Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
Combination of sofosbuvir and daclatasvir in the treatment of genotype 3 chronic hepatitis C virus infection in patients on maintenance hemodialysis
Authors Sperl J , Frankova S, Kreidlova M , Merta D, Tothova M, Spicak J
Received 5 February 2017
Accepted for publication 5 May 2017
Published 22 June 2017 Volume 2017:13 Pages 733—738
DOI https://doi.org/10.2147/TCRM.S133983
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Jan Sperl,1 Sona Frankova,1 Miluse Kreidlova,2 Dusan Merta,3 Monika Tothova,4 Julius Spicak1
1Department of Hepatogastroenterology, Institute for Clinical and Experimental Medicine, 2Institute of Medical Biochemistry and Laboratory Medicine, General University Hospital, Charles University, 3Department of Anesthesiology, Resuscitation and Intensive Care, Institute for Clinical and Experimental Medicine, 4Dialysis Center Motol, Fresenius Medical Care, Prague, Czech Republic
Abstract: Chronic hepatitis C virus infection (HCV) has a negative impact on the long-term survival of recipients of kidney transplants. HCV should be treated in hemodialyzed patients before their enlistment for kidney transplantation in order to avoid the reactivation of virus after transplantation. Direct-acting antivirals represent the current standard of care in hemodialyzed patients with HCV genotypes 1 and 4; in patients with genotypes 2 or 3, the optimal regimen is yet to be established. Sofosbuvir (SOF) and daclatasvir (DCV) represent an antiviral pangenotypic regimen with favorable pharmacokinetics in hemodialyzed patients. We retrospectively evaluated safety and efficacy of the combination of SOF and DCV in the treatment of genotype 3a chronic HCV in six male patients (mean age of 39 years, range 25–53 years) with end-stage renal disease on maintenance hemodialysis; these patients were treated with a reduced dose of SOF (one half of a 400 mg tablet) and 60 mg of DCV once daily. The anticipated treatment duration was 12 weeks. Initial HCV RNA ranged from 120,000 to 11,000,000 IU/mL. Two of the six patients had compensated liver cirrhosis based on shear-wave elastography result. All of the patients completed a 12-week treatment. Viremia became negative on treatment and remained negative 12 weeks after the end of therapy in all the patients. All of them (6/6, 100%) achieved sustained virological response, including two with cirrhosis and two with HCV RNA >6,000,000 IU/mL. The treatment was well tolerated: none of the patients presented with a serious adverse event requiring hospital admission and none had anemia or any significant changes in blood count. One patient had a short period of diarrhea, which was resolved with antibiotic treatment. The combination of reduced-dose SOF and full-dose DCV, daily, was a safe and effective treatment in our group of hemodialyzed patients infected with HCV genotype 3.
Keywords: HCV infection, genotype 3, sofosbuvir, daclatasvir, end-stage renal disease, maintenance hemodialysis
Introduction
Only a minority of hemodialyzed patients infected with hepatitis C virus (HCV) received antiviral treatment during the interferon-based treatment era owing to the risk of adverse events and poor tolerability.1 Hemodialyzed patients experienced severe adverse effects, especially those related to the cardiovascular system, in addition to adverse effects classically associated with interferon-α in patients with normal kidney function. Ribavirin (RBV) increased the severity of side effects, especially severe anemia, even administered in a reduced dose. Substitution of interferon-free combinations for interferon-based regimens in hemodialyzed patients was of high priority when the first direct-acting antivirals (DAAs) combinations became available. Therefore, since 2014, DAAs have represented the standard of care in the treatment of HCV infection in patients on maintenance hemodialysis.2
The initial development of DAAs was focused on genotype 1, and the majority of the then available DAA regimens were only effective in patients infected with genotypes 1 and 4. Nowadays, there are DAAs approved for the treatment of patients on maintenance hemodialysis infected with HCV genotypes 1 and 4, in whom safety and efficacy was confirmed in prospective trials (paritaprevir/ritonavir, ombitasvir and dasabuvir, or grazoprevir and elbasvir combinations).3,4
In spite of the fact that there are no available prospective data on the safety and efficacy of DAA regimens in patients on maintenance hemodialysis infected with HCV genotype 3, the antiviral treatment should not be postponed in this group of patients. There are two important facts supporting the need for priority treatment: first, the HCV-infected patients on maintenance hemodialysis have higher mortality compared with noninfected patients,1,5–9 and second, the virus reactivation under immunosuppressive drugs has a negative impact on survival of these patients after kidney transplantation.10
The best way to prevent HCV reactivation after kidney transplantation is successful treatment before enlistment for transplantation, which means during the period of maintenance hemodialysis.10–13 The selection of the antiviral regimen in patients infected with HCV genotype 3 should be based on the data concerning the efficacy of the particular drug in genotype 3 and on the available pharmacokinetics in patients with kidney failure.
Sofosbuvir (SOF) was the first DAA with pangenotypic activity approved in 2013, and therefore, SOF-based regimens are also effective in patients infected with genotype 3.14 Furthermore, the efficacy, safety, and pharmacokinetics of SOF were assessed in patients on maintenance hemodialysis predominantly infected with genotype 1 in small postapproval studies.15–22 However, a group of hemodialyzed patients infected with genotype 3 has not been evaluated separately so far. We retrospectively evaluated the efficacy and safety of antiviral regimen consisting of a reduced dose of SOF in combination with daclatasvir (DCV) in six hemodialyzed patients infected with HCV genotype 3a.
Patients and methods
Study design and eligibility of patients
We retrospectively evaluated six kidney transplant candidates with end-stage renal disease (ESRD) on maintenance hemodialysis, treated for chronic HCV infection genotype 3 in our outpatient department from April 2015 to April 2016. The cohort consisted of six males with average age of 39 years (range 25–53 years). All patients were hemodialyzed 3 times per week and the mean duration time of hemodialysis was 41 months (range 13–84 months). None of the patients had undergone kidney transplant in the past. All patients were Caucasian, five of them were HCV treatment-naïve and one had undergone a short course (4 weeks) of pegylated interferon-α (PEG-IFN-α) and RBV therapy which had been discontinued prematurely owing to severe anemia. All patients were infected with HCV genotype 3a. The initial viremia (HCV RNA) ranged from 120,000 to 11,000,000 IU/mL (mean 4,088,000 IU/mL). One of the patients had normal serum alanine aminotransferase (ALT) activity, and in the other five patients, all with abnormal ALT activity, the values did not exceed three times the upper limit of normal values. All of them had entered renal replacement therapy with known HCV infection, they had all reported a transient period of intravenous drug use in the past as the source of infection, five had been successfully weaned, and one was on methadone substitution. All six had excellent compliance with the hemodialysis program and with the antiviral therapy.
Pretreatment stage of liver fibrosis was evaluated by shear-wave elastography (Aixplorer®; SuperSonic Imagine, Aix-en-Provence, France); the values corresponded to fibrosis stage F1 in four patients and two patients had fibrosis F4 (cirrhosis) corresponding to Metavir score. Liver fibrosis stage (F1–F4) was derived from the liver stiffness values in kPa obtained by shear-wave elastography on the basis of the table provided by the device manufacturer.23 Both patients with fibrosis F4 had compensated liver disease with no signs of proteosynthetic dysfunction (normal albumin, bilirubin, and prothrombin time values), ascites, or encephalopathy.
Antiviral regimen
The antiviral treatment regimen consisted of one half of a tablet containing 400 mg of SOF (Sovaldi®; Gilead Sciences Inc., Foster City, CA, USA) once daily and one entire tablet containing 60 mg of DCV (Daklinza®; Bristol-Myers Squibb, New York, NY, USA) once daily. The treatment period was 12 weeks. The original tablets Sovaldi containing 400 mg of SOF were split by means of a blade tablet splitting device so that we obtained two equal doses (~200 mg). The splitting was performed by the healthcare personnel. SOF was administered in combination with DCV once daily regularly in the morning independently of the hemodialysis session. SOF administration in patients with glomerular filtration rate ≤30 mL/min and the splitting of the tablets were considered to be off-label use of the drug and the patients gave consent to the off-label therapy before its initiation according to the local law.24,25 Other contraindications included in the Standard Product Characteristics of SOF and DCV, especially drug–drug interactions, were respected. Sustained virological response (SVR) was assessed as HCV RNA negativity 12 weeks posttreatment.
Laboratory assessment
HCV RNA was assessed by the Roche COBAS® AmpliPrep/COBAS TaqMan® HCV Quantitative Test v2.0 (Roche Molecular Systems Inc., Branchburg, NJ, USA). HCV genotyping was conducted at baseline using the SIEMENS Versant® HCV Genotype 2.0 Assay (LiPA) (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA).
Safety evaluation
The patients were carefully and regularly monitored for treatment efficacy and side effects as requested in patients on off-label treatment by local regulatory authorities. The patients’ visits were scheduled as follows: treatment initiation, treatment weeks 4, 8, and 12, and 12 weeks posttreatment. Each visit consisted of a query on medical history and side effects, check of concomitant medication, physical examination, laboratory analyses (blood biochemistry, blood count, prothrombin time, and HCV RNA), and drug dispensing (day 1 and treatment weeks 4 and 8). A regular part of the visit was a check of monthly records provided by the hemodialysis units containing a complete concomitant medication overview and the course of hemodialysis therapy. The structure of recordings of adverse events (AEs) was as follows: any AE or serious adverse event (SAE), which included any event requiring hospitalization, life-threatening event, or death, and the relationship with the administered medication, was also assessed. The hematological side effects (hemoglobin level ≤10 g/dL, abnormal white blood cells ≤40×109/L, or platelet count ≤70×109/L) and liver toxicity (any abnormal ALT, AST, and bilirubin levels during treatment) were of special interest.
Statistical analysis
Data are presented as means and SDs and medians and ranges, as appropriate. The changes in laboratory values at the particular therapy time points and at follow-up were compared by the Kruskal–Wallis test. P-value of <0.05 was considered statistically significant throughout the study. Statistical analysis was performed using the R programming language v.3.2.0 (www.r-project.org).
Ethical standard
This study was approved by the Ethics Committee of Thomayer’s Hospital and Institute for Clinical and Experimental Medicine, Prague, Czech Republic, and was carried out in compliance with the Helsinki Declaration. The requirement for informed consent was waived because of the retrospective design of the study and use of data from which the patients’ identification information had been removed. The patients consented to the off-label use of SOF, as required by local law.
Results
All the hemodialyzed patients infected with HCV genotype 3 referred to our center in the above-mentioned period were included in the study. No referred hemodialyzed patient infected with HCV genotype 3 had been contraindicated to therapy owing to potential drug–drug interactions or for severe liver dysfunction. Pretreatment patients’ characteristics are summarized in Table 1. All six patients completed the planned 12-week course of treatment. Three patients achieved negative viremia at week 4 and all six had negative HCV RNA at week 8 and week 12 of therapy. All six remained HCV RNA negative 12 weeks after the end of treatment and all achieved SVR 12. The five patients with initially abnormal ALT values had normal ALT values from treatment week 4; all patients had normal ALT levels from week 4 of therapy as well as during the follow-up.
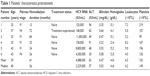  | Table 1 Patients’ characteristics pretreatment |
The treatment was well tolerated, as none of the patients presented with a serious adverse event requiring hospital admission during the treatment period and the 12-week follow-up. Only one nonserious AE was reported, which was probably not related to the administered antivirals: the patient had a short period of diarrhea, which was resolved with a course of antibiotics (7 days of rifaximin) treatment. No further AEs were identified in the patients’ records, and no changes in concomitant medication were required during the course of antiviral therapy.
The initial values of bilirubin, alkaline phosphatase, and gamma-glutamyltransferase activities were normal at baseline and did not elevate during the treatment period and follow-up. No liver toxicity of the administered antivirals was observed. The values of hemoglobin, leukocytes, and platelets at treatment weeks 4, 8, and 12, and 12 weeks posttreatment did not significantly differ from baseline values.
Discussion
Our group of six patients was relatively small, but no larger group of patients on maintenance hemodialysis infected with genotype 3 and treated with an SOF-based regimen has been described so far to the best of our knowledge. The recently published retrospective French multicenter study included five genotype 3-infected patients, but they were not evaluated separately.17 This study included a further 44 patients infected with non-3 genotypes and the overall SVR rate was 86%. Another two articles describing the use of SOF-based regimens in the treatment of patients on maintenance hemodialysis included one and four genotype 3 patients, respectively.16,18
Our decision to treat patients with the SOF and DCV regimen was based on the EASL (The European Association for the Study of the Liver) 2014 Guidelines, which recommended exclusively an interferon-free regimen in hemodialyzed patients.26 As DCV is a drug metabolized in the liver, its dose reduction was not necessary in renal impairment.15,27 However, the EASL Guidelines 2014 stated that the use of SOF in hemodialyzed patients should be postponed until more data were available.26 On the basis of a small study by Gane et al28 describing pharmacokinetics and rapid virological suppression in nonhemodialyzed patients with severe renal impairment treated with 200 mg of SOF daily (in combination with RBV), our decision was to reduce the dose of SOF. There was no other alternative on how to obtain a reduced dose of SOF for daily administration than the nonstandard splitting of the original 400 mg tablets. Unfortunately, we were not able to perform the assessment of SOF blood levels and we were not able to evaluate the difference in SOF exposure caused by the splitting of tablets.
The optimal dose of SOF in hemodialyzed patients remains controversial. SOF is subject to extensive first-pass metabolism in the intestine and in the liver. SOF is hydrolyzed and phosphorylated to form the pharmacologically active nucleoside analog triphosphate GS-461203. Dephosphorylation results in the formation of an inactive metabolite GS-331007.24 Kidneys represent the major site involved in the elimination of SOF and its metabolite. Whereas the pharmacokinetics of the active metabolite GS-461203 has not been characterized, the half-life of the predominant circulating inactive metabolite GS-331007 is 27 hours. The pharmacokinetics of SOF was studied in HCV-negative subjects with ESRD requiring hemodialysis following a single 400 mg dose of SOF. In comparison with subjects with normal renal function, in the subjects with ESRD, the SOF and GS-331007 areas under the curve (AUC0-infinity) were 28% and 1,280% higher, respectively, when SOF was dosed 1 hour before hemodialysis compared with 60% and 2,070% higher, respectively, when SOF was dosed 1 hour after hemodialysis.18 Hemodialysis can efficiently remove the predominant circulating metabolite GS-331007 (53% extraction ratio).29 Furthermore, Desnoyer et al15 recently described that SOF or its inactive metabolite GS-331007 did not accumulate with either full or reduced-dose SOF regimen and they confirmed the GS-331007 hemodialysis extraction ratio of 52%. Consistent with these data, some authors reported safe and effective use of full-dose SOF in patients on maintenance hemodialysis who were infected predominantly with genotype 1.16–18,20–22,30
As we mentioned above, the splitting of the SOF tablets is a controversial point of our study; however, the results of the study by Desnoyer et al15 might support the daily dosage of SOF, which seems to have a higher antiviral activity. The authors described a cohort of 12 patients on maintenance hemodialysis with HCV genotypes 1 or 2, who were treated with SOF in combination with other antiviral agents. Seven of them received full-dose SOF (400 mg daily) and all seven achieved an SVR. Five of them received reduced-dose SOF (400 mg 3 times a week) and three of the five patients in this subgroup relapsed.
We did not add RBV to the SOF and DCV combination even in the cirrhotic patients according to the EASL 2014 Guidelines, which allowed this combination without RBV in cirrhotic patients with normal renal function.
The efficacy of SOF and DCV regimen in noncirrhotic patients without renal impairment infected with genotype 3 was later demonstrated in the ALLY-3 study.31 The SVR rate was 90% in treatment-naïve and 86% in treatment-experienced patients. The SVR rate in patients with cirrhosis was lower (63%) than in noncirrhotic patients in this study; however, both arms of cirrhotic patients (with and without RBV) were evaluated together. We reopened the question of the addition of RBV to the SOF and DCV combination in 2015, when the last patient with liver cirrhosis was referred to antiviral treatment. We decided not to add RBV again, the decision being based on the preliminary data from the two real-life cohorts:32,33 the real-life data demonstrated a higher efficacy of SOF and DCV combination (93.8% SVR rate) also in genotype 3-infected cirrhotic patients in comparison with the ALLY-3 study and the SVR rate in arms with RBV was not significantly better. Nowadays, the EASL 2016 Guidelines2 recommend 12 weeks of SOF and DCV plus RBV or 24 weeks of SOF and DCV as optimal treatment regimen for hemodialyzed patients infected with HCV genotype 3, reflecting the fact that the genotype 3-infected patients with cirrhosis are considered as difficult to treat.
The efficacy of the antiviral regimen consisting of the reduced dose of SOF and the full dose of DCV was excellent in our group of patients: all the patients cleared the virus and achieved an SVR. The SVR was also achieved by two of the six patients who had a very high pretreatment viremia. This fact is consistent with the ALLY-3 study results confirming that pretreatment characteristics such as gender, age, viremia, and IL28B genotype (recently IFNL4) do not impact SVR achievement. The SVR was also achieved in the two patients with compensated liver cirrhosis. The most logical explanation for the efficacy of the reduced dose of SOF in hemodialysed patients seems to be the dose-concentration effect. However, the pharmacokinetics of SOF active metabolite GS-461203 remains unknown and therefore the fact of how the higher concentrations of circulating SOF and its metabolite GS-331007 reflect the concentration of active metabolite GS-461203 in the liver remains unknown.
The regimen consisting of the reduced dose of SOF with DCV was very well tolerated by all patients, they all completed the 12-week course of the treatment, and no SAEs were observed. None of our patients required a higher dose of erythropoietin during the treatment period. In his retrospective study, Dumortier et al17 reported a higher number of AEs in the SOF-based regimens in patients on maintenance hemodialysis. The most frequent AE was anemia, but the study also included patients treated with SOF and RBV or with SOF, RBV, and PEG-IFN-α. However, Saxena et al34 reported a high rate of anemia in patients with severe renal impairment treated with full-dose SOF even in those without RBV addition. The epidemiology of HCV infection has changed during the last decade in our region; the prevalence of genotype 3 rose from <5% to 45%.35 In agreement with the changing epidemiology of HCV, our patients infected with genotype 3 were younger and had a shorter period of hemodialysis and fewer comorbidities in comparison with our historical group of patients infected with genotype 1b.36 This could also contribute to a good tolerance of the antiviral regimen described in our current study.
In contrast to Dumortier et al’s17 cohort, we did not observe gastrointestinal discomfort requiring further SOF dose reduction. Those patients were also given reduced doses of SOF, but the dose reduction was achieved by dosage interval prolongation. We can speculate that the daily administration of the reduced dose of SOF may have contributed to the absence of gastrointestinal discomfort in our patients, thus preventing the saw-toothed ups and downs in the levels of SOF. None of our patients complained of the unpleasant taste of the split tablets.
Considering all the limitations of our study (retrospective design, absence of pharmacokinetics profile, and the splitting of SOF tablets), we may conclude that a reduced dose of SOF and a full dose of DCV showed a high antiviral efficacy and good safety and tolerability in our cohort of hemodialyzed patients infected with HCV, genotype 3. In spite of the fact that both cirrhotic patients in our cohort achieved SVR, we do not recommend this regimen for patients with cirrhosis; either they should be treated for 24 weeks or RBV should be added.
Author contributions
JS and SF provided clinical and laboratory data and wrote and revised the manuscript; MK performed HCV RNA analysis and genotyping; DM performed statistical analysis; MT provided clinical and laboratory data; JS revised the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Goodkin DA, Bieber B, Gillespie B, Robinson BM, Jadoul M. Hepatitis C infection is very rarely treated among hemodialysis patients. Am J Nephrol. 2013;38(5):405–412. | ||
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol. 2017;66(1):153–194. | ||
Pockros PJ, Reddy KR, Mantry PS, et al. Efficacy of direct-acting antiviral combination for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology. 2016;150(7):1590–1598. | ||
Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386(10003):1537–1545. | ||
Espinosa M, Martin-Malo A, Alvarez de Lara MA, Aljama P. Risk of death and liver cirrhosis in anti-HCV-positive long-term haemodialysis patients. Nephrol Dial Transplant. 2001;16(8):1669–1674. | ||
Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18(5):1584–1593. | ||
Butt AA, Skanderson M, McGinnis KA, et al. Impact of hepatitis C virus infection and other comorbidities on survival in patients on dialysis. J Viral Hepat. 2007;14(10):688–696. | ||
Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14(10):697–703. | ||
Fabrizi F, Dixit V, Bunnapradist S, Martin P. Meta-analysis: the dialysis mode and immunological response to hepatitis B virus vaccine in dialysis population. Aliment Pharmacol Ther. 2006;23(8):1105–1112. | ||
Mathurin P, Mouquet C, Poynard T, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29(1):257–263. | ||
Markowitz GS, Cheng JT, Colvin RB, Trebbin WM, D’Agati VD. Hepatitis C viral infection is associated with fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol. 1998;9(12):2244–2252. | ||
Legendre C, Garrigue V, Le Bihan C, et al. Harmful long-term impact of hepatitis C virus infection in kidney transplant recipients. Transplantation. 1998;65(5):667–670. | ||
Bruchfeld A, Wilczek H, Elinder CG. Hepatitis C infection, time in renal-replacement therapy, and outcome after kidney transplantation. Transplantation. 2004;78(5):745–750. | ||
Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–1887. | ||
Desnoyer A, Pospai D, Le MP, et al. Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C. J Hepatol. 2016;65(1):40–47. | ||
Singh T, Guirguis J, Anthony S, Rivas J, Hanouneh IA, Alkhouri N. Sofosbuvir-based treatment is safe and effective in patients with chronic hepatitis C infection and end stage renal disease: a case series. Liver Int. 2016;36(6):802–806. | ||
Dumortier J, Bailly F, Pageaux GP, et al. Sofosbuvir-based antiviral therapy in hepatitis C virus patients with severe renal failure. Nephrol Dial Transplant. Epub October 19, 2016. | ||
Beinhardt S, Al Zoairy R, Ferenci P, et al. DAA-based antiviral treatment of patients with chronic hepatitis C in the pre- and postkidney transplantation setting. Transpl Int. 2016;29(9):999–1007. | ||
Perumpail RB, Wong RJ, Ha LD, et al. Sofosbuvir and simeprevir combination therapy in the setting of liver transplantation and hemodialysis. Transpl Infect Dis. 2015;17(2):275–278. | ||
Saab S, M AJ, S NB, et al. Use of sofosbuvir-based treatment of chronic hepatitis C in liver transplant recipients on hemodialysis. J Clin Gastroenterol. 2017;51(2):167–173. | ||
Nazario HE, Ndungu M, Modi AA. Sofosbuvir and simeprevir in hepatitis C genotype 1-patients with end-stage renal disease on haemodialysis or GFR <30 ml/min. Liver Int. 2016;36(6):798–801. | ||
Hundemer GL, Sise ME, Wisocky J, et al. Use of sofosbuvir-based direct-acting antiviral therapy for hepatitis C viral infection in patients with severe renal insufficiency. Infect Dis (Lond). 2015;47(12):924–929. | ||
Gerber L, Kasper D, Fitting D, et al. Assessment of liver fibrosis with 2-D shear wave elastography in comparison to transient elastography and acoustic radiation force impulse imaging in patients with chronic liver disease. Ultrasound Med Biol. 2015;41(9):2350–2359. | ||
European Medicines Agency. Sovaldi: Příloha I; souhrn údajů o přípravku [Sovaldi: Annex I; summary of product characteristics]. Available from: http://www.ema.europa.eu/docs/cs_CZ/document_library/EPAR_-_Product_Information/human/002798/WC500160597.pdf. Accessed February 1, 2017. Czech. | ||
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. Available from: http://www.easl.eu/research/our-contributions/clinical-practice-guidelines/detail/recommendations-on-treatment-of-hepatitis-c-2015. Accessed February 1, 2017. | ||
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2014. Available from: http://www.easl.eu/discover/news/easl-recommendations-on-treatment-of-hepatitis-c-2014. Accessed February 1, 2017. | ||
Garimella T, Wang R, Luo WL, et al. Single-dose pharmacokinetics and safety of daclatasvir in subjects with renal function impairment. Antivir Ther. 2015;20(5):535–543. | ||
Gane E, Robson RA, Bonacin M. Safety, anti-viral-efficacy and Pharmacokinetics (PK) of Sofosbuvir (SOF) in patients with severe renal impairment. Hepatology. 2014;60 (Suppl S1):667A. | ||
Burra P, Rodriguez-Castro KI, Marchini F, et al. Hepatitis C virus infection in end-stage renal disease and kidney transplantation. Transpl Int. 2014;27(9):877–891. | ||
Gevers TJ, Burger D, Schipper-Reintjes E, Kooistra MP, Richter C. Full-dose sofosbuvir and daclatasvir for chronic hepatitis C infection in haemodialysis patients. Neth J Med. 2016;74(5):225–227. | ||
Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61(4):1127–1135. | ||
Alonso S, Riveiro-Barciela M, Fernandez I, et al. Effectiveness and safety of sofosbuvir-based regimens plus an NS5A inhibitor for patients with HCV genotype 3 infection and cirrhosis. Results of a multicenter real-life cohort. J Viral Hepat. 2017;24(4):304–311. | ||
Welzel TM, Petersen J, Herzer K, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut. 2016;65(11):1861–1870. | ||
Saxena V, Koraishy FM, Sise ME, et al. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int. 2016;36(6):807–816. | ||
Chlibek R, Smetana J, Sosovickova R, et al. Hepatitis C prevalence in the general adult population in the Czech Republic – results of a seroprevalence study. PLoS One. 2017;12(4):e0175525. | ||
Sperl J, Frankova S, Senkerikova R, et al. Relevance of low viral load in haemodialysed patients with chronic hepatitis C virus infection. World J Gastroenterol. 2015;21(18):5496–5504. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
