Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Combination of the CAGE and serum gamma-glutamyl transferase: an effective screening tool for alcohol use disorder and alcohol dependence
Authors Choe YM , Lee BC , Choi IG , Suh GH , Lee DY , Kim JW
Received 1 February 2019
Accepted for publication 2 May 2019
Published 31 May 2019 Volume 2019:15 Pages 1507—1515
DOI https://doi.org/10.2147/NDT.S203855
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Young Min Choe,1,2 Boung Chul Lee,2,3 Ihn-Geun Choi,2,4 Guk-Hee Suh,1,2 Dong Young Lee,5 Jee Wook Kim1,2
1Department of Neuropsychiatry, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Gyeonggi, Republic of Korea; 2Department of Psychiatry, Hallym University College of Medicine, Chuncheon, Republic of Korea; 3Department of Neuropsychiatry, Hallym University Hangang Sacred Heart Hospital, Seoul, Republic of Korea; 4Department of Neuropsychiatry, Hallym University Kangnam Sacred Heart Hospital, Seoul, Republic of Korea; 5Department of Neuropsychiatry, Seoul National University Hospital, Seoul, Republic of Korea
Purpose: The CAGE is a convenient test for alcohol-related disorder due to its brevity, but it is not as effective as the alcohol use disorders identification test (AUDIT). Gamma-glutamyl transferase (GGT) is an objective blood biochemical marker of excessive alcohol intake; however, it has low sensitivity. This study tested the performance of the combined use of CAGE and GGT to screen problem drinking (PD), alcohol use disorder (AUD), and alcohol dependence (AD).
Methods: A total of 394 subjects composed of 91 normal controls and 303 subjects with PD were enrolled in this study. Of the PD subjects, 147 were diagnosed with AUD (77 alcohol abuse and 70 AD). A series of multiple logistic regression models for PD, AUD, and AD discrimination were used to obtain new combined CAGE and GGT scores after adjusting for age and gender (CAGE+GGT). A receiver operating characteristic curve analysis was conducted to determine how well the CAGE+GGT score discriminated between individuals with PD, AUD, and AD.
Results: The discrimination accuracy of the AUDIT for PD was significantly better than that of the CAGE or the CAGE+GGT (z=6.927, p<0.0001; z=5.301, p<0.0001, respectively). The CAGE and the CAGE+GGT were better than the AUDIT at discriminating AUD (z=2.535, p=0.0112; z=2.894, p=0.0038, respectively). The discrimination accuracy of the AUDIT for AD was significantly better than that of the CAGE and GGT (z=3.233, p=0.0012; z=6.529, p<0.0001, respectively), but the CAGE+GGT was comparable with the AUDIT (z=1.652, p=0.0985).
Conclusion: Our findings support the combined use of the CAGE questionnaire and serum GGT level as a sensitive and useful tool for AD screening.
Keywords: CAGE, gamma-glutamyl transferase, alcohol use disorders identification test, alcohol use disorder, alcohol dependence
Introduction
Screening for problem drinking (PD) and alcohol-related disorders is critical because excessive alcohol consumption is associated with individual morbidities and an economic burden.1 Identifying heavy drinkers is also important because they are at increased risk of harming their mental and physical health caused by alcohol, regardless of whether they are diagnosed with alcohol use disorder (AUD) or alcohol dependence (AD).2
The two most widely used alcohol screening questionnaires are the alcohol use disorders identification test (AUDIT) and the CAGE. The AUDIT questionnaire was developed by the World Health Organization to meet the needs of primary health care workers to detect risky drinking, harmful drinking, and AD.3 The AUDIT focuses on recent alcohol use and consists of questions about patterns of alcohol misuse, AD symptoms, and alcohol-related problems. Many studies have shown its validity and reliability,4,5 and the AUDIT performs significantly better than other alcohol screening instruments.6,7 However, the AUDIT is not as easy to administer as the CAGE due to the relatively large number of items, regardless of its high screening accuracy.3 The CAGE, an acronym for questions about cutting down drinking, annoyance at others’ concern about drinking, guilty feeling about drinking, and using alcohol as an eye-opener in the morning, was developed by Ewing and is widely used as a screening and case-finding tool.8 The CAGE asks about alcohol problems ever experienced and it is feasible to use and easily applied in clinical practice due to its brevity.9,10 Despite its brevity, CAGE has a high sensitivity for AUD.11 However, CAGE has drawbacks with regard to insufficient information on alcohol consumption,8 so it may not be more effective than the AUDIT for screening AD.
Although these self-reported questionnaires are accessible and easy to use, they have a critical limitation in that individuals often deny or minimize the quantity and frequency of drinking.12 In addition, the accuracy of self-reporting varies depends on the population13 and interview method.14 To compensate for such limitations, several objective blood biochemical markers, including gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), erythrocyte mean cell volume (MCV), and carbohydrate-deficient transferrin (CDT) have been suggested and some of them are in practical use to assess excessive alcohol consumption and AUD.15 Among the traditional biomarkers, GGT is one of the most representative and commonly used tests for excessive alcohol consumption.16 GGT has the advantage of being objective and widely used, but it has low sensitivity.17 One recent study reported that all biochemical markers including GGT exhibit significantly lower sensitivity and specificity compared to the AUDIT questionnaire.18
To develop an objective and feasible screening tool, we combined the subjective and simplest questionnaire, ie, CAGE score, and an objective and easily available biochemical marker, ie, serum GGT. The combination of CAGE and GGT is expected to bring synergistic efficacy, not a mere summation, because the limitation of the CAGE regarding the lack of information on alcohol consumption may be compensated for by augmenting with GGT, which has a significant correlation with alcohol consumption. Furthermore, the low sensitivity of GGT may be strengthened by augmenting with the CAGE which has high sensitivity. We hypothesized that the combination of the CAGE and GGT would be effective for screening PD, AUD, and AD while retaining its unique benefits of simplicity and objectivity.
Material and methods
Subjects
Subjects were recruited from a pool of individuals registered in the program for early detection and management of alcohol addiction conducted by the Hallym University Hangang Sacred Heart Hospital, and five alcohol-specialized hospitals from March 2010 to January 2012. The diagnoses of alcohol abuse and AD were made according to the criteria of the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders19 by psychiatrists with advanced training in alcohol-related research. Risky drinking was defined as consuming more than 14 drinks per week or five or more drinks on one occasion in the past year in males; more than seven drinks per week or three or more drinks on one occasion in the past year in females. The diagnosis of risky drinking was made by psychiatrists according to the criteria of the National Institute on Alcohol Abuse and Alcoholism20,21 under the condition of not satisfying the AUD criteria. The normal control (NC) was diagnosed after excluding both risky drinking and AUD. Risky drinking, alcohol abuse, and AD were grouped for screening: all three were grouped as PD,22 and the latter two were grouped as AUD.23
The following exclusion criteria were applied to all subjects: 1) major medical illnesses other than an alcohol-related disorder; 2) major psychiatric disorders such as schizophrenia, mood disorder or substance-abuse disorder other than nicotine or caffeine dependence; and 3) the presence of severe behavioral or communication problems that would make a clinical examination difficult.
The Institutional Review Board of the Hallym University Hangang Sacred Heart Hospital approved this study protocol and this study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Clinical assessment
All subjects underwent a clinical interview including a detailed history of alcohol drinking and a family history of AD. The Korean version of the 10-item AUDIT24 and the CAGE25 were also administered by research nurses who were trained in alcohol research and blind to the diagnosis. The Korean version of the 10-item AUDIT consists of three domains: the first three items measure alcohol consumption, items 4–6 assess AD, and items 7–10 consider alcohol-related harm.24 A panel consisting of three psychiatrists with expertise in alcohol-related research made the clinical decisions, including the diagnoses, after having reviewed all available clinical data, except for the AUDIT and CAGE scores.
Laboratory testing
Venous blood was collected in the morning after the subjects had abstained from food and liquids for 12 hrs. Blood specimens were centrifuged immediately, and the serum was stored at 4°C for up to 7 days before testing. Serum GGT levels were determined using a gamma-GT FS kit (DiaSys Diagnostic Systems, Holzheim, Germany) on the Hitachi 7600 automatic analyzer (Tokyo, Japan) using the Szasz-Persijn method.26,27
Statistical analysis
To test the independent contributions of the CAGE and GGT in classifying alcohol use status (NC vs PD, non-AUD vs AUD, and non-AD vs AD), multiple logistic regression analyses were performed after adjusting for age (years) and gender (coded as male=1, female=0). We combined the CAGE and GGT scores using the weighted sum rule suggested by Mackinnon and Mulligan.28 A series of multiple logistic regression models for PD, AUD, and AD discrimination were used to obtain new scores combining the CAGE and GGT after adjusting for age and gender. We conducted a receiver operating characteristic (ROC) curve analysis to determine how well the new scores discriminated individuals with PD, AUD, and AD. The area under the ROC curve (AUROC) was compared using the method of Hanley and McNeil.29 ROC curve analyses were performed using MedCalc for Windows, version 18.11.3 (MedCalc Software, Mariakerke, Belgium). For the ROC curve comparison analyses between AUDIT and others (AUDIT vs CAGE, AUDIT vs GGT, AUDIT vs CAGE+GGT), a Bonferroni-corrected p-value (0.05/number of analyses) was applied and p<0.0167 (0.05/3) was considered significant. All the other analyses, except the ROC curve analysis, were performed using IBM SPSS, version 24 software (IBM Corp., Armonk, NY, USA), and p<0.05 was considered significant.
Results
Demographic and clinical characteristics
The characteristics of the subjects are summarized in Table 1. We recruited 394 subjects (309 males and 85 females) with ages ranging from 23 to 79 years (mean: 42.73, SD: 12.33). Their mean AUDIT score was 11.63 (SD: 9.62), and the mean CAGE score was 1.56 (SD: 1.40). Subjects were composed of 91 (23.1%) NCs and 303 (76.9%) PDs. Of the PD subjects, 147 were diagnosed with AUD, and 156 were classified as risky drinkers. Furthermore, 147 AUD subjects were divided into 70 AD and 77 alcohol abuse individuals.
 | Table 1 Subject characteristics |
Multiple logistic regression analyses treating PD, AUD, and AD as dependent variables
Table 2 shows the results of the multiple logistic regression analyses treating PD, AUD, and AD as dependent variables. Both the CAGE score and GGT level significantly and independently contributed to differentiate PD from NC after adjusting for age and gender [OR=3.709, 95% CI=2.485–5.535; OR=1.027, 95% CI=1.008–1.048, respectively], and also contributed to distinguish AD from non-AD [OR=5.829, 95% CI=3.731–9.106; OR=1.008, 95% CI=1.003–1.013, respectively]. In contrast, subjects with AUD were separated from non-AUD subjects using the CAGE (OR=7.895, 95% CI=5.251–11.870), but not GGT level.
 | Table 2 Multiple logistic regression analyses treating alcohol use status as a dependent variable |
New scores combining the CAGE and GGT after adjusting for age and gender
We used the results of multiple logistic regression analyses treating PD, AUD, and AD as dependent variables to calculate new combined CAGE and GGT scores after adjusting for age and gender (CAGE+GGT score). The equations for the new scores derived from the multiple logistic regressions were as follows:
PD discrimination
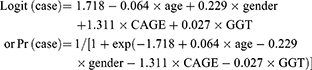
AUD discrimination

AD discrimination
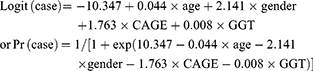
ROC analyses
Table 3 shows the AUROC, sensitivity, specificity, and cutoff points for the AUDIT score, CAGE score, serum GGT level, and the CAGE+GGT score. ROC curves were constructed for each score comparison and are shown in Figure 1. The ROC curve comparisons for NC vs PD, non-AUD vs AUD, and non-AD vs AD are demonstrated in the following sections.
 | Table 3 AUROC and cutoff scores for the AUDIT, CAGE, GGT and the combination of CAGE and GGT |
PD discrimination
The AUROC of the AUDIT score was 0.973, indicating good discrimination of PD subjects from NC individuals. The discrimination accuracy of the AUDIT score was significantly better than those of the CAGE score, GGT level, and the CAGE+GGT score (z=6.927, p<0.0001; z=9.82, p<0.0001; z=5.301, p<0.0001, respectively).
AUD discrimination
The CAGE score and the CAGE+GGT score were better than the AUDIT score to discriminate AUD (z =2.535, p=0.0112; z =2.894, p=0.0038, respectively). Furthermore, no significant difference was found between the CAGE and the CAGE+GGT scores when distinguishing AUD from non-AUD.
AD discrimination
The AUROC of the AUDIT score was 0.970, indicating good discrimination of AD subjects from non-AD individuals. The discrimination accuracy of the AUDIT score was significantly better than that of the CAGE score and GGT level (z=3.233, p=0.0012; z=6.529, p<0.0001, respectively), but the CAGE+GGT score was comparable to the AUDIT score for discriminating AD (z=1.652, p=0.0985).
Discussion
We investigated the efficacy of the combined use of the CAGE score and serum GGT to discriminate between subjects with PD, AUD, and AD, and we examined whether the discriminatory ability was comparable to that of the AUDIT score. The AUDIT showed the highest screening accuracy for PD and AD, whereas the CAGE and the CAGE+GGT were effective for discriminating AUD. The CAGE+GGT score showed a high screening accuracy, comparable to the AUDIT score, for discriminating AD. As far as we know, this is the first study to compare the discrimination accuracy of the AUDIT score, CAGE score, and the CAGE+GGT for distinguishing between PD, AUD, and AD.
The present study revealed that the discrimination accuracy of the AUDIT was better than that of other tools for PD screening. In accordance with our results, one systematic review reported that the AUDIT has increased accuracy, with sensitivity of 57–97%, and specificity of 78–96%, relative to other screening methods, including the CAGE, for detecting PD in primary care.11 The superiority of the AUDIT to the CAGE can be explained by the fact that the AUDIT has questions about the quantity and frequency of alcohol consumption, whereas the CAGE only assesses the consequences of alcohol intake. Although the NIAAA recommends the use of the CAGE with consumption questions,21 such an approach is beyond the scope of our study. The CAGE was superior to the AUDIT for detecting AUD, which was also consistent with a previous systematic review reporting that CAGE performs better than other instruments, with a sensitivity of 43–94% and specificity of 70–97%. The AUDIT performed better than the CAGE for screening AD. In contrast to our findings, a systematic review reported that the CAGE is superior to the AUDIT for screening AD.11 The differences in study characteristics and cultural aspects may account for the disparate findings. Moreover, our AD sample were diagnosed in the clinics of general hospitals, which are different environments from primary care settings.
Questionnaires have a critical limitation in that individuals often deny or minimize the quantity and frequency of drinking as well as harmful behavior related to excessive drinking when individuals seek to avoid blame or legal liability for alcohol misuse.12 To overcome such weaknesses, several blood biochemical markers, including GGT, AST, MCV, and CDT have been suggested and used to detect excess alcohol use.15 However, these biochemical markers are also limited by low sensitivity and specificity for identifying unhealthy drinking.15,30 Only a few studies have attempted the combined use of questionnaires and biochemical markers from laboratory tests to detect alcohol misuse in various settings. The CAGE in combination with GGT presents the best sensitivity for AD in a workplace health examination,31 whereas the AUDIT and CDT are complementary instruments for alcohol screening.32 The early detection of alcohol consumption and CDT may be combined for maximum diagnostic accuracy to identify heavy drinking in males.33 The ability of the AUDIT to predict alcohol withdrawal may increase when used in combination with biochemical markers in a general medical setting.34
In the present study, we attempted to combine GGT and the CAGE to screen PD, AUD, and AD. We expected that such a combination would create a synergistic effect because the CAGE lacks information on alcohol consumption and could be compensated for by augmenting with GGT, which is significantly correlated with alcohol intake. Furthermore, Bataille et al35 reported that the CAGE and GGT are independently related to excessive alcohol use in a large population-based sample. Our findings revealed that the combined use of the CAGE and GGT was comparable to the AUDIT for screening AD. The combination of GGT with CAGE was not as effective as the AUDIT for screening PD. GGT has a short half-life and requires prolonged and heavier alcohol intake to reach an abnormal level.32 It is possible that the combination of CAGE with GGT is effective for screening when alcohol is consumed continuously and severely enough to be diagnosed as AUD or AD.
The present study had some strengths, including a relatively large sample size and clinically strict diagnoses. The clinical evaluations and diagnoses were conducted by a panel consisting of three psychiatrists who were experts in this field. Major medical illnesses and psychiatric disorders were strictly excluded through a case conference. However, this study also had several limitations. First, we did not acquire other biochemical markers, including CDT, MCV, GOT, or GPT. The use of CDT, in particular, is important because it is more specific marker for identifying chronic excessive alcohol use,36–38 and Sillanaukee and Olsson39 reported that the combined use of CDT and GGT is a powerful tool to discriminate alcohol abusers. In addition to traditional biomarkers, direct alcohol markers, such as phosphatidylethanol in the blood, and fatty acid ethyl esters or ethylglucuronide in hair have drawn attention as they are highly specific for chronic alcohol use.40,41 Additional studies using biomarkers other than GGT are still needed. Second, caution should be taken to interpret the contribution of age and gender in comparison of combined the CAGE and GGT with AUDIT. We included age and gender in the equations for the combined CAGE and GGT score, because they are powerful biological factors that affect the GGT level.42 Further analyses were conducted to exclude the effects of age and gender on the ROC curve comparison and similar results were found. Third, GGT can be elevated by causes other than alcohol consumption.43 Although we excluded subjects with major medical illnesses, there still remains the possibility that the level of GGT was related to other unmeasured etiological factors that might be linked to AD. Finally, the clinical diagnosis was made by experts but mainly based on interviews, so there remains the possibility of diagnostic error due to false reporting by the subjects.
Conclusion
Our findings support combined use of the CAGE questionnaire and serum GGT level as a sensitive, useful tool for AD screening given the brevity of the CAGE and objectivity of GGT. Particularly, in specific situations, where reliability of the CAGE becomes a key concern, augmenting with the serum GGT level would enhance performance for AUD screening.
Acknowledgments
This study was supported by Hallym University Research Fund, 2018 (HURF-2018-45). The funding source had no role in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit it for publication.
Author contributions
JWK and IGC designed the study and JWK wrote the study protocol. YMC undertook the statistical analyses, and YMC and BCL wrote the draft of the manuscript. BCL, IGC, GHS, DYL, and JWK collected and analyzed the data. All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Crombie IK, Irvine L, Elliott L, Wallace H. How do public health policies tackle alcohol-related harm: a review of 12 developed countries. Alcohol Alcohol. 2007;42(5):492–499. doi:10.1093/alcalc/agl083
2. Bradley KA, Donovan DM, Larson EB. How much is too much? Advising patients about safe levels of alcohol consumption. Arch Intern Med. 1993;153(24):2734–2740. doi:10.1001/archinte.1993.00410240036004
3. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88(6):791–804. doi:10.1111/add.1993.88.issue-6
4. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56(4):423–432.
5. Piccinelli M, Tessari E, Bortolomasi M, et al. Efficacy of the alcohol use disorders identification test as a screening tool for hazardous alcohol intake and related disorders in primary care: a validity study. Bmj. 1997;314(7078):420–424. doi:10.1136/bmj.314.7078.420
6. Clements R. A critical evaluation of several alcohol screening instruments using the CIDI-SAM as a criterion measure. Alcohol Clin Exp Res. 1998;22(5):985–993. doi:10.1111/j.1530-0277.1998.tb03693.x
7. Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res. 1997;21(4):613–619.
8. Ewing JA. Detecting alcoholism. The CAGE questionnaire. Jama. 1984;252(14):1905–1907. doi:10.1001/jama.1984.03350140051025
9. Knight JR, Sherritt L, Harris SK, Gates EC, Chang G. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67–73. doi:10.1097/01.ALC.0000057944.57330.65
10. Adams WL, Barry KL, Fleming MF. Screening for problem drinking in older primary care patients. Jama. 1996;276(24):1964–1967. doi:10.1001/jama.1996.03540240042028
11. Fiellin DA, Reid MC, O’Connor PG. Screening for alcohol problems in primary care: a systematic review. Arch Intern Med. 2000;160(13):1977–1989. doi:10.1001/archinte.160.13.1977
12. Freeman WM, Vrana KE. Future prospects for biomarkers of alcohol consumption and alcohol-induced disorders. Alcohol Clin Exp Res. 2010;34(6):946–954. doi:10.1111/j.1530-0277.2010.01169.x
13. Frank D, DeBenedetti AF, Volk RJ, Williams EC, Kivlahan DR, Bradley KA. Effectiveness of the AUDIT-C as a screening test for alcohol misuse in three race/ethnic groups. J Gen Intern Med. 2008;23(6):781–787. doi:10.1007/s11606-008-0594-0
14. Steinweg DL, Alcoholism WH. the keys to the CAGE. Am J Med. 1993;94(5):520–523. doi:10.1016/0002-9343(93)90088-7
15. Liangpunsakul S, Qi R, Crabb DW, Witzmann F. Relationship between alcohol drinking and aspartate aminotransferase:alanine aminotransferase (AST:ALT) ratio, mean corpuscular volume (MCV), gamma-glutamyl transpeptidase (GGT), and apolipoprotein A1 and B in the U.S. population. J Stud Alcohol Drugs. 2010;71(2):249–252. doi:10.15288/jsad.2010.71.249
16. Peterson K. Biomarkers for alcohol use and abuse–a summary. Alcohol Res Health. 2004;28(1):30–37.
17. Conigrave KM, Davies P, Haber P, Whitfield JB. Traditional markers of excessive alcohol use. Addiction. 2003;98(Suppl 2):31–43. doi:10.1046/j.1359-6357.2003.00581.x
18. Coulton S, Drummond C, James D, et al. Opportunistic screening for alcohol use disorders in primary care: comparative study. Bmj. 2006;332(7540):511–517. doi:10.1136/bmj.38743.421574.7C
19.
20.
21. Friedmann PD, Saitz R, Gogineni A, Zhang JX, Stein MD. Validation of the screening strategy in the NIAAA “Physicians’ guide to helping patients with alcohol problems”. J Stud Alcohol. 2001;62(2):234–238. doi:10.15288/jsa.2001.62.234
22. Bradley KA, Bush KR, McDonell MB, Malone T, Fihn SD. Screening for problem drinking: comparison of CAGE and AUDIT. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. J Gen Intern Med. 1998;13(6):379–388. doi:10.1046/j.1525-1497.1998.00118.x
23. Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Care. Geneva, Switzerland: World Health Organization; 2001.
24. JS OM K, Park BK, Lee MK, Kim GJ. Screening criteria of alcoholism by alcohol use disorders identification test(AUDIT) in Korea. J Korean Acad Fam Med. 1999;20(9):1152–1159.
25. BK LD P, Lee TY, Cho YC, Kwon YH. Comparison of screening tests for alcoholism in terms of reliability, sensitivity and specificity. Chungnam Med J. 2000;27:37–47.
26. Szasz G. New substrates for measuring gamma-glutamyl transpeptidase activity. Z Klin Chem Klin Biochem. 1974;12(5):228.
27. Persijn JP, van der Slik W. A new method for the determination of gamma-glutamyltransferase in serum. J Clin Chem Clin Biochem. 1976;14(9):421–427.
28. Mackinnon A, Mulligan R. Combining cognitive testing and informant report to increase accuracy in screening for dementia. Am J Psychiatry. 1998;155(11):1529–1535. doi:10.1176/ajp.155.11.1529
29. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–843. doi:10.1148/radiology.148.3.6878708
30. Liangpunsakul S, Lai X, Ross RA, et al. Novel serum biomarkers for detection of excessive alcohol use. Alcohol Clin Exp Res. 2015;39(3):556–565. doi:10.1111/acer.12654
31. Do Amaral RA, Malbergier A. Effectiveness of the CAGE questionnaire, gamma-glutamyltransferase and mean corpuscular volume of red blood cells as markers for alcohol-related problems in the workplace. Addict Behav. 2008;33(6):772–781. doi:10.1016/j.addbeh.2007.12.006
32. Hermansson U, Helander A, Huss A, Brandt L, Ronnberg S. The Alcohol Use Disorders Identification Test (AUDIT) and carbohydrate-deficient transferrin (CDT) in a routine workplace health examination. Alcohol Clin Exp Res. 2000;24(2):180–187. doi:10.1111/j.1530-0277.2000.tb04589.x
33. Harasymiw J, Bean P. The combined use of the early detection of alcohol consumption (EDAC) test and carbohydrate-deficient transferrin to identify heavy drinking behaviour in males. Alcohol Alcohol. 2001;36(4):349–353. doi:10.1093/alcalc/36.4.349
34. Dolman JM, Hawkes ND. Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients. Alcohol Alcohol. 2005;40(6):515–519. doi:10.1093/alcalc/agh189
35. Bataille V, Ruidavets JB, Arveiler D, et al. Joint use of clinical parameters, biological markers and CAGE questionnaire for the identification of heavy drinkers in a large population-based sample. Alcohol Alcohol. 2003;38(2):121–127. doi:10.1093/alcalc/agg051
36. Golka K, Sondermann R, Reich SE, Wiese A. Carbohydrate-deficient transferrin (CDT) as a biomarker in persons suspected of alcohol abuse. Toxicol Lett. 2004;151(1):235–241. doi:10.1016/j.toxlet.2004.01.023
37. Reynaud M, Schellenberg F, Loisequx-Meunier MN, et al. Objective diagnosis of alcohol abuse: compared values of carbohydrate-deficient transferrin (CDT), gamma-glutamyl transferase (GGT), and mean corpuscular volume (MCV). Alcohol Clin Exp Res. 2000;24(9):1414–1419.
38. Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001-2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841(1–2):96–109. doi:10.1016/j.jchromb.2006.05.005
39. Sillanaukee P, Olsson U. Improved diagnostic classification of alcohol abusers by combining carbohydrate-deficient transferrin and gamma-glutamyltransferase. Clin Chem. 2001;47(4):681–685.
40. Viel G, Boscolo-Berto R, Cecchetto G, Fais P, Nalesso A, Ferrara SD. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788–14812. doi:10.3390/ijms131114788
41. Hastedt M, Buchner M, Rothe M, et al. Detecting alcohol abuse: traditional blood alcohol markers compared to ethyl glucuronide (EtG) and fatty acid ethyl esters (FAEEs) measurement in hair. Forensic Sci Med Pathol. 2013;9(4):471–477. doi:10.1007/s12024-013-9416-8
42. Tynjala J, Kangastupa P, Laatikainen T, Aalto M, Niemela O. Effect of age and gender on the relationship between alcohol consumption and serum GGT: time to recalibrate goals for normal ranges. Alcohol Alcohol. 2012;47(5):558–562. doi:10.1093/alcalc/ags072
43. Mihas AA, Tavassoli M. Laboratory markers of ethanol intake and abuse: a critical appraisal. Am J Med Sci. 1992;303(6):415–428. doi:10.1097/00000441-199206000-00014
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

