Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Combination of tadalafil and finasteride for improving the symptoms of benign prostatic hyperplasia: critical appraisal and patient focus
Authors Elkelany O, Owen R, Kim E
Received 5 January 2015
Accepted for publication 17 February 2015
Published 30 March 2015 Volume 2015:11 Pages 507—513
DOI https://doi.org/10.2147/TCRM.S80353
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Garry Walsh
Osama O Elkelany, Ryan C Owen, Edward D Kim
Department of Surgery, Division of Urology, University of Tennessee Graduate School of Medicine, Knoxville, TN, USA
Abstract: The evidence suggests that combination therapy for benign prostatic hyperplasia (BPH)-lower urinary tract symptoms (LUTS) using an α-blocker and a 5α-reductase inhibitor has become well accepted. The combination of daily tadalafil and an α-blocker has also demonstrated benefit. This paper addresses combination therapy with daily tadalafil and finasteride for the treatment of BPH-LUTS. Our results demonstrate that use of tadalafil and finasteride represents a logical extension of combination therapies. We analyze a landmark study by Casabé et al that demonstrates improved voiding symptoms as assessed by International Prostate Symptom Scores with a combination of tadalafil and finasteride compared with finasteride and placebo. Study patients had moderate to severe LUTS and prostate volumes >30 g. The additional benefit of improved erectile function as assessed by International Index of Erectile Function-erectile function domain scores with the addition of tadalafil was a secondary benefit. We propose that the ideal patient for combination therapy with tadalafil and finasteride has a prostate volume >30 g and desires additional benefit over monotherapy. For these men, improved erectile function without sexual side effects was a secondary benefit.
Keywords: benign prostatic hyperplasia, lower urinary tract symptoms, tadalafil, finasteride
Introduction
The prevalence of benign prostatic hyperplasia (BPH)-lower urinary tract symptoms (LUTS) is currently reported as 10%–25% for the male population and is estimated to rise to 1.1 billion affected males by the year 2018.1–4 The treatment of BPH, also commonly referred to as LUTS, has evolved considerably over the last 25 years. Prior to the early 1990s, symptomatic BPH was commonly treated with surgery, most notably transurethral prostatectomy. In the early 1990s, as a result of medical therapy using non-selective α-blockers, use of transurethral prostatectomy declined significantly. Around this time, 5α-reductase inhibitor therapy with finasteride was introduced, but initially struggled to find its ideal target patient. Combination therapies with α-blockers and 5α-reductase inhibitors have evolved, and have now become a safe and effective treatment for men with larger prostate volumes.5 Tadalafil was approved for daily use in the treatment of BPH in October 2011 and represents a novel mechanism of action for treatment of the signs and symptoms of BPH.6 As a natural progression, tadalafil has been used in combination with α-blockers and 5α-reductase inhibitors. This article reviews recent clinical data and makes recommendations as to ideal patients for therapy.
Monotherapies for LUTS
Alpha-blockers
Alpha-blockers (α1-adrenoreceptor antagonists) improve urinary symptoms by inhibiting the effects of α-adrenoreceptors on the prostate. There are two types of α-receptors, ie, α1 and α2, and three subtypes of α1-receptor, ie, 1a, 1b, and 1d. The α1a-receptors are the main receptors located in stromal smooth muscle cells and represent the main target for medical therapy.7
The earliest α-blocker used for the treatment of LUTS associated with BPH was phenoxybenzamine, a non-selective agent that inhibits both α1a-receptors and α1b-receptors. Alfuzosin, doxazosin, terazosin, tamsulosin, and silodosin are currently the most common α-blockers in use and are selective for the α1-receptor. Tamsulosin and silodosin are selective for the α1a-receptor.7 Alpha-blockers are similar in efficacy, as assessed by improvements in symptom scores and maximum urinary flow rate (Qmax).8 Several examples of key studies are presented below.
In a 12-week study conducted by van Kerrebroeck et al 447 patients were randomized to one of three treatments, ie, placebo, alfuzosin 2.5 mg three times daily, or alfuzosin 10 mg daily.9 At the end of the study, symptom scores (American Urological Association [AUA]/International Prostate Symptom Score [I-PSS]) had improved significantly (P=0.005) by −4.9, −6.4, and −6.9 points, respectively. Qmax increased significantly by 1.4 and 3.2 (P<0.0001), and 2.3 (P=0.03) mL/sec, respectively. A 4.5-year study by McConnell et al focused on the effects of doxazosin compared with placebo.5 Men were randomized to doxazosin 4–8 mg versus placebo. Symptom scores improved for the doxazosin 4–8 mg group by −6.6 points versus −4.9 points for the placebo group (P<0.001). In addition, Qmax increased by 1.4 and 2.5 mL/sec, respectively (P<0.001). Chapple et al performed a 12-week study of treatment with a daily dose of tamsulosin 0.4 mg as compared with placebo.10 Tamsulosin improved symptom scores by −3.3 points while men on placebo improved by −2.4 points (P=0.002). Qmax increased by 1.6 mL/sec for tamsulosin and by 0.6 mL/sec for placebo (P=0.002).
5α-reductase inhibitors
Finasteride and dutasteride are the two 5α-reductase inhibitors available to treat BPH-LUTS. Finasteride inhibits the type II isoenzyme, which has a much higher activity level in the prostate than the type I isoform. Dutasteride inhibits both type I and II isoenzymes. The 5α-reductase enzyme converts testosterone into dihydrotestosterone in the stromal cells of the prostate, thereby mediating the effects of androgens on the prostate. Finasteride has a half-life of 6–8 hours, while dutasteride has a half-life of 5 weeks.7
According to the AUA guidelines, 5α-reductase inhibitors should be used with prostate volumes larger than 30–40 mL.7 In PLESS (the Finasteride Long-Term Efficacy and Safety Study), McConnell et al reported that after 234 weeks, finasteride improved the AUA symptom score by −3.3 points as compared with an improvement of −1.3 points in the placebo group (P<0.001).11 With regard to prostate volume, finasteride decreased the size of the prostate by 19%, while the prostate size increased by 24% while on placebo (P<0.001). These findings correlated with a significant reduction in the need for surgery and development of acute urinary retention. Using dutasteride, Roehrborn et al identified a decrease in AUA symptom score of 4.5 points versus 2.3 points in the placebo group (P<0.001).12 Prostate volumes decreased by 25.7% in the dutasteride group and increased by 1.5% in the placebo group (P<0.001). When comparing α-blockers (tamsulosin 0.4 mg daily) and 5α-reductase inhibitors (dutasteride 0.5 mg daily), Roehrborn et al reported a decrease in AUA symptom score of 3.8 points and 5.3 points (P<0.001), respectively.13 However, dutasteride decreased prostate volumes by 28% while tamsulosin resulted in a 4.6% increase in prostate volumes.
Phosphodiesterase-5 inhibitors: tadalafil daily
Nitric oxide (NO) is a non-adrenergic neurotransmitter that converts guanosine triphosphate into cyclic guanosine monophosphate (cGMP).14 cGMP initiates calcium efflux from smooth muscle cells, with resulting relaxation in cavernosal and arterial smooth muscle tone. Type 5 phosphodiesterase (PDE5) converts cGMP to 5-GMP, thereby resulting in smooth muscle contraction and degradation of the erection. Inhibition of PDE5 effectively maintains cGMP activity in the penis, resulting in improved erections. The PDE5 isoenzyme is highly prevalent in the penis, as well as in the prostate, bladder, and urethra, and their supporting vasculature.
In a 2013 review, Giuliano et al reported that lower urinary tract tissue under various types of neural control exhibits relaxation when affected by PDE5 inhibition.14 Briefly, urothelial and endothelial smooth muscle cells receive signals from the NO/cGMP cascade. This neural system is modulated by PDE5 isoenzymes, which have been isolated within the lower urinary tract.15 Disease states such as detrusor overactivity, benign prostatic enlargement, and altered pelvic vascular supply contribute to the development of LUTS. Underlying mechanisms include decreased pelvic NO synthase, pelvic atherosclerosis, tissue ischemia, local inflammation, and altered androgen activity within the lower urinary tract.16,17 Thus, it has been proposed that PDE5 inhibition allows smooth muscle cell relaxation and increased blood flow to the pelvic tissue, which provides symptomatic relief. The benefit from daily tadalafil may be the result of sustained smooth muscle relaxation at the bladder neck, prostate, and urethra, and their supporting vascular supply.
In a Phase III clinical trial, Porst et al compared tadalafil 5 mg with placebo for 12 weeks.18 From a mean baseline score of 17.1 points for tadalafil and 16.6 points for placebo, I-PSS scores decreased by 5.6 points for tadalafil compared with 3.6 points for placebo (P=0.004). This improvement was statistically significant and clinically meaningful. Regarding improvements in urinary flow, Roehrborn et al compared tadalafil 5 mg daily versus placebo.19 Starting from a mean baseline value of 10.7 and 10.5 mL/sec, respectively, mean endpoint Qmax values were 11.9 and 12.3 mL/sec. While not significantly different from placebo, their findings hinted that the difference in Qmax for tadalafil increases with high baseline voiding volumes. In a subsequent double-blind, randomized, placebo-controlled trial comparing tadalafil with tamsulosin, Oelke et al assessed men older than 45 years with an I-PSS score >13 and a Qmax between 4 and 15 mL/sec.20 A key finding of the study was that Qmax increased significantly versus placebo for both tadalafil (2.4 mL/sec; P=0.009) and tamsulosin (2.2 mL/sec; P=0.014). They reported an improvement in I-PSS score of −2.1 (P=0.001) for tadalafil compared with −1.5 (P=0.23) for tamsulosin, with results seen as early as 1 week. The authors also noted that tadalafil has the ability to improve erectile function (change in International Index of Erectile Function [IIEF] of 4.0, P<0.001 versus −0.4, P=0.699) when comparing tadalafil with tamsulosin, whereas other BPH therapies have the potential for sexual side effects, including erectile dysfunction and ejaculatory dysfunction. The AUA guidelines have recognized that there is a poor correlation between symptom scores and Qmax (see Table 1).7
Combination medical therapies: mechanism of action and key studies
Alpha-blockers and 5α-reductase inhibitors
The use of combination therapy with finasteride and α-blockers represented a breakthrough for the treatment of men who were refractory to monotherapy or were at risk for BPH progression. The first major study of combination therapy was the randomized, placebo-controlled, 4-year MTOPS (Medical Therapy of Prostatic Symptoms) trial5 that investigated whether a combination of both doxazosin and finasteride had a greater effect than monotherapy alone. Over 3,000 patients were randomized to daily placebo, doxazosin 1–8 mg, finasteride 5 mg, or a combination of doxazosin and finasteride. Symptom scores decreased by 4.0, 6.0 (P<0.001), 5.0 (P=0.001), and 7.0 (P<0.001) points, respectively (see Table 2). Qmax increased by 1.4, 2.5 (P<0.001), 2.2 (P<0.001), and 3.7 (P<0.001) mL/sec, respectively. Prostate volumes increased by 24% in the placebo and doxazosin groups and decreased by 19% in the finasteride and combination therapy groups. In this study, both finasteride and combination therapy resulted in a significant decrease in the risk of acute urinary retention and the risk of surgical therapy related to prostatic obstruction. In contrast, doxazosin did not decrease these endpoint parameters. The importance of this paper is that it demonstrates that combination therapy can work synergistically to delay or prevent the progression of BPH.
  | Table 2 Comparison of year 4 treatment results for MTOPS study |
The second major study was CombAT (Combination of Avodart® and Tamsulosin).14 Nearly 5,000 patients were randomized to daily tamsulosin 0.4 mg, dutasteride 0.5 mg, or combination therapy. After 2 years, I-PSS scores decreased by 4.3, 4.9, and 6.2 (P<0.001) points, respectively. Qmax increased by 0.9, 1.9 (P<0.006), and 2.4 mL/sec (P<0.001), respectively. Prostate volumes did not change for the tamsulosin group and decreased by 28% and 26.9% for the dutasteride group and combination group, respectively.
Both MTOPS and CombAT demonstrated that combination therapy with an α-blocker and a 5α-reductase inhibitor was more effective than monotherapy in improving symptom scores as well as decreasing prostate volumes. The differences between the results of the two studies were likely due to the different criteria required for the inclusion or exclusion of patients and not the different types of treatment.8 These studies established and validated the concept of combination medical therapy for BPH-LUTS.
Tadalafil and α-blockers
Combination therapy with tadalafil and α-blockers reduces smooth muscle tone in the prostate and in cavernosal smooth muscle tissue.21 Bechara et al conducted a 6-week study in which they compared the effects of monotherapy with tamsulosin 0.4 mg once daily versus tamsulosin 0.4 mg daily combined with tadalafil 20 mg once daily.22 In this small study, 15 patients were on monotherapy while another 15 were on the combination therapy. At the end of the study, symptom scores decreased by 6.7 and 9.2 points (P<0.05), respectively. Qmax increased by 2.1 and 3.0 mL/sec (P<0.001), respectively. This study suggested that combination therapy including high-dose tadalafil has additive value compared with tamsulosin alone.
Singh et al conducted a similar study comparing the results of treatment with tamsulosin or tadalafil monotherapies against tamsulosin/tadalafil combination therapy.23 Endpoint I-PSS scores decreased by 10.7, 6.8, and 11.7 points for the tamsulosin group, tadalafil group, and combination group, respectively; further, Qmax increased by 3.1, 2.6, and 3.7 mL/sec. Another tamsulosin/tadalafil combination study done by Regadas et al also reported decreases in I-PSS scores as well as increases in Qmax.24
Tadalafil and 5α-reductase inhibitors: a critical appraisal
Casabé et al recently published their findings for combination therapy with tadalafil and finasteride.25 In their randomized, double-blind study, men with I-PSS scores ≥13 and prostate volumes ≥30 mL were randomized to two groups for a 26-week study. Three hundred and fifty patients were on finasteride and placebo therapy, while another 345 patients were administered combination therapy of tadalafil and finasteride. The study determined improvement in BPH-LUTS, as assessed by the I-PSS, as well as improved erectile function, as assessed by the International Index of Erectile Function-erectile function domain (IIEF-EF) for sexually active men. The primary objective was to determine whether combination therapy for 12 weeks was superior to placebo/finasteride in improving I-PSS scores.
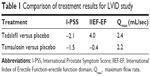
I-PSS scores at 4, 12, and 26 weeks decreased by an average of 2.3, 3.8, and 4.5 points for the placebo group and by 4.0 (P<0.001), 5.2 (P=0.001), and 5.5 (P=0.022) points for the combination group (see Figure 1). These differences in I-PSS score improvements proved to be significantly better for the combination therapy group. Improvements in I-PSS score were seen as early as 4 weeks (the first planned checkpoint), although symptomatic relief from tadalafil therapy may be recognized as early as the first week.25,26 These data provide an insight into the onset of action, which can be used for clinical counseling. Combination therapy with finasteride and tadalafil will provide more timely relief of voiding symptoms than finasteride alone.
  | Figure 1 Comparison of treatment results with regard to I-PSS scores reported by Casabé et al.25 |
In addition, combination therapy with tadalafil improved erectile function, as reported in a secondary analysis from the study by Casabé et al.25 IIEF-EF changed by an average of −1.1, 0.6, and −0.0 for the placebo group at 4, 12, and 26 weeks, respectively. In the tadalafil/finasteride group, the average changes were 3.7 (P<0.001), 4.7 (P<0.001), and 4.7 (P<0.001), respectively. Notably, at 4 weeks, patients in the finasteride/placebo group demonstrated a decrease in erectile function, with a least square mean change of −1.0, while men in the combination arm recognized an improvement in IIEF-EF score of 3.7 for a total of 4.9 least square mean change. This difference in erectile function remained essentially the same throughout the duration of the trial, indicating the significant benefit of tadalafil. Further, when the groups were stratified according to baseline erectile function prior to entering the study, Glina et al reported that men without baseline erectile dysfunction recognized improvement, as documented by IIEF score (see Figure 2).27 Interestingly, men without prior erectile dysfunction in the combination therapy group demonstrated improvement in sexual desire, erectile function, orgasmic function, and overall satisfaction with intercourse. These unexpected improvements are suspected to have occurred as a result of engaging the NO cascade, which improves blood flow and the neural response to male genitalia, therefore expanding the ability for sexual stimulation, ejaculation, and orgasm. A caveat is described at the conclusion of the article, ie, that the secondary analysis was not designed to detect changes in IIEF scores, so conclusions regarding improvement in these scores should be interpreted with caution. However, each study indicates that combination therapy with tadalafil improves erectile function.
  | Figure 2 Comparison of treatment results with regard to IIEF scores reported by Glina et al.27 |
Differences in side effects when comparing combination therapy with finasteride monotherapy were largely attributed to adverse effects from tadalafil. Casabé et al reported back pain, headache, dyspepsia, and flu-like symptoms in 1.7%, 3.4%, 0.6%, and 2.3% of men on finasteride/placebo versus 4.6%, 3.5%, 2.3%, and 2.3% of men on combination therapy.25 In a comparison of sexual-related adverse events, men in the placebo group were found to have erectile dysfunction, delayed ejaculation, ejaculatory failure, decreased libido, Peyronie’s disease, and decreased semen volume at a rate of 1.1%, −0.3%, −0.3%, −1.4%, −0.3%, and −0.3%, respectively, when compared with the combination therapy group. Importantly, tadalafil is contraindicated in men taking nitrates for cardiac disease and in men who have a history of serious hypersensitivity reactions to tadalafil.6 Also, combination therapy is not indicated for patients with primary complaints of nocturia.25
Defining the patient for combination therapy with tadalafil/finasteride
Based on the mechanisms of action and the study by Casabé et al,25 combination therapy with daily tadalafil and finasteride is ideally suited for men with moderate to severe BPH-LUTS and a prostate volume >30 g. In addition, men with coexisting erectile dysfunction will benefit from PDE5 inhibition. In this group of men, finasteride results in a reduced prostate volume, while tadalafil mediates lower urinary tract smooth muscle relaxation via PDE5 inhibition. This dual mechanism of action results in additive clinical improvements when compared with finasteride alone. The safety profile is consistent with that seen with daily tadalafil therapy.
Scenario 1: suboptimal response to 5α-reductase inhibitor therapy with prostatic enlargement
Based on the study by Casabé et al,25 this patient represents an ideal candidate for combination therapy. He has moderate to severe urinary symptoms, a prostate volume >30 g, and a suboptimal clinical improvement with 5α-reductase inhibitor therapy. He wants to avoid surgical intervention and the potential sexual side effects of α-blocker therapy. If he has pre-existing erectile dysfunction, then he may have improved erections with daily tadalafil.
Scenario 2: suboptimal response to 5α-reductase inhibitor and α-blocker combination therapy
Although the combination of tadalafil and finasteride has not been well studied in men who have failed on a 5α-reductase inhibitor and α-blocker combination, it is reasonable to recommend this therapy from an efficacy standpoint. From a side effect standpoint, this patient may avoid the adverse events of α-blockers, such as retrograde ejaculation and hypotension. When compared with the combination of finasteride and an α-blocker, tadalafil offers a lower risk of retrograde ejaculation. Although not mentioned in the side effect profile for tadalafil,6 Casabé et al,25 reported decreased semen volume in one of 306 patients in the finasteride/tadalafil arm. This is a significant difference when compared with the reported rates of retrograde ejaculation for commonly used α-blockers, such as silodosin (28%) or tamsulosin (8%–18%).28,29
Scenario 3: suboptimal response to tadalafil and α-blocker
As in scenario 2, it is reasonable to recommend tadalafil and a 5α-reductase inhibitor from an efficacy standpoint. If the patient has a prostate volume >30 g, he may obtain greater benefit from administration of finasteride compared to administration of an α-blocker. Further, he may avoid α-blocker-related side effects, as mentioned in the previous scenario.
Scenario 4: 5α-reductase inhibitor responder but with erectile dysfunction
In addition to improvement in BPH-LUTS, combination therapy with tadalafil may improve or maintain erectile function in men on finasteride monotherapy. When combined with finasteride, which may have sexual side effects, tadalafil provides an alternative for men wanting to improve their erectile function. With the addition of tadalafil, IIEF-EF scores improved significantly.25
Scenario 5: treatment-naïve patient with larger prostate and moderate to severe LUTS
In males with BPH-LUTS as a result of prostatic enlargement, 5α-reductase inhibitor therapy prevents progression of BPH, promotes a reduction in prostate size, reduces the risk of acute urinary retention, and avoids the need for subsequent surgery.7 Further, men with a larger baseline prostatic volume recognize a more pronounced symptomatic benefit than men with smaller prostate volumes.8 Although effective, treatment with 5α-reductase inhibitors can take 6–12 months before clinical effects are realized.8 Addition of an agent such as tadalafil or an α-blocker that has a more rapid onset of action may provide more timely relief of symptoms. A discussion with the patient to elicit symptoms of erectile dysfunction can aid in the decision as to which rapid-acting agent should be initiated.
Clinical recommendation
Combination therapy with daily tadalafil and finasteride is ideally suited for men with moderate to severe BPH-LUTS and a prostate volume >30 g. In addition, men with coexisting erectile dysfunction will benefit from PDE5 inhibition.
Disclosure
The authors report no conflicts of interest in this work.
References
Verhamme KM, Dieleman JP, Bleumink GS, et al; for Triumph Pan European Expert Panel. Incidence and prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in primary care – the Triumph project. Eur Urol. 2002;42(4):323–328. | ||
Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306–1314. | ||
Coyne KS, Sexton CC, Thompson CL, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009;104(3):352–360. | ||
Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138. | ||
McConnell JD, Roehrborn CG, Bautista OM, et al; for Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387–2398. | ||
Cialis® (tadalafil) [package insert]. Indianapolis, IN, USA: Eli Lilly and Company; 2011. | ||
McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185(5):1793–1803. | ||
Oelke M, Bachmann A, Descazeaud A, et al. Guidelines on the management of male lower urinary tract symptoms (LUTS), including benign prostatic obstruction (BPO). Eur Urol. 2013;64(1):118–140. | ||
van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol. 2000;37(3):306–313. | ||
Chapple CR, Wyndaele JJ, Nordling J, Boeminghaus F, Ypma AF, Abrams P. Tamsulosin, the first prostate-selective alpha 1A-adrenoceptor antagonist. A meta-analysis of two randomized, placebo-controlled, multicenter studies in patients with benign prostatic obstruction (symptomatic BPH). European Tamsulosin Study Group. Eur Urol. 1996;29(2):155–167. | ||
McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338(9):557–563. | ||
Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G; for ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60(3):434–441. | ||
Roehrborn CG, Siami P, Barkin J, et al; for CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57(1):123–131. | ||
Giuliano F, Ückert S, Maggi M, Birder L, Kissel J, Viktrup L. The mechanism of action of phosphodiesterase type 5 inhibitors in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Eur Urol. 2013;63(3):506–516. | ||
Morelli A, Sarchielli E, Comeglio P, et al. Phosphodiesterase type 5 expression in human and rat lower urinary tract tissues and the effect of tadalafil on prostate gland oxygenation in spontaneously hypertensive rats. J Sex Med. 2011;8(10):2746–2760. | ||
Andersson KE, de Groat WC, McVary KT, et al. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: pathophysiology and mechanism(s) of action. Neurourol Urodyn. 2011;30(3):292–301. | ||
Gacci M, Eardley I, Giuliano F, et al. Critical analysis of the relation- ship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. 2011;60(4):809–825. | ||
Porst H, Kim ED, Casabé AR, et al; for LVHJ Study Team. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of an international randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60(5):1105–1113. | ||
Roehrborn CG, Chapple C, Oelke M, Cox D, Esler A, Viktrup L. Effects of tadalafil once daily on maximum urinary flow rate in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. J Urol. 2014;191(4):1045–1050. | ||
Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomized, parallel, placebo-controlled clinical trial. Eur Urol. 2012;61(5):917–925. | ||
McNicholas TA, Kirby RS, Lepor H. Evaluation and nonsurgical management of benign prostatic hyperplasia. In: Wein AJ, editor. Campbell-Walsh Urology. Philadelphia, PA, USA: Saunders Elsevier; 2011. | ||
Bechara A, Romano S, Casabé A, et al. Comparative efficacy assessment of tamsulosin vs tamsulosin plus tadalafil in the treatment of LUTS/BPH. Pilot study. J Sex Med. 2008;5(9):2170–2178. | ||
Singh DV, Mete UK, Mandal AK, Singh SK. A comparative randomized prospective study to evaluate efficacy and safety of combination of tamsulosin and tadalafil vs. tamsulosin or tadalafil alone in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. J Sex Med. 2014;11(1):187–196. | ||
Regadas RP, Reges R, Cerqueira JB, et al. Urodynamic effects of the combination of tamsulosin and daily tadalafil in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: a randomized, placebo-controlled clinical trial. Int Urol Nephrol. 2013;45(1):39–43. | ||
Casabé A, Roehrborn CG, Da Pozzo LF, et al. Efficacy and safety of the coadministration of tadalafil once daily with finasteride for 6 months in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia. J Urol. 2014;191(3):727–733. | ||
Egerdie RB, Auerbach S, Roehrborn CG, et al. Tadalafil 2.5 or 5 mg administered once daily for 12 weeks in men with both erectile dysfunction and signs and symptoms of benign prostatic hyperplasia: results of a randomized, placebo-controlled, double-blind study. J Sex Med. 2012;9(1):271–281. | ||
Glina S, Roehrborn CG, Esen A, et al. Sexual function in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia: results of a 6-month, randomized, double-blind, placebo-controlled study of tadalafil coadministered with finasteride. J Sex Med. 2015;12(1):129–138. | ||
Rapaflo® (silodosin) [package insert]. Parsippany, NJ, USA: Watson Pharma, Inc; 2013. | ||
Flomax® (tamsulosin) [package insert]. Brunswick, NJ, USA: Citron Pharma LLC; 2014. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.