations per megabase) while low TMB was defined as as <16 (mutations per megabase). ORR in patients with high TMB vs low TMB was 28.6% compared to 4.4%. PFS in TMB high vs TMB low patients was 4.6 months compared to 3.7 months (HR: 0.66; 90%CI: 0.42–1.02).15 Based on these interim results there is an ongoing randomized Phase III study, the B-FAST trial (NCT03178552) comparing atezolizumab to platinum-based chemotherapy in patients with high TMB based on a blood based assay. In addition, the BIRCH trial was a Phase II trial designed to examine the use of atezolizumab 1,200 mg every 3 weeks as monotherapy in patients with advanced NSCLC who had received zero to up to two lines of treatment and had PD-L1 expression ≥5% based on the SP142 immunohistochemistry assay. The study found that patients achieved ORR around 20% and OS was 23.5 months (95%CI: 18.1 months to not estimable (NE)) with front-line treatment, 15.5 (95%CI: 12.3–19.3 months) and 13.2 (95%CI: 10.3–17.5 months) months in patients treated with second-line or third-line treatment, respectively.16
Durvalumab
Durvalumab, a human monoclonal PD-L1 antibody, is also being studied in patients with advanced NSCLC. Previously, durvalumab was FDA approved for use in patients with unresectable, stage III NSCLC who have not progressed following concurrent radiation and platinum-based chemotherapy based on the PACIFIC trial.17 In the advanced stage setting, the MYSTIC trial is a Phase III study that enrolled 1,118 patients with metastatic NSCLC and randomly assigned them to durvalumab alone, durvalumab plus tremelimumab (at CTLA-4 inhibitor) or chemotherapy. Primary endpoints were OS for durvalumab vs chemotherapy and OS and PFS for durvalumab plus tremelimumab vs chemotherapy in patients with 25% or greater PD-L1 expression on tumor cells. Results demonstrated that of the 488 patients with ≥25% PD-L1 tumor cell expression, durvalumab alone (16.3 months vs 13.9 months; HR: 0.75; 97.5%CI: 0.564–1.019; P=0.036) or in combination with tremelimumab (11.9 vs 12.9 months; HR: 1.05; 99.5%CI: 0.722–1.534; P=0.705) did not improve OS or PFS as compared to chemotherapy.18
Chemotherapy plus pembrolizumab
Until recently, chemotherapy or immunotherapy alone had been standard first-line treatment for patients with advanced NSCLC without a targetable genetic alteration. Some studies suggest that many of the antitumor effects of chemotherapy are mediated through the immune system. Chemotherapy reduces T-regulatory cell activity and increases the ratio of cytotoxic T-lymphocytes to T-regulatory cells.19,20 Another study demonstrated that chemotherapy inhibits myeloid-derived suppressor cells.21 Cytotoxic chemotherapy also enhances presentation of tumor antigens as well as the potential for dendritic cell tumor antigen presentation following destruction of tumor cells.22,23 Further, PD-L1 expression on tumor cells can be upregulated by chemotherapy.24,25 Therefore, chemotherapy can act as a sensitizing agent to induce increased antitumor activity of PD-L1 and PD-1 antagonist antibodies. It is not clear if these immune based effects of chemotherapy drugs are clinically relevant or whether they significantly differ among different chemotherapeutic agents but formed the basis for evaluation of drugs targeting PD(L)-1 in combination with chemotherapy.
One of the first randomized studies to evaluate the combination of chemotherapy with a checkpoint inhibitor was Keynote 21G. In the Phase I portion of Keynote 21G toxicity and clinical activity of combination chemotherapy and immunotherapy were explored.26–29 The KEYNOTE-021 study demonstrated that addition of pembrolizumab to either carboplatin plus paclitaxel (cohort A); carboplatin, paclitaxel and bevacizumab (cohort B); or carboplatin plus pemetrexed (cohort C) had manageable safety profiles in cohorts A and C with the greatest antitumor activity in cohort C (75% ORR, PFS of 10.2 months; 95%CI: 6.5–13.9).27 Therefore, Langer et al30 sought to compare the efficacy and safety of pembrolizumab plus carboplatin and pemetrexed verus carboplatin and pemetrexed as first-line treatment in patients with advanced NSCLC with nonsquamous histology in the randomized Phase II study, Keynote 21G.
In this trial 123 patients were randomized (1:1), stratified by PD-L1 tumor proportion score (<1% or ≥1%) to receive either four cycles of pembrolizumab plus carboplatin and premetrexed followed by pemetrexed and pembrolizumab maintenance therapy for 24 months or four cycles of carboplatin and pemetrexed alone followed by indefinite pemetrexed maintenance therapy. The primary endpoint was ORR with secondary endpoints of PFS, duration of response, OS and correlation between PD-L1 expression levels and antitumor activity. ORR was superior in patients treated with pembrolizumab plus carboplatin and pemetrexed (55%; 95%CI: 42–68) compared to chemotherapy alone (29%; 95%CI: 18–41). PFS was significantly longer with combination therapy compared to chemotherapy alone (HR: 0.53; 95%CI: 0.31–0.91). Median PFS was 13 months for combination therapy vs 8.9 months with chemotherapy alone with estimated 6 month PFS of 77% vs 63%. At the time of data cut-off no difference in OS was noted between treatment groups (HR: 0.90; 95%CI: 0.81–0.96) and 6-month survival was 92% in both treatment groups.30 Updated results from 24 months demonstrated a PFS of 24 months in patients treated with pembrolizumab plus chemotherapy vs 9.3 months for patients treated with chemotherapy alone (HR: 0.53; 95%CI: 0.33–0.86). The median OS was not reached in the pembrolizumab plus chemotherapy group while OS in the chemotherapy alone group was 21.1 months (HR: 0.56; 95%CI: 0.32–0.95).31 The most common treatment-related events were fatigue (64% in pembrolizumab + chemotherapy group vs 40% in chemotherapy alone group), nausea (58% vs 44%), and anemia (32% vs 53%). Patients treated with pembrolizumab plus chemotherapy had increased incidence of rash (27% vs 15%) and alopecia (14% vs 3%). Incidence of adverse events (AEs) based on presumed immunological mechanisms was 22% in the pembrolizumab plus chemotherapy group vs 11% in the chemotherapy alone group. However, the incidence of immune related AEs were no more than what would be expected with pembrolizumab alone. There was a low number of grade 3 skin reactions (2% in both groups) and grade 3 pneumonitis (2% in pembrolizumab plus chemotherapy group). The most common immune-mediated AEs of any grade in the pembrolizumab plus chemotherapy group were hypothyroidism (15%), hyperthyroidism (8%) and pneumonitis (5%). Given these results, carboplatin, pemetrexed, and pembrolizumab were granted accelerated FDA approval for patients with advanced, untreated, nonsquamous NSCLC.
More recently, in a Phase III, double-blind, placebo-controlled KEYNOTE-189 trial, Gandhi et al compared the combination of pembrolizumab or placebo plus pemetrexed and a platinum-based drug in patients with advanced, nonsquamous NSCLC with any level of PD-L1 level expression. The co-primary endpoints of the study were OS and PFS. Six hundred and sixteen patients with metastatic nonsquamous NSCLC who were treatment-naïve were randomized (2:1). At median follow-up (10.5 months) the estimated OS at 12 months was 69.2% (95%CI: 64.1–73.8) in the pembrolizumab-combination group, compared to 49.4% in the placebo combination. The median OS was not reached in the pembrolizumab-combination group and was 11.3 months in the placebo-combination group (HR: 0.49; 95%CI: 0.38–0.64; P<0.0001). The benefit of pembrolizumab combination was noted across all levels of tumor PD-L1 expression in patients (Table 1). Median PFS was 8.8 months in the pembrolizumab-combination group (95%CI: 7.6–9.2) while 4.9 months in the placebo-combination group (95%CI: 4.7–5.5) (HR: 0.52; 95%CI: 0.43–0.64; P<0.0001).32 OS and PFS outcomes did not change depending on platinum used. Adverse events of any cause occurred in 99.8% of the patients in pembrolizumab-combination group vs 99% of patients in the placebo-combination group. Grade 3 or higher events occurred in 67.2% vs 65.8% of patients, respectively. AEs led to 27 (6.7%) deaths in the pembrolizumab group vss 12 (5.9%) in placebo group. The most common adverse effects were nausea (55.6% vs 52.0%), anemia (46.2% vs 46.5%) and fatigue (40.7% vs 38.1%). Diarrhea and rash were reported more frequently in at least 10% of patients in the pembrolizumab group compared to the placebo group. Immune-mediated AEs occurred in 22.7% of patients treated with pembrolizumab combination vs 11.9% of patients treated with placebo combination. These events were grade 3 or higher in 8.9% vs 4.5%, respectively. AEs led to discontinuation of all trial drugs in 13.8% of patients in the pembrolizumab-combination group and 7.9% of patients in the placebo-combination group. Three immune-mediated AEs (pneumonitis) led to death in the pembrolizumab combination group. From these studies, it is now an accepted approach to treat patients with advanced, nonsquamous NSCLC with pemetrexed and a platinum-based drug plus pembrolizumab.
 | Table 1 Summary of results from chemotherapy + immunotherapy trials for patients with advanced nonsquamous NSCLC |
Recently, combination immunotherapy and chemotherapy has been studied in patients with advanced squamous NSCLC (Table 2). The Phase III KEYNOTE 407 trial randomized 559 patients with untreated metastatic squamous NSCLC in a 1:1 ratio to receive either pembrolizumab or saline placebo, plus carboplatin and either paclitaxel or nab-paclitaxel. At median follow-up of 7.8 months, PFS was 6.4 months in the pembrolizumab-combination group vs 4.8 months in the placebo-combination group (HR: 0.56; 95%CI: 0.45–0.70). OS was 15.9 months vs 11.3 months (HR: 0.64; 95%CI: 0.49–0.85) in patients treated with pembrolizumab plus chemotherapy vs placebo plus chemotherapy.33 PFS and OS outcomes did not change based on taxane used. This lead to FDA approval for pembrolizumab plus chemotherapy (carboplatin plus nab-paclitaxel or paclitaxel) in patients with advanced NSCLC with squamous histology.
 | Table 2 Summary of results from chemotherapy + immunotherapy trials for patients with advanced squamous NSCLC |
One outstanding question that remains to be answered is whether patients treated with pembrolizumab-combination have better survival compared to patients treated with pembrolizumab monotherapy, in those patients with ≥50% PD-L1 tumor expression. In a subset analysis of KEYNOTE 189 based on PD-L1 expression, survival benefit with chemotherapy and pembrolizumab was greater in patients with tumor PD-L1 expression ≥50% (HR: 0.42) than in patients with lower PD-L1 expression (HR: 0.55) or no PD-L1 expression (HR: 0.59). In addition, this survival benefit among patients with tumor PD-L1 expression of ≥50% appears to be greater than the survival benefit with pembrolizumab alone compared to chemotherapy in KEYNOTE 024 (HR: 0.60) and KEYNOTE 042 (HR: 0.69). Similar differential efficacy of the combination of pembrolizumab with chemotherapy was not observed in KEYNOTE 407 conducted in squamous cell patients and the survival benefit in patients with tumor PD-L1 expression ≥50% (HR: 0.64) in this study does not appear to be better than pembrolizumab alone. Thus, it is possible that in patients with nonsquamous NSCLC, combination pembrolizumab with chemotherapy may provide an added survival benefit in patients with tumor PD-L1 expression ≥50% than pembrolizumab alone, however, this benefit may not necessarily be observed in squamous cell patients. Such inferences based on across-trial comparisons should be viewed with a high level of caution. Studies specifically assessing the role of combination chemotherapy in addition to pembrolizumab in patients with tumor PD-L1 expression ≥50% are warranted.
It will also be important to understand if TMB plays a role in helping to determine which patients with advanced, nonsquamous NSCLC will respond more robustly to pembrolizumab-combination treatment. Altogether, chemotherapy plus pembrolizumab appears to be more efficacious than chemotherapy alone in patients with advanced, nonsquamous NSCLC.
Chemotherapy + immunotherapy (other combinations)
Other combinations of chemotherapy and immunotherapy agents have recently been studied in patients with advanced NSCLC (Table 1). The IMpower150 trial randomized 1,202 patients irrespective of PD-L1 status with advanced, nonsquamous NSCLC to first-line carboplatin and paclitaxel every 3 weeks combined with either atezolizumab (1,200 mg IV every 3 weeks), atezolizumab plus bevacizumab or bevacizumab alone. Primary endpoints were PFS and OS and compared atezolizumab, bevacizumab, carboplatin, paclitaxel vs bevacizumab, carboplatin, paclitaxel. PFS was noted to be improved in patients treated with the atezolizumab combination as compared to non-atezolizumab combination (8.3 months vs 6.8 months; HR for disease progression or death: 0.62; 95%CI: 0.52–0.74). Interim analysis of OS was 19.2 months in patients treated with the atezolizumab combination compared to 14.7 months with non-atezolizumab combination (HR due death: 0.78; 95%CI: 0.64–0.96). Overall survival was also measured in patients treated with atezolizumab plus carboplatin and paclitaxel vs carboplatin, paclitaxel and bevacizumab and no significant difference was observed (HR: 0.88; 95%CI: 0.72–1.08). It should be noted that this trial enrolled patients with EGFR or ALK positive NSCLC (who had received at least one line of targeted therapy) and in subgroup analysis OS and PFS was noted to benefit these patients treated with the atezolizumab combination compared to the non-atezolizumab combination.34 Recently the FDA approved the carboplatin, paclitaxel, bevacizumab and atezolizumab as front-line therapy for nonsquamous NSCLC patients without EGFR or ALK genetic alterations.
The IMpower132 study also assessed atezolizumab plus combination chemotherapy combination. This was a Phase III randomized trial of patients with advanced, nonsquamous NSCLC. Patients were randomized to either atezolizumab plus pemetrexed and platinum-based chemotherapy (cisplatin or carboplatin) or chemotherapy alone. Interim analysis demonstrated increased PFS with atezolizumab combination vs chemotherapy alone, 7.6 vs 5.2 months (HR: 0.60; 95%CI: 0.49–0.72). OS was also improved, 18.1 vs 13.6 months (HR: 0.81; 95%CI: 0.64–1.03) however, this was not significant at interim analysis.35 It should be noted that these results appear to be similar to the results observed in KEYNOTE-189.
Treatment with atezolizumab plus chemotherapy has also been evaluated in patients with squamous NSCLC (Table 2). The IMpower131 trial is a randomized Phase III trial which enrolled patients with advanced squamous cell lung cancer. Patients received either atezolizumab plus carboplatin and paclitaxel or nab-paclitaxel vs chemotherapy (carboplatin and nab-paclitaxel). Interim analysis demonstrated improved PFS of 6.3 months vs 5.6 months in patients treated with atezolizumab plus carboplatin and nab-paclitaxel vs chemotherapy alone (HR: 0.715; 95%CI: 0.603–0.848).36 PFS benefit was irrespective of PD-L1 status. However, a statistically significant OS benefit was not observed at interim analysis.
Nivolumab has also been studied in combination with chemotherapy in patients with advanced NSCLC. The Phase I CheckMate 012 trial enrolled patients with Stage IIIb and IV, chemotherapy-naïve NSCLC and randomized patients to nivolumab 1 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks, nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks, or nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks until disease progression, unacceptable toxicities or withdrawl of consent. The study demonstratd that nivolumab plus ipilimumab had a tolerable safety profile and demonstrated a confirmed ORR of approximately 47% (95%CI: 31–64) in patients treated with 12 week ipilimumab and 38% (95%CI: 23–55) in patients treated with 6-week ipilimumab.37
More recently, results from the CheckMate 568 trial were published. This Phase II trial evaluated the efficacy and safety of nivolumab 3 mg/kg every 2 weeks plus low-dose ipilimumab (1 mg/kg every 6 weeks) in patients with advanced or metastatic NSCLC. Primary end point was ORR in patients with 1% or more and less than 1% PD-L1 expression. Efficacy on the basis of TMB was also assessed as a secondary endpoint. ORR was 41% in patients with 1% or more PD-L1 expression and 15% in patients with less than 1% expression. Patients with TMB of 10 or more mutations/megabase portended a higher ORR, 48% vs patients with less than 10 mutations/megabase, 18%. PFS was also longer in patients with high TMB (10 or more mutations/megabase) vs lower TMB (fewer than 10 mutations/Mb), 7.1 (95%CI: 3.6–11.3) vs 2.6 months (95%CI: 1.4–5.4).38
The CheckMate 227 trial randomized patients with advanced, untreated NSCLC to histology-matched, platinum doublet chemotherapy, nivolumab plus ipilimumab or either nivolumab alone in patients with tumor PD-L1 expression ≥1% or nivolumab plus chemotherapy in patients with tumor PD-L1 expression ≤1% (Table 3). Across all the arms, 1,739 patients were enrolled on the trial. One of the co-primary end points of the study was to assess PFS based on TMB. In this trial TMB was determined using the Foundation CDx assay. Previous studies utilizing whole exome sequencing had demonstrated significant clinical benefit with PD-1/PD-L1 inhibitors in patients with high TMB. The results of the first part of the study comparing nivolumab plus ipilimumab to chemotherapy in patients with high TMB (≥10 mutations per megabase) demonstrated increased PFS with nivolumab plus ipilimumab as compared to chemotherapy (7.2 months vs 5.4 months, HR for disease progression or death: 0.58; 97.5%CI: 0.41–0.81). Of the 679 evaluable patients, 44% (299 patients) had high TMB. It was also noted in the study that outcomes were irrespective of PD-L1 tumor expression status. ORR in patients with high TMB was also higher with nivolumab plus ipilimumab (45.3% vs 26.9%).39 Further preliminary results from CheckMate 227 assessing nivolumab and chemotherapy compared to chemotherapy alone in patients with tumor PD-L1≤1% demonstrated improved PFS with combination nivolumab and chemotherapy (5.6 months vs 4.7 months; HRL 0.74l 95%CI: 0.58–0.94). In subgroup analysis patients receiving nivolumab plus chemotherapy combination therapy who had high TMB tumors derived benefit while those with low TMB tumors did not.40 Survival advantage has not yet been demonstrated in any of the treatment arms. These studies suggest that TMB may be a biomarker predictive of benefit from nivolumab combinations both with ipilimumab and with chemotherapy. In the future it will be important to understand how both tumor TMB and PD-L1 expression can be used to make treatment decisions.
 | Table 3 Summary of results from CheckMate 227 trial |
Conclusion
In conclusion, immunotherapy has demonstrated a significant survival benefit in patients with advanced NSCLC. Recent studies combining chemotherapy and checkpoint inhibition have demonstrated OS benefits in the first-line setting. This combination has become standard of care for patients with advanced NSCLC (Figure 1). The excitement surrounding immunotherapy and checkpoint inhibition stems from the durable responses that are observed in some patients with advanced NSCLC. Given these results, understanding how to improve responses and outcomes in patients with advanced NSCLC will be extremely important given the potential for durable responses with immunotherapy. Additional research is needed to further our understanding of the biology underlying those patients that are “exceptional-responders” to immunotherapy.
It will also be important to understand more fully how to incorporate immunotherapy with chemotherapy to improve patient outcomes not only in advanced staged lung cancers but also in early stage disease as well. Already, durvalumab is FDA approved for patients with unresectable, stage III NSCLC. Other studies have explored the use of immunotherapy in the adjuvant setting in patients with early stage NSCLC with results pending.41 Neoadjuvant immunotherapy is also being studied in early stage NSCLC. A pilot study using neoadjuvant nivolumab in patients with early stage NSCLC demonstrated a 43% response rate as determined by major pathologic response at time of surgery.41–43 More recently a phase I study is underway testing pembrolizumab for Stage I and II NSCLC in the neoadjuvant setting and has demonstrated promising results.44
It should also be noted that several studies included EGFR-positive patients with advanced NSCLC and a recent meta-analysis was completed to assess outcomes in these patients.45 As mentioned previously the CheckMate 057 trial assessed the use of nivolumab vs docetaxel in the second-line setting for treatment of advanced NSCLC. Approximately 14% of patient enrolled were EGFR-positive median OS was 12.2 vs 9.4 months in patients treated with nivolumab vs docetaxel, however, this was not statistically significant (HR: 1.18; 95%CI: 0.69–2.00).46 Pembrolizumab has also been assessed in EGFR-positive patients. The KEYNOTE-010 as previously mentioned was a study assessing previously treated patients with advanced NSCLC randomized to pembrolizumab (2 mg/kg and 10 mg/kg) vs docetaxel. Patients treated with either dose of pembrolizumab demonstrated improve OS as compared to docetaxel, however, this was statistically significant (HR: 0.88; 95%CI: 0.45–1.70). Finally, two studies using atezolizumab in the second-line setting for treatment of patients with advanced NSCLC have included EGFR-positive patients. Both these studies failed to demonstrate a significant improvement in OS.13,47 Further studies are needed to assess whether combination or sequential immunotherapy and EGFR tyrosine kinase inhibitor (standard of care for EGFR-positive advanced stage NSCLC) treatment will lead to better outcomes in EGFR-positive patients. Altogether, understanding how to optimize the benefits of immunotherapy for patients with NSCLC will be extremely important in improving patient outcomes.
Disclosure
Dr Shirish Gadgeel reports personal fees from Genentech/Roche, Astra-Zeneca and Takeda. The authors report no other conflicts of interest in this work.
References
1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
2. Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–567. doi:10.1038/nm863
3. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736.
4. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239
5. Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10(10):1563–1572.
6. Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–322. doi:10.1126/science.291.5502.319
7. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi:10.1056/NEJMoa1501824
8. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-7
9. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
10. Brahmer J, Rodríguez-Abreu D, Robinson A, et al. OA 17.06 updated analysis of KEYNOTE-024: pembrolizumab vs platinum-based chemotherapy for advanced NSCLC with PD-L1 TPS ≥50%. J Thorac Oncol. 2017;12(11):S1793–S1794. doi:10.1016/j.jtho.2017.09.431
11. Lopes G, Wu Y-L, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018;36(18_suppl):LBA4–LBA4. doi:10.1200/JCO.2018.36.18_suppl.LBA4
12. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi:10.1056/NEJMoa1613493
13. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi:10.1016/S0140-6736(16)32517-X
14. Fehrenbacher L, von Pawel J, Park K, et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase iii study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2018;13(8):1156–1170. doi:10.1016/j.jtho.2018.04.039
15. Kim ES, Velcheti V, Mekhail T, et al. Primary efficacy results from B-F1RST, a prospective Phase II trial evaluating blood-based tumour mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC).
16. Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. 2017;35(24):2781–2789. doi:10.1200/JCO.2016.71.9476
17. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi:10.1056/NEJMoa1709937
18. Rizvi NA, Chul Cho B, Reinmuth N, Lee KH, et al. Durvalumab with or without tremelimumab vs platinum-based chemotherapy as first-line treatment for metastatic non-small cell lung cancer: MYSTIC. Ann Oncol. 2018;29(suppl_10):x39–x43. doi:10.1093/annonc/mdy511.005
19. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi:10.1016/j.immuni.2013.06.014
20. Roselli M, Cereda V. di Bari MG, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology. 2013;2(10):e27025. doi:10.4161/onci.27025
21. Wang Z, Till B, Gao Q. Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology. 2017;6(7):e1331807. doi:10.1080/2162402X.2017.1331807
22. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714. doi:10.1016/j.ccell.2015.10.012
23. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. doi:10.1038/cdd.2013.67
24. Peng J, Hamanishi J, Matsumura N, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-kappab to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75(23):5034–5045. doi:10.1158/0008-5472.CAN-14-3098
25. Zhang P, Ma Y, Lv C, et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci. 2016;107(11):1563–1571. doi:10.1111/cas.13072
26. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(25):2969–2979. doi:10.1200/JCO.2016.66.9861
27. Gadgeel SM, Stevenson J, Langer CJ, et al. Pembrolizumab (pembro) plus chemotherapy as front-line therapy for advanced NSCLC: KEYNOTE-021 cohorts A-C. J Clin Oncol. 2016;34(15_suppl):9016. doi:10.1200/JCO.2016.34.15_suppl.9016
28. Liu SV, Powderly JD, Camidge DR, et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with platinum-based doublet chemotherapy in patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2015;33(15_suppl):8030.
29. Gadgeel SM, Stevenson JP, Langer CJ, et al. Pembrolizumab and platinum-based chemotherapy as first-line therapy for advanced non-small-cell lung cancer: phase 1 cohorts from the KEYNOTE-021 study. Lung Cancer. 2018;125:273–281. doi:10.1016/j.lungcan.2018.08.019
30. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi:10.1016/S1470-2045(16)30498-3
31. Borghaei H, Langer CJ, Gadgeel S, et al. 24-month overall survival from keynote-021 cohort g: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2019;14(1):124–129. doi:10.1016/j.jtho.2018.08.004
32. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005
33. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi:10.1056/NEJMoa1810865
34. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi:10.1056/NEJMoa1716948
35. IMpower132 Study Shows Atezolizumab in Combination With Carboplatin and Pemetrexed Reduced the Risk of Disease Worsening or Death (PFS) in Stage IV Non-Squamous NSCLC [press release]. 2018.
36. Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol. 2018;36(18_suppl):LBA9000–LBA9000. doi:10.1200/JCO.2018.36.18_suppl.LBA9000
37. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31–41. doi:10.1016/S1470-2045(16)30624-6
38. Ready N, Hellmann MD, Awad MM. et al. First-Line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol;2019. JCO1801042. doi:10.1200/JCO.18.01042
39. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi:10.1056/NEJMoa1801946
40. Borghaei H, Hellmann MD, Paz-Ares LG, et al. Nivolumab (Nivo) + platinum-doublet chemotherapy (Chemo) vs chemo as first-line (1L) treatment (Tx) for advanced non-small cell lung cancer (NSCLC) with <1% tumor PD-L1 expression: results from CheckMate 227. J Clin Oncol. 2018;36(15_suppl):9001.
41. Owen D, Chaft JE. Immunotherapy in surgically resectable non-small cell lung cancer. J Thorac Dis. 2018;10(Suppl 3):S404–S411. doi:10.21037/jtd.2017.12.93
42. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant anti-PD1, nivolumab, in early stage resectable non-small cell lung cancer.
43. Chaft JE, Forde PM, Smith KN, et al. Neoadjuvant nivolumab in early-stage, resectable non-small cell lung cancers. J Clin Oncol. 2017;35(15_suppl):8508. doi:10.1200/JCO.2017.35.15_suppl.8508
44. Ben Nun A, Golan N, Ofek E, et al. Neoadjuvant pembrolizumab (Pembro) for early stage non-small cell lung cancer (NSCLC) – initial report of a phase I study, MK3475-223. Ann Oncol. 2018;29(suppl8):viii483–viii487. doi:10.1093/annonc/mdy290.011
45. Cavanna L, Citterio C, Orlandi E. Immune checkpoint inhibitors in EGFR-mutation positive TKI-treated patients with advanced non-small-cell lung cancer network meta-analysis. Oncotarget. 2019;10(2):209–215. doi:10.18632/oncotarget.26541
46. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643
47. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi:10.1016/S0140-6736(16)00587-0
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
