Back to Journals » International Journal of General Medicine » Volume 15
Combination of the BISAP Score and miR-155 is Applied in Predicting the Severity of Acute Pancreatitis
Authors Wu B, Yang J, Dai Y, Xiong L
Received 28 July 2022
Accepted for publication 14 September 2022
Published 24 September 2022 Volume 2022:15 Pages 7467—7474
DOI https://doi.org/10.2147/IJGM.S384068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Bing Wu, Jun Yang, Yonghong Dai, Le Xiong
Department of Critical Care Medicine, Jiangjin Central Hospital, Chongqing, People’s Republic of China
Correspondence: Le Xiong, Department of Critical Care Medicine, Jiangjin Central Hospital, No. 725, Jiangzhou Road, Dingshan Street, Jiangjin District, Chongqing, 402260, People’s Republic of China, Tel +86-2347521342, Email [email protected]
Purpose: To evaluate the predictive value of combination of Bedside Index for Severity in AP (BISAP) score and miR-155 for the severity of acute pancreatitis (AP).
Patients and Methods: A total of 1046 AP patients were divided into control group and case group according to the severity of AP [mild and moderately severe AP vs severe AP (SAP)]. Demographic data, comorbidities, clinical characteristics and laboratory data were collected. Multivariate analysis was conducted for the variables with two-sided P< 0.10 in univariate analysis to identify independent associated factors for progression to SAP in AP patients. The predictive values were evaluated using receiver operating characteristic (ROC) curve, and the area under curve (AUC) was compared using Z test.
Results: A total of 117 (11.2%) patients were evaluated as SAP. Univariate analysis showed that there were significant differences in age, hypertension, ICU admission, hospital stay, Leukocytes, CRP, BUN, BISAP score and miR-155 between case group and control group (P< 0.05), and the P value of Fibrinogen was < 0.10. Multivariate analysis showed that the BISAP score, BUN, Leukocytes, age and CRP were independent risk factors for progression to SAP among AP patients after adjusting for hypertension, ICU admission, hospital stay and Fibrinogen, while miR-155 was a protective factor. The ROC curves demonstrated the AUCs of BISAP score, miR-155 and their combination were 0.842 (SE: 0.017, 95% CI: 0.809– 0.874), 0.751 (SE: 0.022, 95% CI: 0.708– 0.793) and 0.945 (SE: 0.007, 95% CI: 0.931– 0.959), respectively. Z test showed that the AUC of combination prediction was significantly higher than that of individual predictions (0.945 vs 0.842, Z=5.602, P< 0.001; 0.945 vs 0.751, Z=8.403, P< 0.001). The sensitivity, specificity and negative predictive value (NPV) of combination prediction were 95.7%, 93.6% and 99.4%, respectively.
Conclusion: The combination of the BISAP score and miR-155 should be utilized to elevate the predictive value for the severity of AP in clinic.
Keywords: severe acute pancreatitis, Bedside Index for Severity in AP score, miR-155, prediction
Introduction
Acute pancreatitis (AP) is the acute inflammation of the pancreas, which is associated with sudden activation of pancreatic enzymes and resulting self-digestion and self-destruction of the pancreas itself.1,2 Majority of AP patients have a self-limited and mild course with no sequelae,3 but around 30% of patients will progress to severe acute pancreatitis (SAP) characterized by systemic inflammatory response syndrome (SIRS). The mortality of SAP can reach to 30% due to life-threatening necrosis of the pancreas and multi-organ failure.4,5 Therefore, it is urgent to find an accurate tool for the early prediction of the development of SAP in AP patients.
A number of scoring systems and biomarkers have been applied in the prediction of the severity of AP. As a simple and effective method, the Bedside Index for Severity in AP (BISAP) is proven to have high specificity and negative predictive value (NPV), and moreover, incremental rise in the BISAP score from 3 and above has been demonstrated an significant association with increased risk of pancreatic necrosis which can result in multi-organ failure.6 MicroRNAs (miRNAs) are a class of single-stranded, non-coding, 21–23 nucleotide long, evolutionarily highly conserved small RNA molecule nucleotides. They are involved in plenty of physiological and pathological processes via regulating gene expression.7–9 Many miRNAs have been shown to be dysregulated in a variety of cell types associated with AP such as lymphocytes, macrophages and acinar cells.10
Among them, miR-155 has a significantly lower expression in severe and critical AP patients compared with mild and moderate AP patients, indicating a significant correlation with the progression of AP.11 However, no previous studies have evaluated the predictive value of the combination of BISAP score and miR-155 for SAP in AP patients.
Patients and Methods
Patients
This was a prospective observational study, enrolling a consecutive cohort of patients admitted to Jiangjin Central Hospital due to the first attack of AP between March 2020 and September 2021. We excluded these patients with pregnancy, malignancies, hematological system diseases, immune system diseases, severe organ dysfunction, chronic pancreatitis and in-hospital mortality within 48 hours after hospital stay in this study. This study was conducted according to the guidelines of the Declaration of Helsinki and received the approval of the Ethical Committee of Jiangjin Central Hospital (JJ2020017029). Written informed consents were obtained from all enrolled patients.
Definitions
AP was diagnosed based on the presence of two out of these 3 criteria at admission: ① abdominal pain conforming to AP, which had an acute onset and usually radiated to the back; ② at least a threefold increase of serum amylase and/or lipase levels compared with the upper normal limit; and ③ imaging evidence suggesting AP on abdomen computed tomography (CT) or ultrasound.12,13 The severity of AP was evaluated as described by the 2012 revision of the Atlanta classification,14 and the patients were allocated to control group (mild and moderately severe AP) and case group (SAP).
Data Collection
We collected demographic data, comorbidities, clinical characteristics and laboratory data. Laboratory tests were performed at admission. The BISAP score was computed at admission.
qRT-PCR Detection of miR-155
Fasting peripheral vein blood was collected and centrifuged at 3000 rpm/min for 10 min after stored for 30 min at 4℃. Total RNA was extracted from the obtained supernatant fluid using TRIzol kit (Invitrogen, Waltham, USA). Agarose gel electrophoresis and ultraviolet spectrophotometry were employed to detect its concentration, purity and integrality. The reverse transcription was performed with TransScript Green miRNA Two-Step qRTPCR SuperMix (AQ202-01, Beijing TransGen Biotech Company, China). The qRT-PCR amplified system was 20 μL, including 1 μL of cDNA, 0.8 μL of upstream and downstream primers (each 0.4 μL), 10 μL of 2×TransTaq® Tip Green qPCR SuperMix, 0.4 μL of Passive Reference Dye (50×) and 7.8 μL of ddH2O. The qRT-PCR amplification procedures were as follows: 30s of initial denaturation at 94℃, 5 s of degeneration at 94℃, and 30s of annealing and extending at 60℃, with a total of 40 cycles. The expression level of miR-155 was assessed through the 2–ΔΔCt method with U6 as an internal parameter.
Statistical Analysis
The SPSS version 18.0 (SPSS Inc., USA) was employed to conduct statistical analysis, and a two-sided P value of <0.05 was considered statistically significant. Continuous variables were evaluated for their normality using Kolmogorov–Smirnov test. Univariate analysis was conducted using Student’s t-test for the normally distributed variables, and using Mann–Whitney U-test for the non-normally distributed variables, and using Chi-square test for categorical variables. Multivariate analysis was conducted for the variables with two-sided P<0.10 in univariate analysis through binary logistic regression model to identify independent associated factors for progression to SAP among AP patients. The predictive values were evaluated using receiver operating characteristic (ROC) curve, and the area under curve (AUC) was compared using Z test.
Results
General Data
During the study period, a consecutive cohort of 1098 pancreatitis patients were enrolled. Among them, 4 patients were excluded due to pregnancy, 11 patients were excluded due to malignancies, 6 patients were excluded due to hematological system diseases, 8 patients were excluded due to immune system diseases, 5 patients were excluded due to severe organ dysfunction, 17 patients were excluded due to chronic pancreatitis, and 1 patient was excluded due to in-hospital mortality within 48 hours after hospital stay. Finally, a total of 1046 AP patients were included in this study. They included 621 (59.4%) males and 425 (40.6%) females with a mean age of (51.67 ± 11.92) years old and body mass index (BMI) of 25.80 ± 1.36. As for the etiology, biliary AP accounted for 25.0% (261 cases), hyperlipidemic 31.3% (327 cases), alcoholic 9.3% (97 cases) and undetermined 34.5% (361 cases). They were followed up for 9.9 ± 3.7 days. A total of 117 (11.2%) patients were evaluated as SAP, 89 (8.5%) developed organ failure, 140 (13.4%) were admitted to ICU, and 2 (0.2%) died.
Univariate Analysis
As shown in Table 1, there were significant differences in age, hypertension, ICU admission, hospital stay, Leukocytes, CRP, BUN, BISAP score and miR-155 between case group and control group (P < 0.05), and there were no significant differences in the rest variables (P > 0.05). But the P value of Fibrinogen was <0.10.
 |
Table 1 Results of Univariate Analysis Between Case Group and Control Group |
Multivariate Analysis
Age, hypertension, ICU admission, hospital stay, Leukocytes, CRP, BUN, BISAP score, miR-155 and Fibrinogen were included in the binary logistic regression model to identify independent associated factors for progression to SAP in AP patients. As shown in Table 2, the BISAP score, BUN, Leukocytes, age and CRP were independent risk factors for progression to SAP in AP patients after adjusting for hypertension, ICU admission, hospital stay and Fibrinogen, while miR-155 was a protective factor.
 |
Table 2 Results of Multivariate Analysis Between Case Group and Control Group |
Predictive Value
The ROC curves demonstrated that the predictive value of BISAP score for SAP in AP patients was high with the AUC of 0.842 (SE: 0.017, 95% CI: 0.809–0.874, Figure 1) and miR-155 was moderate with the AUC of 0.751 (SE: 0.022, 95% CI: 0.708–0.793, Figure 2). In order to further enhance the predictive value, the combination of BISAP score and miR-155 was employed to predict SAP in AP patients. The ROC curve demonstrated that the value of combination prediction was elevated with the AUC of 0.945 (SE: 0.007, 95% CI: 0.931–0.959, Figure 1). Z test showed that the AUC of combination prediction was significantly higher than that of individual predictions (0.945 vs 0.842, Z=5.602, P<0.001; 0.945 vs 0.751, Z=8.403, P<0.001). Table 3 shows the clinical utility indexes of the three methods for the prediction of SAP in AP patients.
 |
Table 3 Clinical Utility Indexes of the BISAP Score, miR-155 and Their Combination for the Prediction of SAP in AP Patients |
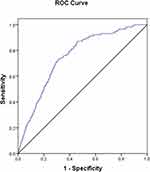 |
Figure 2 ROC curve of miR-155 for predicting SAP among AP patients. Abbreviations: ROC, receiver operating characteristic; SAP, severe acute pancreatitis; AP, acute pancreatitis. |
Discussion
There are multiple scoring systems that are available to predict the severity of AP, including computed tomography severity index (CTSI), modified CTSI (mCTSI), Acute Physiology and Chronic Health Evaluation (APACHE) II, Ranson criteria, etc.15–18 The pooled AUC for the prediction of mortality in AP was 0.91 (95% CI: 0.88~0.93) for the APACHE II score, 0.87 (95% CI: 0.81~0.92) for the Ranson score, 0.79 (95% CI: 0.73~0.86) for CTSI, and 0.80 (95% CI: 0.72~0.89) for mCTSI; and the AUC for the prediction of severity of AP were 0.80 (95% CI: 0.77~0.83) for APACHE II score, 0.81 (95% CI: 0.75~0.87) for Ranson score, 0.80 (95% CI: 0.76~0.85) for CTSI, and 0.83 (95% CI: 0.75~0.91) for mCTSI.18 Therefore, the APACHE II score is the most accurate prediction tool of mortality, and CTSI is a reliable prediction tool of both AP severity and mortality. However, these scoring systems are either complex or need data which are not routinely collected in the early stages of AP, which makes the early prediction of SAP difficult.13,19 Additionally, Kui et al developed a clinical prediction model of severity in AP in 2022, ie, the EASY prediction score. This score consists of indicators easily accessible on admission with an accuracy of 89.1% and a mean AUC score of 0.81 ± 0.033. But it still needs a lot of external validation.20
The BISAP scoring system, proposed by Wu et al in 2008,6 is a simple and effective prognostic scoring system for assessing the severity of AP in early stages. It improves the difficulties and drawbacks of the aforementioned scoring systems. The BISAP scoring system consists of the following variables, including age >60 years, impaired mental status, blood urea nitrogen level >25 mg/dl, and presence of pleural effusion and SIRS. The required data of the BISAP scoring system are easy to obtain at admission, and this scoring system can predict the in-hospital death in early stages of AP.19,21,22 SIRS, GCS and age are employed in both APACHE II and BISAP, but BISAP obtains a high predictive value for SAP and mortality with only addition of pleural effusion and BUN, which is equivalent to the complicated APACHE II. ROC analysis demonstrates that the BISAP score is correlated with SAP, more organ failure and higher mortality. But its cutoff remains controversial. Some researchers used ≥3 as cutoff, while others used ≥2.21–25 Kapadia et al showed that the BISAP score was very reliable for identifying AP patients at increased risk of severity with sensitivity of 100% and specificity of 94.62%.2 Valverde-López et al demonstrated that the AUC of the BISAP score for predicting SAP at admission was up to 0.90 (95% CI: 0.83–0.97).26 In our study, the AUC of the BISAP score for predicting SAP was 0.842, demonstrating a high predictive value. Its optimal cutoff was 3.02 with sensitivity of 89.7%, specificity of 88.8% and negative predictive value (NPV) of 98.6%.
As a multifunctional miRNA, miR-155 is regulated by multiple inflammatory mediators. The expression of miR-155 can be induced by TNF-α, IFN-β and bacterial lipopolysaccharide (LPS) in human monocyte cell strain. Notably, the imbalance of miR-155 expression is closely correlated with colorectal carcinoma, inflammatory intestinal disease and Helicobacter pylori-related gastropathy because of its involvement in the molecular changes of signal pathways and key targets.27–29 Liu et al demonstrated that down-regulated expression of miR-155 was significantly correlated with the severity of AP through mouse models of moderate/severe acute pancreatitis and mild acute pancreatitis (MAP), and miR-155 mediated the deterioration of pancreatic acinar cells via the Rela/Traf3/Ptgs2 signaling pathway through in vitro experiments.30 Hu et al reported that the expression of miR-155 in circulating blood was lower in AP patients than in healthy controls with an AUC of 0.775 for the prediction of AP.11 In addition, miR-155 has a significantly lower expression in severe and critical AP patients compared with mild and moderate AP patients, indicating a significant correlation with the progression of AP. In our study, the AUC of miR-155 for predicting SAP was 0.751, demonstrating a moderate predictive value. Its optimal cutoff was 1.58 with sensitivity of 81.2%, specificity of 79.9% and NPV of 97.1%.
In order to further elevate the predictive value for SAP, the combination of the BISAP score and miR-155 was employed to perform the prediction for SAP. The ROC curve showed that their combination had a higher AUC compared with individual predictions. The sensitivity, specificity and NPV were 95.7%, 93.6% and 99.4%, respectively. Thus, the combination of the BISAP score and miR-155 should be utilized to elevate the predictive value for the severity of AP in clinic.
In this study, the researchers responsible for collecting demographic data, comorbidities and clinical characteristics were trained uniformly before data collection to ensure the accuracy, and the laboratory indexes except for miR-155 are routine laboratory testing items. This study had some limitations, including a small sample size of the case group and no inclusion of all associated variables. Additionally, the miR-155 test is not accessible in smaller hospitals. In the next step, we will evaluate the predictive value of the BISAP score combined with routine laboratory test indexes for the severity of AP.
Conclusion
The BISAP score, miR-155, BUN, Leukocytes, age and CRP were independent associated factors for progression to SAP in AP patients. The combination of the BISAP score and miR-155 had a higher AUC compared with individual predictions, and thus their combination should be utilized to elevate the predictive value for the severity of AP in clinic.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zaheer A, Singh VK, Qureshi RO, et al. The revised Atlanta classification for acute pancreatitis: updates in imaging terminology and guidelines. Abdom Imaging. 2013;38(1):125–136. doi:10.1007/s00261-012-9908-0
2. Kapadia NN, Siddiqui E. Bedside index (BISAP) v/s Ranson scores in predicting mortality and severity in patients with acute pancreatitis. J Pak Med Assoc. 2021;71(8):1988–1991. doi:10.47391/JPMA.03-417
3. Hagjer S, Kumar N. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis – a prospective observational study. Int J Surg. 2018;54(Pt A):76–81. doi:10.1016/j.ijsu.2018.04.026
4. Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375(20):1972–1981. doi:10.1056/NEJMra1505202
5. Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386(9988):85–96. doi:10.1016/S0140-6736(14)60649-8
6. Wu BU, Johannes RS, Sun X, et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57(12):1698–1703. doi:10.1136/gut.2008.152702
7. Fabian MR, Sonenberg N. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol. 2012;19(6):586–593. doi:10.1038/nsmb.2296
8. Samir M, Vaas LA, Pessler F. MicroRNAs in the host response to viral infections of veterinary importance. Front Vet Sci. 2016;3:86. doi:10.3389/fvets.2016.00086
9. Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi:10.1016/j.addr.2015.05.001
10. Yang Y, Huang Q, Luo C, et al. MicroRNAs in acute pancreatitis: from pathogenesis to novel diagnosis and therapy. J Cell Physiol. 2020;235(3):1948–1961. doi:10.1002/jcp.29212
11. Hu L, Han D, Yu D, et al. Circulating blood miR-155 and miR-21 promote the development of acute pancreatitis and can be used to assess the risk stratification of pancreatitis. J Healthc Eng. 2021;2021:2064162. doi:10.1155/2021/2064162
12. Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400–1415. doi:10.1038/ajg.2013.218
13. Pezzilli R, Zerbi A, Di Carlo V, et al.; Working Group of the Italian Association for the Study of the Pancreas on Acute Pancreatitis. Practical guidelines for acute pancreatitis. Pancreatology. 2010;10(5):523–535. doi:10.1159/000314602
14. Banks PA, Bollen TL, Dervenis C, et al.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi:10.1136/gutjnl-2012-302779
15. Balthazar EJ, Ranson JH, Naidich DP, et al. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156(3):767–772. doi:10.1148/radiology.156.3.4023241
16. Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi:10.1097/00003246-198510000-00009
17. Ranson JH, Pasternack BS. Statistical methods for quantifying the severity of clinical acute pancreatitis. J Surg Res. 1977;22(2):79–91. doi:10.1016/0022-4804(77)90045-2
18. Mikó A, Vigh É, Mátrai P, et al. Computed tomography severity index vs. other indices in the prediction of severity and mortality in acute pancreatitis: a predictive accuracy meta-analysis. Front Physiol. 2019;10:1002. doi:10.3389/fphys.2019.01002
19. Cho YS, Kim HK, Jang EC, et al. Usefulness of the Bedside Index for severity in acute pancreatitis in the early prediction of severity and mortality in acute pancreatitis. Pancreas. 2013;42(3):483–487. doi:10.1097/MPA.0b013e318267c879
20. Kui B, Pintér J, Molontay R, et al.; Hungarian Pancreatic Study Group. EASY-APP: an artificial intelligence model and application for early and easy prediction of severity in acute pancreatitis. Clin Transl Med. 2022;12(6):e842. doi:10.1002/ctm2.842
21. Park JY, Jeon TJ, Ha TH, et al. Bedside index for severity in acute pancreatitis: comparison with other scoring systems in predicting severity and organ failure. Hepatobiliary Pancreat Dis Int. 2013;12(6):645–650. doi:10.1016/s1499-3872(13)60101-0
22. Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol. 2009;104(4):966–971. doi:10.1038/ajg.2009.28
23. Zhang J, Shahbaz M, Fang R, et al. Comparison of the BISAP scores for predicting the severity of acute pancreatitis in Chinese patients according to the latest Atlanta classification. J Hepatobiliary Pancreat Sci. 2014;21(9):689–694. doi:10.1002/jhbp.118
24. Papachristou GI, Muddana V, Yadav D, et al. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105(2):435–441; quiz 442. doi:10.1038/ajg.2009.622
25. Khanna AK, Meher S, Prakash S, et al. Comparison of Ranson, Glasgow, MOSS, SIRS,BISAP,APACHE-II,CTSI Scores,IL-6, CRP, and procalcitonin in predicting severity, organ failure, pancreatic necrosis, and mortality in acute pancreatitis. HPB Surg. 2013;2013:367581. doi:10.1155/2013/367581
26. Valverde-López F, Matas-Cobos AM, Alegría-Motte C, et al. BISAP, RANSON, lactate and others biomarkers in prediction of severe acute pancreatitis in a European cohort. J Gastroenterol Hepatol. 2017;32(9):1649–1656. doi:10.1111/jgh.13763
27. Vigorito E, Kohlhaas S, Lu D, et al. miR-155: an ancient regulator of the immune system. Immunol Rev. 2013;253(1):146–157. doi:10.1111/imr.12057
28. Faraoni I, Antonetti FR, Cardone J, et al. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta. 2009;1792(6):497–505. doi:10.1016/j.bbadis.2009.02.013
29. Wan J, Xia L, Xu W, et al. Expression and function of miR-155 in diseases of the gastrointestinal tract. Int J Mol Sci. 2016;17(5):709. doi:10.3390/ijms17050709
30. Liu S, Zou H, Wang Y, et al. miR-155-5p is negatively associated with acute pancreatitis and inversely regulates pancreatic acinar cell progression by targeting rela and Traf3. Cell Physiol Biochem. 2018;51(4):1584–1599. doi:10.1159/000495648
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

