Back to Journals » Cancer Management and Research » Volume 10
Combination of recurrent oral aphthae and dry eye syndrome may constitute an independent risk factor for oral cavity cancer in elderly women
Authors Qin L, Kao YW, Lin YL , Peng BY, Deng WP, Chen TM, Lin KC, Yuan KSP, Wu ATH , Shia BC, Wu SY
Received 17 March 2018
Accepted for publication 25 June 2018
Published 5 September 2018 Volume 2018:10 Pages 3273—3281
DOI https://doi.org/10.2147/CMAR.S168477
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Lei Qin,1,* Yi-Wei Kao,2,* Yueh-Lung Lin,3 Bou-Yue Peng,4 Win-Ping Deng,5 Tsung-Ming Chen,6 Kuan-Chou Lin,7 Kevin Sheng-Po Yuan,8 Alexander TH Wu,9 Ben-Chang Shia,10 Szu-Yuan Wu11–14
1School of Statistics, University of International Business and Economics, Beijing, China; 2Graduate Institute of Business Administration, Fu Jen Catholic University, Taipei, Taiwan; 3School of Mathematical Sciences, University of Nottingham, Ningbo, China; 4Department of Dentistry, Taipei Medical University Hospital, Taipei, Taiwan; 5Graduate Institute of Biomedical Materials and Engineering, Taipei Medical University, Taipei, Taiwan; 6Department of Otorhinolaryngology, Shuang-Ho Hospital, Taipei Medical University, Taipei, Taiwan; 7Department of Oral and Maxillofacial Surgery, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan; 8Department of Otorhinolaryngology, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan; 9PhD Program for Translational Medicine, Taipei Medical University, Taipei, Taiwan; 10College of Management, Taipei Medical University, Taipei, Taiwan; 11Institute of Clinical Science, Zhongshan Hospital, Fudan University, Shanghai, China; 12Department of Radiation Oncology, Wanfang Hospital, Taipei Medical University, Taipei, Taiwan; 13Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; 14Epidemiology and Bioinformatics Center, Wanfang Hospital, Taipei Medical University, Taipei, Taiwan
*These authors contributed equally to this work
Background: Few studies have evaluated the risk of oral cavity cancer (OC) in patients with recurrent oral aphthae (ROA) and dry eye syndrome (DES). This study assessed the risk of OC in patients who had received diagnoses of ROA and DES in Taiwan.
Methods: A population-based frequency-matched case–control study was conducted in which data were analyzed from the National Health Insurance Research Database of Taiwan. Patients with ROA and DES were identified as the case cohort. Patients and controls without ROA and DES were frequency matched (1:4) on the basis of age, sex, monthly income, geographical location, and urbanization level. Chi-squared tests were conducted to compare demographic factor distributions between the patients and controls. Cox proportional hazards models were used to calculate the adjusted hazard ratios (aHRs) and 95% CI of OC diagnoses among the patients and controls. Risk consistency between the two cohorts was determined using subgroup analysis.
Results: A total of 7,110 patients with ROA and DES and 28,388 controls were identified. The OC risk was significantly higher for female patients than controls (aHR=3.41, 95% CI=1.69–6.86). Furthermore, women aged 50–69 years exhibited a higher risk of OC than those in the other age groups. Female patients aged 50–59 years exhibited the highest aHR for OC (aHR=5.56, 95% CI=1.70–18.25), followed by those aged 60–69 years (aHR=4.34, 95% CI=1.26–15.99).
Conclusion: ROA and DES may be associated with a high risk of OC in elderly women.
Keywords: risk factor, oral cavity cancer, recurrent oral aphthae, dry eye syndrome, women
Introduction
Oral cavity cancer (OC) is the fourth leading cause of cancer-related death and sixth most common cancer in Taiwan according to the Taiwan Cancer Registry Report (2011 edition) published on the website of the Health Promotion Administration, Ministry of Health and Welfare.1–6 In Taiwan, most patients with head and neck cancers are men who habitually chew betel nut and whose median age is 53 years; these patients are often the primary contributors of their family incomes.1–9 Taiwan’s national OC incidence rate is the highest in the world;8,9 however, the incidence rate of OC in women in Taiwan is low. Moreover, the risk factors for OC are unclear and OC surveillance is unsatisfactory.2–6,8
In clinical practice, recurrent oral aphthae (ROA) and dry eye syndrome (DES) have been frequently observed before OC is diagnosed in patients, particularly in women with autoimmune diseases.10–14 The development of modern therapies and suitable methods of care for patients with autoimmune diseases has increased the survival rate of these patients. However, autoimmune diseases may be associated with a high risk of cancer;13,14 such a risk is mainly attributable to the high level of inflammatory activity and the severity of the autoimmune diseases in question.10 In this study, we estimated the risk of OC in patients with ROA and DES.
We conducted a population-based frequency-matched case–control study in a clinical setting to determine whether a combination of ROA and DES is a risk factor for OC.
Patients and methods
Database
Our population-based cohort study was conducted using the National Health Insurance Research Database (NHIRD) of Taiwan. The NHIRD contains large amounts of valuable medical data concerning outpatient visits, inpatient care, and catastrophic illnesses; the database was made public under the National Health Insurance (NHI) program and is available to the public for research purposes after identification numbers and personal information have been removed. The NHI program, initiated in 1995, covers more than 99% of Taiwanese NHI beneficiaries (23 million people). Owing to the comprehensiveness and authenticity of NHIRD data, the database is a crucial research resource for epidemiology, and results extracted from it can be used as references for the development of medical and health policies. The dataset used in this study contained all NHIRD claims data between 1996 and 2013. Our original dataset was a subset consisting of one million individuals that were randomly selected from the NHIRD. Permission from the Institutional Review Board of Taipei Medical University was obtained before this study commenced (TMU-JIRB No. 201402018).
Selection of patients and controls
This retrospective cohort study was conducted using two cohorts, namely a ROA–DES case cohort and a matched non-ROA–DES control cohort. Identification of patients with ROA or DES was based on a minimum of two outpatient visits for oral aphthae (OA) (ICD, Ninth Revision, Clinical Modification [ICD-9-CM] code 528.2). The ROA–DES cohort consisted of patients who received a first diagnosis of either ROA or DES (ICD-9-CM code 375.15) between January 2000 and December 2011. Members of the non-ROA–DES cohort consisted of individuals who did not receive a diagnosis of either ROA or DES throughout the entire follow-up period. We excluded patients aged <40 years; those with human immunodeficiency virus, Epstein–Barr virus, or human papillomavirus infection; those who habitually consumed alcohol, chewed betel nut, or smoked cigarettes; those who had undergone an organ transplantation or eye surgery; and those with a history of OC (ICD-9-CM codes 140–145, excluding 142) (Figure 1). The date of the initial ROA or DES diagnosis was designated as the index date.
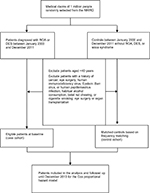  | Figure 1 Flowchart of patient and control enrollment. Abbreviations: NHIRD, National Health Insurance Research Database; ROA, recurrent oral aphthae; DES, dry eye syndrome. |
Among the non-ROA–DES controls without OA and DES medical claims or a history of OC, we excluded patients with sicca syndrome (ICD-9-CM code 710.2) or keratoconjunctivitis sicca (ICD-9-CM code 370.33) to prevent the inclusion of patients with ROA or DES in the control cohort. We selected matched controls for each patient in the ROA–DES case cohort at a 1:4 ratio, based on the year of index date, age group (18–29, 30–39, 40–49, 50–59, 60–69, or ≥70), sex (male or female), monthly income (NT$0–15,840, NT$15,841–25,000, or ≥NT$25,001), geographical region (northern, central, eastern, or southern Taiwan), and urbanization level (a total of seven levels in which one is the most urbanized and seven is the least urbanized) to create the control cohort. The first use of medical services in the matched year was defined as the index date for the controls.
Statistical analysis
All statistical analyses in this study were performed using SAS for Windows version 9.2 (SAS Institute, Cary, NC, USA). Baseline characteristics were analyzed first, followed by outcome measurements. Statistical significance was set at P≤0.05.
For demographic analysis, chi-squared testing was conducted to compare the distributions of baseline characteristics. The major comorbidities of interest, all of which were considered potential risk factors, were identified prior to the index date, and consisted of rheumatoid arthritis (RA) (ICD-9-CM code 714.0), systemic lupus erythematosus (SLE) (ICD-9-CM code 710.0), systemic sclerosis (SSc) (ICD-9-CM code 710.1), idiopathic inflammatory myositis (IIM) (ICD-9-CM codes 710.3 and 710.4), type one diabetes mellitus (DM) (ICD-9-CM codes 250.01, 250.03, 250.11, 250.13, 250.21, 250.23, 250.31, 250.33, 250.41, 250.43, 250.51, 250.53, 250.61, 250.63, 250.71, 250.73, 250.81, 250.83, 250.91, and 250.93), multiple sclerosis (MS) (ICD-9-CM code 340), myasthenia gravis (MG) (ICD-9-CM code 358), Crohn’s disease (CD) (ICD-9-CM code 555), ulcerative colitis (UC) (ICD-9-CM code 556), and vasculitis (ICD-9-CM codes 446.2 and 446.29).
The major outcome of interest was the diagnosis of OC, and all patients in this study were followed until December 2013. Cox proportional hazards model analysis was used to model the survival time condition on baseline characteristics and to calculate and compare the hazard ratios (HRs), adjusted HRs (aHRs), and 95% CI of the case and control cohorts. Subgroup analyses were performed after the cohorts had been stratified on the basis of sex and age to investigate potential differences between subgroups.
Results
Table 1 presents the demographic characteristics of the case and control cohorts. Our study population consisted of 7,110 patients and 28,388 controls. After frequency matching to select controls, no significant differences were observed in age (P=1.000), sex (P=0.942), monthly income (P=0.976), geographical region (P=1.000), and urbanization level (P=1.000). However, of the comorbidities of interest, patients in the case cohort were more likely to have autoimmune diseases such as SLE (P=0.001), SSc (P=0.001), IIM (P=0.001), type 1 DM (P=0.001), MS (P=0.009), MG (P=0.001), CD (P=0.01), UC (P=0.001), and vasculitis (P=0.004) than RA (P=0.159).
The risk factor analysis for OC, which was conducted using a Cox proportional hazards model, is summarized in Table 2. The multivariate Cox proportional hazards model revealed that the risk of OC among men was more than six-times higher than among women (HR=6.20, 95% CI=4.06–9.54); however, no statistically significant differences were observed when the cohorts were stratified on the basis of factors other than sex.
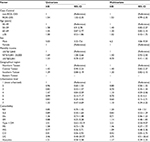  | Table 2 Cox proportional hazards model for oral cavity cancer risk in the patient group Note: All variables in Table 1 were used in the multivariate analysis. Abbreviations: HR, hazard ratio; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; IIM, idiopathic inflammatory myositis; DM, diabetes mellitus; MS, multiple sclerosis; MG, myasthenia gravis; CD, Crohn’s disease; UC, ulcerative colitis; N/A, not applicable. |
Table 3 presents the risk analysis for the overall study population and the subgroups. The combination of ROA and DES may not be an overall risk factor for OC because its aHR was 1.53 (95% CI=0.99–2.35, P=0.052). Because sex significantly affected the risk of OC among patients with ROA–DES, the subgroup analysis focused on stratification of the study population based on sex. The risk of OC was significantly higher in female patients than female controls (aHR=3.41, 95% CI=1.69–6.86); however, the risk of OC was similar between male patients and male controls (aHR=1.00, 95% CI=0.57–1.77). Female patients aged 50–59 years exhibited the highest HR for OC (aHR=5.56, 95% CI=1.70–18.25), followed by female patients aged 60–69 years (aHR=4.34, 95% CI=1.26–15.00); however, a significantly high risk was not observed in females aged ≥70 years.
  | Table 3 Stratified Cox proportional hazards model for risk of oral cavity cancer among patients and controls, considering both sex and age Note: All variables in Table 1 were used in the multivariate analysis. Abbreviations: OC, oral cavity cancer; HR, hazard ratio; N/A, not applicable. |
Discussion
In ROA, painful, localized oral lesions develop and appear as shallow round-to-oval-shaped ulcers with a grayish base. ROA is the most common cause of mouth ulcers,15 and may be observed in patients with celiac disease or inflammatory bowel disease.16 The pathogenesis of ROA is not well defined; one hypothesis posits that ROA results from a dysregulation of local cell-mediated immunity, which causes the development of subsets of T cells and proinflammatory cytokines.17 Factors that predispose an individual to develop ROA include family history, trauma, hormonal factors, and emotional stress.18 Dysregulation of local cell-mediated immunity and the consequent accumulation of subsets of T cells and proinflammatory cytokines might be associated with squamous cell carcinomas in the oral cavity.17 In the presence of tumor necrosis factor-alpha (TNF-α) and interleukin (IL)–6, naïve CD4 T cells are believed to develop into Th22 cells that secrete IL-22 and TNF-α.19,20 Evidence has suggested that Th22 cells are involved in the development of squamous cell carcinomas.19–21 Furthermore, proinflammatory cytokines and Th1-associated chemokine receptors are involved in the development of squamous cell carcinoma at the protein level in patients with ROA and in those with OC.17,22,23 The expression levels of Th2 cytokine IL-4 in the oral lesions of patients with oral ulcers suggest that the antigenic stimuli in patients with ROA are more complex than those in patients with oral ulcers.17,24 Moreover, in addition to the effects of immune mediators in ROA and OC, family history, trauma, hormonal factors, and emotional stress have been associated with ROA and a risk of OC.25,26
Individual autoimmune diseases are associated with a high risk of malignancies such as dermatomyositis, polymyositis, SSc with multiple malignancies and RA, Sjögren syndrome (SS), and SLE with malignancies.27,28 Patients with myositis and SSc who possess specific antibodies (such as RNA polymerase III antibodies in SSc) are at a relatively high risk of developing malignancies near the onset time of the autoimmune disease. SS with DES is a chronic autoimmune inflammatory disorder characterized by diminished lacrimal and salivary gland function, which causes dryness of the eyes and mouth.27,28 In addition, various other disease manifestations that affect multiple organs and organ systems may occur; the clinical features of SS can be divided into two broad categories, namely exocrine glandular features and extraglandular features.29 Age- and sex-appropriate screening should be conducted on these patients who are at a high risk of cancers related to their autoimmune diseases. Moreover, an additional screening step based on the individual risk of a patient may be included in the screening process. DES may be a risk factor for OC, owing to one of several mechanisms that indicate the involvement of platelet-derived and other growth factors. For example, megakaryocytes can release growth factors in the distal extremities and perform a circulatory bypass of the lung.11,12 The tumor-derived production and release of a factor such as the vascular endothelial growth factor could promote features of the condition such as vascular proliferation, edema formation, and new bone formation.30,31 Additionally, an elevated level of circulating prostaglandin E2—a bioactive lipid that induces various biological effects associated with inflammation and cancer—is believed to play a role in primary hypertrophic osteoarthropathy.32
Table 2 indicates that the patients in this study with ROA or DES were more likely to have autoimmune diseases such as SLE (P=0.001), SSc (P=0.001), IIM (P=0.001), type 1 DM (P=0.001), MS (P=0.009), MG (P=0.001), CD (P=0.001), UC (P=0.001), and vasculitis (P=0.004) than RA (P=0.159). The exact pathophysiology of OC in relation to autoimmune diseases has yet to be determined, but several mechanisms that contribute to the high susceptibility of distinct groups of patients to specific types of malignancies have been implicated,33,34 namely impaired genetic stability, genetic predisposition, immune dysregulation, impaired clearance of oncogenic viruses, and iatrogenic causes.13,14 Similarities in the pathologies of autoimmune diseases and cancer have been recognized for at least 30 years.13,14 Inflammatory cytokines and growth factors mediate cell proliferation, and proteinases—particularly the collagenase matrix metalloproteinase-1 (MMP-1)—contribute to disease progression through remodeling the disease’s extracellular matrix and modulating the microenvironment.35,36 An autoimmune disorder might activate stromal cells present in autoimmune diseases and tumor formation.13,14,35,36 MMP-1 was originally thought to degrade interstitial collagen, but is now believed to have novel roles that involve various G protein-coupled receptors, namely C-X-C chemokine receptor type 4 (CXCR-4) and protease activated receptor-1 (PAR-1). Cooperativity between MMP-1 and CXCR-4 or stromal-cell-derived factor one signaling, influences the behavior of activated fibroblasts in autoimmune diseases and cancer.13,14,35,36 Furthermore, MMP-1 is a crucial part of an autocrine–paracrine MMP-1–PAR-1 signal transduction axis, and amplifies its potential to remodel the matrix and modify cell behavior.35,36 Finally, new therapeutic agents targeting MMP-1 and G protein-coupled receptors are emerging as treatments that emphasize fundamental similarities between autoimmune disorders and some cancers.35,36
Investigating estrogen deficiency as a risk factor for OC in postmenopausal women may provide insights into the etiology of oral malignancies.37 The findings of other studies are consistent with ours, which concluded that female patients aged 50–59 years had the highest HR for OC, followed by those aged 60–69 years (Table 3).37 Another study supported the estrogen deficiency theory of cancer initiation, and determined that estrogen deficiency and high fasting glucose levels were risk factors for OC in postmenopausal women.38 In addition, estrogen deficiency was associated with ROA and DES.15,39–42 The novel hypothesis regarding associations between estrogen deficiency and ROA, DES, and the risk of OC in postmenopausal women may provide new insights into the etiology and prevention of oral malignancies.
No studies have reported that a combination of ROA and DES might be an independent risk factor for OC in elderly women; the present study is the first to detail such an association. Estrogen deficiency could be the etiology. Additional clinical studies or animal model studies are necessary to verify whether estrogen deficiency causes OC; such studies could provide insight regarding the potential role of estrogen supplements for cancer prevention in elderly women. The exploratory nature of this study implies the necessity of conducting future studies to corroborate and prove the hypothesis positing the role of estrogen deficiency in OC.
In the future, physicians should pay close attention to ROA and DES in elderly women; detailed surveillance of the oral cavity or a close follow-up may be necessary for the early detection of OC. Symptoms and signs such as ROA and DES are easily detectable, and close monitoring of the oral cavity in elderly women is a relatively straightforward method for the early detection of OC in susceptible populations.
The strengths of this study were its large sample size and the balance afforded by the frequency-matched design that included patients with ROA and DES and controls. The results suggested that elderly women with ROA and DES are susceptible to OC. Table 3 indicates that female patients aged 50–59 years exhibited the highest HR for OC (aHR=5.56, 95% CI=1.70–18.25), followed by female patients aged 60–69 years (aHR=4.34, 95% CI=1.26–15.00). The present study is the first to discern that a combination of ROA and DES is an independent risk factor for OC in elderly women. Our results indicate that close surveillance of the oral cavity in elderly women with ROA and DES might be beneficial for early OC detection. These findings should be taken into account in future clinical practice.
This study had some limitations. First, all the patients with ROA and DES included in this study belonged to an Asian population. The corresponding ethnic susceptibility is unclear, and thus our results should be cautiously extrapolated to non-Asian populations. Second, the relatively small number of patients with OC might have limited the generalizability of our conclusions. Therefore, to obtain crucial information concerning population specificity and disease occurrence, a large-scale randomized trial that carefully compares selected patients undergoing suitable surveillance is essential. Third, all diagnoses of comorbid conditions depended entirely on ICD-9-CM codes; however, the NHI Administration randomly reviews charts and interviews patients to verify the accuracy of diagnoses, and hospitals with non-standard charges or practices are liable to audits and heavy penalties if malpractice or discrepancies are detected. Considering the magnitude and statistical significance of the observed outcomes in this study, these limitations are unlikely to have affected the conclusions.
Conclusion
A combination of ROA and DES might be an independent risk factor for OC in elderly women. The findings and recommendations of this study could promote the early detection of OC in elderly women with ROA and DES.
Data sharing statement
We used data from the National Health Insurance Research Database (NHIRD). The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. The data utilized in this study cannot be made available in the manuscript, the supplementary files, or in a public repository due to the “Personal Information Protection Act” executed by Taiwan’s government, starting from 2012. Requests for data can be sent as a formal proposal to obtain approval from the ethics review committee of the appropriate governmental department in Taiwan. Specifically, links regarding contact info for which data requests may be sent to are as follows: http://nhird.nhri.org.tw/en/Data_Subsets.html#S3 and http://nhis.nhri.org.tw/point.html.
Acknowledgments
LQ work is supported by the Beijing Natural Science Foundation (No. 4164100) and the National Natural Science Foundation of China (Grant No. 61603092). Szu-Yuan Wu’s work is supported by funding from Taipei Medical University (TMU105-AE1-B26) and Wanfang Hospital (107-wf-swf-08).
Author contributions
SYW was responsible for the study concept and design. LQ, YWK, YLL, BYP, WPD, TMC, KCL, KSPY, ATHW, BCS, and SYW were responsible for data collections and organization. LQ, YWK, BCS, and SYW were responsible for data analysis and interpretation. All authors contributed toward drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work
References
Health Promotion Administration MoHaW. Taiwan Cancer Registry report, 2011 edition; 2011. Available from: http://www.hpa.gov.tw/BHPNet/Web/Stat/StatisticsShow.aspx?No=201404160001. Accessed March 17, 2018. | ||
Chen JH, Yen YC, Chen TM, et al. Survival prognostic factors for metachronous second primary head and neck squamous cell carcinoma. Cancer Med. 2017;6(1):142–153. | ||
Chang CL, Yuan KS, Wu SY, Sy W. High-dose or low-dose cisplatin concurrent with radiotherapy in locally advanced head and neck squamous cell cancer. Head Neck. 2017;39(7):1364–1370. | ||
Chen JH, Yen YC, Liu SH, et al. Dementia risk in irradiated patients with head and neck cancer. Medicine. 2015;94(45):e1983. | ||
Chen JH, Yen YC, Yang HC, et al. Curative-Intent Aggressive Treatment Improves Survival in Elderly Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma and High Comorbidity Index. Medicine. 2016;95(14):e3268. | ||
Chang JH, Wu CC, Yuan KS, Wu ATH, Wu SY, Cc W, Ath W, Sy W. Locoregionally recurrent head and neck squamous cell carcinoma: incidence, survival, prognostic factors, and treatment outcomes. Oncotarget. 2017;8(33):55600–55612. | ||
Chen YJ, Chang JT, Liao CT, et al. Head and neck cancer in the betel quid chewing area: recent advances in molecular carcinogenesis. Cancer Sci. 2008;99(8):1507–1514. | ||
Lin YS, Jen YM, Wang BB, Lee JC, Kang BH. Epidemiology of oral cavity cancer in taiwan with emphasis on the role of betel nut chewing. ORL J Otorhinolaryngol Relat Spec. 2005;67(4):230–236. | ||
Ko YC, Huang YL, Lee CH, Chen MJ, Lin LM, Tsai CC. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J Oral Pathol Med. 1995;24(10):450–453. | ||
Boussios S, Pentheroudakis G, Somarakis G, Markatseli TE, Drosos AA, Pavlidis N. Cancer diagnosis in a cohort of patients with Sjogren’s syndrome and rheumatoid arthritis: a single-center experience and review of the literature. Anticancer Res. 2014;34(11):6669–6676. | ||
Dickinson CJ. The aetiology of clubbing and hypertrophic osteoarthropathy. Eur J Clin Invest. 1993;23(6):330–338. | ||
Pedersen NT. Occurrence of megakaryocytes in various vessels and their retention in the pulmonary capillaries in man. Scand J Haematol. 1978;21(5):369–375. | ||
Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–625. | ||
de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. | ||
Ship JA. Recurrent aphthous stomatitis. An update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(2):141–147. | ||
Lankarani KB, Sivandzadeh GR, Hassanpour S. Oral manifestation in inflammatory bowel disease: a review. World J Gastroenterol. 2013;19(46):8571–8579. | ||
Dalghous AM, Freysdottir J, Fortune F. Expression of cytokines, chemokines, and chemokine receptors in oral ulcers of patients with Behcet’s disease (BD) and recurrent aphthous stomatitis is Th1-associated, although Th2-association is also observed in patients with BD. Scand J Rheumatol. 2006;35(6):472–475. | ||
Akintoye SO, Greenberg MS. Recurrent aphthous stomatitis. Dent Clin North Am. 2014;58(2):281–297. | ||
Fujita H. The role of IL-22 and Th22 cells in human skin diseases. J Dermatol Sci. 2013;72(1):3–8. | ||
Zhuang Y, Peng LS, Zhao YL, et al. Increased intratumoral IL-22-producing CD4(+) T cells and Th22 cells correlate with gastric cancer progression and predict poor patient survival. Cancer Immunol Immunother. 2012;61(11):1965–1975. | ||
Zhang S, Fujita H, Mitsui H, et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLoS One. 2013;8(5):e62154. | ||
Freysdottir J, Zhang S, Tilakaratne WM, Fortune F. Oral biopsies from patients with orofacial granulomatosis with histology resembling Crohn’s disease have a prominent Th1 environment. Inflamm Bowel Dis. 2007;13(4):439–445. | ||
Rhodus NL, Cheng B, Ondrey F. Th1/Th2 cytokine ratio in tissue transudates from patients with oral lichen planus. Mediators Inflamm. 2007;2007:19854–5. | ||
Poulter LW, Lehner T. Immunohistology of oral lesions from patients with recurrent oral ulcers and Behçet’s syndrome. Clin Exp Immunol. 1989;78(2):189–195. | ||
Abetz LM, Savage NW. Burning mouth syndrome and psychological disorders. Aust Dent J. 2009;54(2):84–93quiz 173. | ||
Soto Araya M, Rojas Alcayaga G, Esguep A. Association between psychological disorders and the presence of Oral lichen planus, Burning mouth syndrome and Recurrent aphthous stomatitis. Med Oral. 2004;9(1):1–7. | ||
Ramos-Casals M, Tzioufas AG, Font J. Primary Sjögren’s syndrome: new clinical and therapeutic concepts. Ann Rheum Dis. 2005;64(3):347–354. | ||
Pertovaara M, Korpela M, Uusitalo H, et al. Clinical follow up study of 87 patients with sicca symptoms (dryness of eyes or mouth, or both. Ann Rheum Dis. 1999;58(7):423–427. | ||
Asmussen K, Andersen V, Bendixen G, Schiødt M, Oxholm P. A new model for classification of disease manifestations in primary Sjögren’s syndrome: evaluation in a retrospective long-term study. J Intern Med. 1996;239(6):475–482. | ||
Abe Y, Kurita S, Ohkubo Y, et al. A case of pulmonary adenocarcinoma associated with hypertrophic osteoarthropathy due to vascular endothelial growth factor. Anticancer Res. 2002;22(6B):3485–3488. | ||
Olán F, Portela M, Navarro C, et al. Circulating vascular endothelial growth factor concentrations in a case of pulmonary hypertrophic osteoarthropathy. Correlation with disease activity. J Rheumatol. 2004;31(3):614–616. | ||
Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol. 2013;35(2):123–137. | ||
Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev. 2017;16(10):1049–1057. | ||
Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 2012;32(4):1119–1136. | ||
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. | ||
Eck SM, Blackburn JS, Schmucker AC, Burrage PS, Brinckerhoff CE. Matrix metalloproteinase and G protein coupled receptors: co-conspirators in the pathogenesis of autoimmune disease and cancer. J Autoimmun. 2009;33(3-4):214–221. | ||
Suba Z. Gender-related hormonal risk factors for oral cancer. Pathol Oncol Res. 2007;13(3):195–202. | ||
Suba Z, Maksa G, Mihályi S, Takács D. Role of hormonal risk factors in oral cancer development. Orv Hetil. 2009;150(17):791–799. | ||
Mccartan BE, Sullivan A. The association of menstrual cycle, pregnancy, and menopause with recurrent oral aphthous stomatitis: a review and critique. Obstet Gynecol. 1992;80(3 Pt 1):455–458. | ||
Tarakji B, Gazal G, Al-Maweri SA, Azzeghaiby SN, Alaizari N. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Oral Health. 2015;7(5):74–80. | ||
Peck T, Olsakovsky L, Aggarwal S. Dry Eye Syndrome in Menopause and Perimenopausal Age Group. J Midlife Health. 2017;8(2):51–54. | ||
Versura P, Campos EC. Menopause and dry eye. A possible relationship. Gynecol Endocrinol. 2005;20(5):289–298. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

