Back to Journals » Breast Cancer: Targets and Therapy » Volume 13
Combination of Pertuzumab and Trastuzumab in the Treatment of HER2-Positive Early Breast Cancer: A Review of the Emerging Clinical Data
Received 3 November 2020
Accepted for publication 23 March 2021
Published 14 June 2021 Volume 2021:13 Pages 393—407
DOI https://doi.org/10.2147/BCTT.S176514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Megan Jagosky, Antoinette R Tan
Department of Solid Tumor Oncology and Investigational Therapeutics, Levine Cancer Institute, Atrium Health, Charlotte, NC, USA
Correspondence: Antoinette R Tan
Levine Cancer Institute, Atrium Health, 1021 Morehead Medical Drive, Charlotte, NC, 28204, USA
Tel +1 980-442-6400
Fax +1 980-442-6321
Email [email protected]
Abstract: Human epidermal growth factor receptor type 2 (HER2) is a relevant and effective target in breast cancer. The development of monoclonal antibodies against HER2 has revolutionized the treatment of HER2-positive breast cancer. The humanized monoclonal antibody, trastuzumab, was the first in its class to be widely adopted. It was initially studied in the metastatic setting and then in the treatment of early-stage disease, demonstrating significant improvement in overall survival in both settings. The addition of pertuzumab further improved upon results achieved with trastuzumab and chemotherapy, specifically extending overall survival in patients with metastatic disease, lessening the risk of recurrence when used in the adjuvant setting, and improving pathologic complete response rate when utilized in the neoadjuvant setting. In this article, we review the studies that support the use of HER2-directed monoclonal antibodies in early-stage breast cancer both in the adjuvant and neoadjuvant settings and focus on the success of dual HER2-targeted therapy achieved with the combination of trastuzumab and pertuzumab. A newer way to administer these agents, specifically the subcutaneous formulation of pertuzumab and trastuzumab with recombinant human hyaluronidase, will also be discussed.
Keywords: breast cancer, HER2, trastuzumab, pertuzumab
Introduction
Human epidermal growth factor receptor type 2 (HER2) belongs to a category of epidermal growth factors including HER1, HER3, and HER4.1 HER2 is unique to other members of the HER family as it can constitutively dimerize without ligand binding. It forms homodimers and heterodimers with other epidermal growth factor receptor (EGFR) proteins leading to downstream activation of multiple pathways crucial to cellular regulations including proliferation, differentiation, migration, and apoptosis. Specific pathways activated via phosphorylation include Ras/Raf/mitogen-activated protein kinase, phosphatidylinositol-3-kinase (PI3K)/AKT, and phospholipase Cγ (PLCγ)/protein kinase C (PKC).1
The HER2-HER3 heterodimer likely promotes the strongest signal transduction, particularly via the PI3K/AKT/mTOR pathway.2 HER2 is overexpressed in 15 to 20% of all invasive breast cancers.3,4 HER2-positivity has traditionally been associated with worse prognosis, early metastases, decreased disease-free survival (DFS), and decreased overall survival (OS).5,6 With the advent of anti-HER2 therapy, the outcomes of patients with HER2-amplified breast cancer have improved.
The Advent of Trastuzumab
Trastuzumab was the first recombinant humanized monoclonal antibody that dramatically changed the treatment of HER2-positive breast cancer. Originally named 4D5, trastuzumab was studied in the mid to late 1990s, demonstrating cytotoxic effects as monotherapy in breast cancer cell lines as well as synergism when paired with multiple chemotherapeutic agents.7 It targets subdomain IV of the HER2 extracellular domain, exerting its antitumor effect by blocking HER2 cleavage, downstream proliferation pathways, and promoting apoptosis. Additionally, it stimulates endocytosis and prevents shedding of the extracellular domain of HER2. Lastly, it triggers antibody-dependent cellular cytotoxicity.8 It is typically dosed by body weight and given intravenously weekly or every three weeks, depending on the treatment setting.9
Trastuzumab was first approved by the US Food and Drug Association (FDA) in 1998 to treat patients with metastatic breast cancer whose tumors overexpress the HER2 protein and who have received at least one chemotherapy regimen for metastatic disease. Slamon et al reported the results of a phase III study that demonstrated the benefit of adding trastuzumab to chemotherapy (anthracycline and cyclophosphamide or paclitaxel) in the first-line metastatic setting.10 The addition of trastuzumab to chemotherapy improved the median time to progression to 7.4 months compared to 4.6 months in the chemotherapy alone group (p<0.001). This translated to an improvement in median survival of about 5 months (p=0.046).
In addition to the efficacy of trastuzumab, it is also well tolerated by patients. The most concerning adverse effect is cardiotoxicity which is increased when trastuzumab is combined with anthracyclines. Early studies demonstrated that New York Heart Association (NYHA) class III or IV heart failure occurred in up to 27% of patients treated with anthracycline, cyclophosphamide, and trastuzumab.10 Evaluation of left ventricular ejection fraction (LVEF) is recommended prior to initiation of adjuvant trastuzumab, every 3 months while on treatment, at completion, and every 6 months for 2 years after treatment is completed.9 Most cardiac toxicity is reversible with vigilant monitoring and early cessation of trastuzumab when it occurs.11
Of note, there have been efforts to assess the cardiac safety of HER2-targeted therapy in breast cancer patients with mildly reduced ejection fraction. There are two small single-arm prospective studies that have been conducted evaluating the intervention with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) and beta blockers and close cardiac monitoring in HER2-positive breast cancer patients with compromised cardiac function.12,13 Both show the feasibility of continuation of HER2 treatments in patients with mild cardiotoxicity in the setting of cardiac monitoring and intervention with cardioprotective medications.
The Addition of Pertuzumab
Pertuzumab is a HER2-targeted monoclonal antibody that belongs to the class of HER dimerization inhibitors. It binds to a different domain of HER2 than trastuzumab (subdomain II), preventing the HER2/HER3 heterodimerization and homodimerization.14 Preclinical studies, both in vitro and xenograft models, have demonstrated synergistic effects when pertuzumab and trastuzumab are given together.15,16 Similar to trastuzumab, pertuzumab’s efficacy was initially demonstrated in the metastatic setting. The Clinical Evaluation of Pertuzumab and Trastuzumab (CLEOPATRA) study was a large, phase III randomized trial of 808 breast cancer patients that demonstrated the added benefit of pertuzumab to trastuzumab and docetaxel in the first-line metastatic setting.17,18 The addition of pertuzumab increased the median OS from 40.8 months with docetaxel and trastuzumab alone to 56.5 months with pertuzumab added to docetaxel and trastuzumab (32% reduction in mortality; hazard ratio [HR] of 0.68 favoring pertuzumab group). In addition, because crossover was allowed, the efficacy of pertuzumab may have been underestimated. Remarkably, at 8-years of long-term follow-up, 37% of patients with metastatic disease treated with the combination were still alive.19 This regimen was established as the standard of care for first-line treatment of HER2-positive metastatic breast cancer.20 Of note, only 10% of the study population received adjuvant trastuzumab and therefore an interesting question is whether this subset of patients also derived the same benefit as those not previously treated with adjuvant trastuzumab, but the analysis is limited by the small sample size.
Unlike trastuzumab, the incidence of cardiotoxicity is not increased with the addition of pertuzumab to chemotherapy. Diarrhea is an adverse effect that frequently occurs with pertuzumab treatment. In the CLEOPATRA trial, the incidence of all grade diarrhea was 68% in patients treated in the pertuzumab arm vs 49% in the placebo arm. After completion of docetaxel, diarrhea persisted at twice the incidence in the pertuzumab treated group compared to the placebo group (28% versus 14%). The exact mechanism by which pertuzumab causes diarrhea is not entirely known but it is theorized to be a secretory process related to increased activity of the intestinal epithelial chloride channels.21 Proactive follow-up and early intervention should be employed to help manage pertuzumab-related diarrhea, particularly in the first cycle when diarrhea most commonly occurs.22 Particular attention should be paid to older and/or Asian patients as studies have shown an increased incidence of grade 3 diarrhea in subgroup analysis.23 Management of pertuzumab-related diarrhea includes aggressive rehydration and antidiarrheal medications.
Adjuvant HER2-Targeted Trials
Adjuvant Trastuzumab in Addition to Chemotherapy
The benefit of adding trastuzumab to chemotherapy was also observed in the adjuvant setting. Four major trials demonstrated treatment with adjuvant trastuzumab for one year with chemotherapy was superior in both DFS and OS in comparison to adjuvant chemotherapy alone.24–27 The results from these pivotal randomized phase III trials which included Herceptin Adjuvant (HERA) Trial, National Surgical Adjuvant Breast and Bowel Project (NSABP B-31), the North Central Cancer Treatment Group (NCCTG) N9831 and Breast Cancer International Research Group 006 (BCIRG-006), led to the FDA approval of adjuvant trastuzumab in HER2-amplified early-stage breast cancer in 2006.
The N9831 and NSABP B-31 trials evaluated the benefit and tolerability of adding trastuzumab to paclitaxel in the standard AC-T (doxorubicin and cyclophosphamide followed by paclitaxel) regimen in HER2-positive early breast cancer.26 The study arms that included trastuzumab had fewer events compared to the chemotherapy alone groups (HR, 0.48; p<0.0001), prompting an early stop. The 3-year DFS was improved by 12% in the trastuzumab group with a 33% reduction in death (p=0.015). At a median 8.4 years follow-up, the benefit of trastuzumab remained significant, with a relative risk reduction of 40% in DFS and 37% in OS.26 Long-term cardiac follow-up at a median of 9.2 years in the N9831 trial demonstrated that the AC-T alone arm had the lowest incidence of cardiac events at 0.6%.28 Treatments with trastuzumab plus AC-T and trastuzumab plus paclitaxel were associated with a 2.8% and 3.4% cardiac event incidence, respectively. Antihypertensives, baseline LVEF less than 65%, and age of 60 and older were associated with increased cardiac events. These factors may be used to guide treatment decisions.
HERA was another important adjuvant study evaluating the benefit of trastuzumab after adjuvant chemotherapy.27,29 It included 5102 patients with early-stage HER2-positive breast cancer. Patients with small (≤1 cm in diameter), node-negative invasive tumors were not eligible for this trial. Patients were randomized to one year of adjuvant trastuzumab, two years of adjuvant trastuzumab or observation after they had completed primary therapy, consisting of surgery, radiation, and chemotherapy. The justification for extended duration of trastuzumab therapy was that most relapses occur around 18 to 24 months post-surgery. Patients received a minimum of four courses of chemotherapy (either adjuvant (89%) or neoadjuvant (5%) or both (6%)). HR for risk of events in the trastuzumab vs observation group was 0.54 (P<0.0001) translating to a DFS improvement of 8.4% at two years. There was also a reduce risk for distant recurrence in the trastuzumab group with an HR of 0.49 (P<0.0001). Analyses performed at later follow-up time points (median of 8 and 11 years) demonstrated no DFS or OS benefit between 1 and 2 years of adjuvant trastuzumab.30 Therefore, this study confirmed one year of adjuvant trastuzumab is better than none, but two years is not better than one.
The previously described adjuvant trials included anthracycline-based regimens, all of which reduce the rate of recurrence by approximately one-half and improved survival by about 30%. The combination of trastuzumab with anthracycline increases the risk of congestive heart failure by a factor of 4 to 5 and approximately 10% of patients treated with trastuzumab have a substantial decrease in their LVEF.26 This finding led to the study of an anthracycline-sparing adjuvant regimen of docetaxel and carboplatin with the addition of trastuzumab in BCIRG-006, in which 3222 early-stage HER2-positive breast cancer subjects were randomly assigned to three arms: 1) AC- docetaxel (D) alone, 2) AC-D with 52 weeks of trastuzumab, or 3) docetaxel and carboplatin with 52 weeks of trastuzumab (TCH).24 At the final analysis, both trastuzumab arms had superior outcomes with DFS and OS compared to the anthracycline arm. In the final analysis, presented at the 2016 annual CTRC-AACR San Antonio Breast Cancer Symposium 10-year DFS was 74.6% with AC-DH (p < 0.0001), 73.0% with TCH (p= 0.0011), and 67.9% with AC-D. Overall survival at 10 years was 85.9% with AC-DH, 83.3% with TCH and 78.7% with AC-D, representing risk reductions of 27% (p < 0.0001) and 24% (p = 0.0075), respectively.31 Of note, while the difference in efficacy was not significant between the anthracycline arm of ACDH and the non-anthracycline arm of TCH, the study was not powered to demonstrate a difference. Additionally, there were less cardiac toxicities with the non–anthracycline regimen; the rate of grade 3-4 congestive heart failure was 0.4% for TCH and 2% for AC-DH.31 Based on these findings, TCH is an acceptable adjuvant regimen that may lessen the risk of cardiotoxicity.
Minimizing cardiotoxicity is particularly paramount in the setting of node-negative HER2-positive breast cancer measuring ≤3 cm in which the risk of recurrence is low, but not negligible. Tolaney et al conducted the Adjuvant Paclitaxel and Trastuzumab (APT) phase II single-arm trial, in this lower risk group to determine the benefit of omitting doxorubicin and administering adjuvant trastuzumab and paclitaxel.32,33 There were 406 patients that received 12 weeks of the combination followed by the completion of 1 year of trastuzumab as a single-agent. The DFS rate at 7 years was 93% and the 7-year OS rate was 95%.33 Cardiac toxicity was noted in 15 patients with two symptomatic cases, 13 asymptomatic cases, and the former requiring trastuzumab discontinuation and the latter, necessitating a hold in therapy. Two patients in which treatment was held did not have their ejection fraction normalize and therefore they could not complete a year of trastuzumab. The strategy evaluated in this trial shows that treatment de-escalation is possible in patients with Stage I HER2-positive breast cancer.
Overall, the HERA trial, NSABP B-31/NCCTG N9831 joint analysis and BCIRG-006 trial demonstrate that the number needed to treat (NNT) to avoid one disease recurrence or death ranges from 9 to 20 when trastuzumab is added to standard chemotherapy in the adjuvant setting. The NNT to save one life was similar and ranged from 11 to 22.
Adjuvant Trastuzumab-Drug Conjugate
Von Minckwitz et al reported the results of the KATHERINE trial, which studied the efficacy of adjuvant trastuzumab emtansine (T-DM1), an antibody-drug conjugate of trastuzumab linked to the microtubule inhibitor, emtansine, in comparison to adjuvant trastuzumab in early-stage HER2-positive breast cancer.34 This was a phase III, open-label trial that enrolled patients with early HER2-positive breast cancer who were found to have residual invasive disease in the breast or axilla at surgery after receiving neoadjuvant therapy containing a taxane (with or without anthracycline) and trastuzumab. Patients were randomly assigned to receive adjuvant T-DM1 or trastuzumab for 14 cycles. The primary end point was invasive disease-free survival (iDFS). At a median follow-up of 41 months, invasive disease or death had occurred in 12.2% of patients in the T-DM1 group in comparison to 22.2% in the trastuzumab group. The estimated percentages of patients free of invasive disease at 3 years were 88.3% in the T-DM1 group and 77.0% in the trastuzumab group and iDFS was significantly higher among patients who received T-DM1 than among those who received trastuzumab (HR, 0.50; P<0.001). Overall, the NNT with T-DM1 compared to trastuzumab to avoid one disease recurrence or death was 9. These results were practice-changing and led to the FDA approval of T-DM1 in May 2019 for patients with HER2-positive breast cancer with residual disease at the time of surgery after neoadjuvant taxane and trastuzumab-based neoadjuvant chemotherapy.35
Combination of Trastuzumab and Pertuzumab in the Adjuvant Setting
While adjuvant trastuzumab is beneficial, approximately one-third of patients with HER2-positive early-stage breast cancer recur. Based on the benefits of dual HER2-blockade in the treatment of HER2-positive metastatic breast cancer, pertuzumab added to trastuzumab was evaluated in the adjuvant setting. APHINITY was a phase III trial of 4805 women with HER2-positive, node-positive or high-risk node-negative operable breast cancer in which pertuzumab added to trastuzumab and chemotherapy in the adjuvant setting was compared to trastuzumab and chemotherapy alone.36 The chemotherapy included anthracycline-based and taxane-based chemotherapy regimens or a taxane with carboplatin regimen. Patients received pertuzumab or placebo with trastuzumab for a year. At accrual completion, two-thirds of the patients had node-positive disease and one-third had hormone receptor-negative disease. The primary endpoint was iDFS. At the time of publication, the estimated difference in 3-year iDFS was less than one percent (94.1% in the pertuzumab group and 93.2% in the placebo group). The node-positive disease subgroup had a 92% iDFS with pertuzumab added compared to 90.2% in the placebo group (p=0.02). The hormone receptor-positive subgroup had a 2% improvement in estimated 3-year iDFS (94.8 vs 92.8%), but this was not statistically significant. Toxicity was increased with dual HER2-blockade particularly in the form of diarrhea with a 9.8% rate of grade 3 or higher diarrhea in the pertuzumab group compared to 3.7% in the placebo group. The risk of diarrhea was even greater among patients treated with the docetaxel and carboplatin regimen at 18% incidence, demonstrating concern for using docetaxel compared to paclitaxel. Cardiac toxicity was similar between treatment arms.36
A planned six-year analysis was published showing slight widening of iDFS to 91% in the pertuzumab group vs 88% in the placebo group, which was statistically significant (HR 0.76; 95% CI, 0.64 to 0.91).37,38 Most events were distant recurrences. Pertuzumab continued to offer the greatest benefit in the node-positive cohort with an absolute iDFS improvement 4.5% (88% vs 83%); HR of 0.72. The 6-year OS difference remains insignificant with 95% in the pertuzumab group compared to 94% in the placebo group. The NNT with adjuvant pertuzumab in combination with trastuzumab to avoid one disease recurrence or death was 36, while the NNT to save one life was 100.
In practice, this regimen should be offered to node-positive patients regardless of hormone receptor status. A challenge in incorporating the data from the APHINITY trial is that in clinical practice most Stage II and higher HER2-positive breast cancer patients receive neoadjuvant chemotherapy, with trastuzumab and pertuzumab, and APHINITY is an adjuvant trial. In patients who achieve a pCR, it is generally recommended that pertuzumab be continued with trastuzumab after surgery to complete 1 year of treatment as has been studied in neoadjuvant trials such as TRYPHAENA and BERENICE (which will be reviewed).
Harbeck et al conducted the phase III KAITLIN trial to evaluate whether T-DM1 could be utilized in place of adjuvant trastuzumab and taxane chemotherapy to allow for a potentially less toxic treatment option.39 The study enrolled patients who had completely resected HER2-positive disease that was node-positive or node-negative, hormone-receptor negative, and tumor size >2 cm. There were two cohorts that both received anthracycline-based chemotherapy followed by either T-DM1 and pertuzumab or taxane chemotherapy and concurrent trastuzumab and pertuzumab. Notably, after a median of 57 months of follow-up, there was no significant difference in the primary endpoint, iDFS, between the two treatments in the full intention-to-treat population (3-year iDFS 94.2% vs 93.1%).
While the intent was to impart less toxicity with the TDM-1 regimen, there was a greater incidence of hepatotoxicity, thrombocytopenia, and more discontinuation in the TDM-1 arm (18.4%) than the taxane plus HER2 targeted antibody arm (3.8%). The most common reason for discontinuation in the TDM-1 arm was an increased bilirubin (4.6%), followed by peripheral neuropathy (3.1%), and thrombocytopenia (2.6%). Therefore, adjuvant trastuzumab and pertuzumab with chemotherapy remains the standard of care adjuvant regimen for patients with high-risk, HER2-positive early-stage breast cancer. Currently, trials are evaluating de-escalation or optimization approaches. EA1181 (NCT042662) is a single-arm de-escalation trial and the first study in the CompassHER2 research program, which aims to optimize treatment for patients with HER2-positive breast cancer. Eligible Stage II and IIIA HER2-positive breast cancer will be treated with a neoadjuvant combination of 12 weeks of a taxane (paclitaxel, docetaxel, or nab-paclitaxel), trastuzumab, and pertuzumab (THP) before surgery followed by 13 cycles of HP if they achieve a pCR or standard of care treatment if they do not achieve a pCR. This trial has an accrual goal of 1250 participants.
HER2-Targeted Therapies in the Neoadjuvant Setting
The benefits of neoadjuvant chemotherapy for early-stage breast cancer have been described elsewhere.40 It has been well supported that pathologic complete response (pCR), commonly defined as lack of viable cancer in the breast or axilla (ypT0ypN0), predicts less chance of local and distant recurrence.41,42 Also, the use of neoadjuvant therapy can guide the need for adjuvant therapy as patients with residual disease may receive greater benefit from additional systemic treatment (capecitabine for HER2-negative disease and T-DM1 for HER2-positive disease).34,43
Neoadjuvant Trastuzumab in Addition to Chemotherapy
Many neoadjuvant studies have proven the benefit of adding trastuzumab to standard neoadjuvant chemotherapy. Gianni et al conducted the NeOAdjuvant Herceptin (NOAH) trial, which demonstrated an association of pCR and event-free survival (EFS) using HER2-based neoadjuvant therapy.44 Between 2002 and 2012, the NOAH trial randomized 235 women with locally advanced or inflammatory HER2-positive disease to receive either preoperative chemotherapy with concurrent trastuzumab (with a full year completion of trastuzumab post-operatively) or preoperative chemotherapy alone.44 The chemotherapy regimen used was doxorubicin, paclitaxel, cyclophosphamide, methotrexate and fluorouracil (H+AT-CMF). A similar cohort of 99 patients with HER2-negative disease was included and treated with chemotherapy alone (AT-CMF). After a median of 5.4-years follow-up, the primary endpoint, EFS, was significantly greater in the trastuzumab-containing arm at 58% compared to 43% in the chemotherapy only arm with an HR of 0.64 (p=0.016). In addition, the incidence of total pCR (ypT0 ypN0) was 38% in the trastuzumab arm versus 19% in the chemotherapy alone arm (p=0.001). EFS was highly associated with pCR, supporting the concept that pCR is a strong biomarker for improved long-term outcomes.45
The relationship between pCR and disease outcomes was also demonstrated in a phase II trial by Untch et al, entitled Taxol Epirubicin Cyclophosphamide Herceptin NeOadjuvant (TECHNO).46 The chemotherapy regimen used included epirubicin, cyclophosphamide, and paclitaxel (ECT) and all patients received perioperative trastuzumab. The pCR rate was almost identical to what was reported in the NOAH study at 38.7%. Those with pCR had a significantly improved 3-year DFS (88% in patients with pCR compared to 73% in patients without pCR (P = 0.01) and OS (96% in patients with pCR compared to 86% in patients without pCR (P = 0.025))). Notably, the duration of time receiving neoadjuvant trastuzumab was shorter at 12 weeks than in the NOAH trial but the attainment of pCR was similar (38.7% in TECHNO, 39% in patients on trastuzumab arm in NOAH).
Even when the perioperative chemotherapy differs, pCR improves with the addition of trastuzumab. GeparQuattro, a prospective trial of HER2-positive patients enrolled approximately 1500 patients with early-stage disease and treated them preoperatively with EC-D followed by docetaxel ± capecitabine with trastuzumab.47 Remarkably, the pCR was almost identical to the TECHNO and NOAH study at 40% using the definition of no invasive residual tumor in breast or lymph nodes. The outcomes were better than those in the HER2-negative disease reference group (17.3% with pCR).
Over the years there have been many neoadjuvant trials enrolling patients with HER2-overexpressing early-stage breast cancer. A pivotal meta-analysis by Cortazar et al, CTneoBC, helped consolidate the findings from 12 international studies of almost 12,000 patients to look at responders.41 While the studies were heterogeneous and not all involved targeted therapy with trastuzumab, a consistent theme was noted; when targeted therapy with trastuzumab was given, pCR improved, specifically to 40% of patients who received trastuzumab as part of their neoadjuvant regimen in comparison to 23% who had chemotherapy alone. This translated into a significantly improved EFS HR of 0.39, regardless of hormone receptor status.
Neoadjuvant Trastuzumab Plus Pertuzumab and Chemotherapy
To enhance HER2-inhibition, the NeoSphere trial by Gianni et al evaluated dual HER2 blockade with multiple incorporations of pertuzumab with trastuzumab and chemotherapy in the neoadjuvant setting.48 There were four arms: 1) docetaxel and trastuzumab, 2) docetaxel and pertuzumab, 3) docetaxel with pertuzumab and trastuzumab, and 4) a chemotherapy-free arm with pertuzumab and trastuzumab alone. Eligible patients were early-stage, locally advanced or inflammatory HER2-positive breast cancer and they received adjuvant chemotherapy with concomitant trastuzumab for 1 year. Triplet therapy with chemotherapy and dual HER2-targeted agents had the greatest pCR rate at 45.8%. The pCR rates were 29% and 24% in the trastuzumab and docetaxel arm and in the pertuzumab and docetaxel arm, respectively. Interestingly, the chemotherapy-free arm demonstrated a pCR rate of 16.8%, indicating there is a subset of patients for whom chemotherapy can be omitted, but this requires identification with accurate biomarkers to correlate with response. Estrogen receptor (ER) status of the tumors predicted response to the docetaxel, pertuzumab and trastuzumab arm with the ER-negative group having a 63.2% pCR rate vs 26% in the ER-positive group. One limitation of this trial is the exclusion of primary endocrine therapy for the ER-positive group. Historically, HR-positive, HER2-positive tumors are less likely to attain a pCR to neoadjuvant chemotherapy compared to HR-negative tumors as was seen in NeoSphere. Neoadjuvant endocrine therapy compared to chemotherapy has been evaluated in phase II trials and while it appears inferior to chemotherapy in premenopausal females, it has demonstrated similar response rates to chemotherapy in the postmenopausal cohort.49
In September 2013, the FDA approved the combination of pertuzumab and trastuzumab with chemotherapy for the neoadjuvant treatment of HER2-positive breast cancer. This was the first time a drug had been approved based on the surrogate endpoint of pCR. Its accelerated approval was initially contingent on the results of the adjuvant APHINITY study. The 5-year follow-up of NeoSphere demonstrated that 5-year PFS was improved in those patients treated with the triplet compared to trastuzumab and docetaxel alone.50 This long-term follow-up also demonstrated that patients with pCR had longer PFS compared with those who did not with an HR of 0.54 compared to those who did not meet this marker. To determine which patients may preferentially benefit from pertuzumab added to trastuzumab-based regimen, biomarkers in both tissue and sera were evaluated from patients.51 Proteins downstream from HER2 were analyzed as well as the presence of the truncated forms of HER2 (p95HER2) which has demonstrated poorer response to HER2-targeted therapy and outcomes. No predictive biomarkers were discovered across cohorts to be associated with pCR.
There has been a theoretical concern that molecular alterations in phosphatidylinositol 3-kinase (PI3K), seen in 12 to 39% of HER2-positive breast cancer, can lead to resistance to trastuzumab therapy. Alterations in PI3K leads to constitutively active p110α, one of the targets of anti-HER2 therapy.52 Preclinical studies have demonstrated trastuzumab resistance with activating mutations in the helical or kinase domains of PIK3CA.53 This effect has not been reported in an analysis of tissue specimens from clinical trials, such as NSABP B-31, in which no variation in trastuzumab response has been associated with the presence of PIK3CA mutations.54 An explanation for this could be due to other mechanisms of action of trastuzumab including antibody-dependent cellular cytotoxicity.
Like NeoSphere, other studies have evaluated chemotherapy-free neoadjuvant therapy for the treatment of HER2-positive early breast cancer. The phase II West German Study Group-Adjuvant Dynamic Marker-Adjusted Personalized Therapy (WSG-ADAPT) trial randomized 134 patients to 12 weeks of pertuzumab and trastuzumab ± paclitaxel.55 While the dual HER2 blockade with paclitaxel outperformed dual HER2 blockade alone (pCR 90.5% vs 34.4%, respectively), the possibility of de-escalation and chemotherapy omission in a select group of patients is feasible.
In a comparable population of patients (HER2+ HR-), the GeparSepto (GBG 69) phase III trial investigated whether exchanging 12 cycles of nab-paclitaxel (150 mg/m2 and 125 mg/m2) for 12 cycles of paclitaxel (80 mg/m2) weekly with pertuzumab and trastuzumab followed by 4 cycles of epirubicin and cyclophosphamide improves pCR rates.56 There were multiple cohorts, including TNBC, luminal, and HER2-positive disease. HER2-positive patients received pertuzumab and trastuzumab and continued after surgery with trastuzumab up to one year. In the HER2-positive HR-negative cohort, pCR was significantly higher at 38% for nab-paclitaxel compared to paclitaxel 29%. After approximately 4 years of follow-up there was improved iDFS in the nab-paclitaxel arm at 84% vs 76.3% in the paclitaxel arm (p=0.002), but similar OS (89.7% vs 87.2%, respectively).57 Even in patients who did not achieve a pCR, outcomes were still better with nab-paclitaxel with disease-free survival HR of 0.67 (p=0.015). However, no difference in overall survival has been noted. The benefit of nab–paclitaxel was accompanied by an increased incidence of adverse effects, particularly grade 3-4 peripheral sensory neuropathy (2.7% paclitaxel, 14.5% nab-paclitaxel 150 mg/m2, 8.3% nab-paclitaxel 125 mg/m2). There was no difference in time to resolution of neuropathy between paclitaxel and nab-paclitaxel. Non-hematologic adverse events were more common in the nab-paclitaxel group however those treated with the 125 mg/m2 dose had less high-grade neutropenia and infection and less discontinuation than the higher nab-paclitaxel dose.
The I-SPY trial evaluated numerous novel agents with standard therapy in the neoadjuvant setting for various subtypes of early breast cancer in a phase II trial. The goal was to determine which combination has the highest rate of success if advanced to phase III study based on initial results. This trial included a treatment arm of pertuzumab added to paclitaxel and trastuzumab (THP) in 44 patients followed by AC compared to trastuzumab and paclitaxel alone (TH) followed by AC in 31 patients.58 THP met the Bayesian predictive probability criterion for achievement of pCR among all subgroups (HR ± HER2+) with pCR of 22% with TH and 54% with THP therefore the latter graduated. Another treatment arm utilized T-DM1 and pertuzumab (52 patients) in comparison to paclitaxel and trastuzumab (31 patients).59 Among all HER2-positive patients, pCR was more than twice as likely achieved in the T-DM1 and pertuzumab arm compared to the paclitaxel and trastuzumab arm (52% vs 22%, respectively). Similar benefits were noted among all subgroups regardless of hormone receptor status.
An anthracycline–sparing regimen commonly used in the neoadjuvant setting is docetaxel, carboplatin, trastuzumab and pertuzumab (TCHP). There was no definitive study that led to its adoption into the treatment paradigm, but rather a series of studies and experiences.60–63 The longest followed neoadjuvant study of TCH was done by Kolberg et al and demonstrated a pCR rate of 43.6% with a median 4-year follow-up DFS of 84.6% and OS of 91%. This was the impetus to add dual HER2 blockade to TCH to enhance outcomes.64 When studied in TRYPHAENA, six neoadjuvant cycles of TCHP led to a pCR of 66.2% with low rates of cardiac toxicity.65
Like the TCHP regimen, van Ramshorst et al investigated the anthracycline-sparing regimen of carboplatin, paclitaxel, trastuzumab and pertuzumab (PTC + Ptz) in the neoadjuvant setting to avoid the short- and long-term cardiotoxicity of anthracycline chemotherapy.66 The results from this study (TRAIN2) were published in 2018 with a 3-year update provided at ASCO in 2020.67 The 438 patients with early-stage HER2-positive breast cancer were randomized to either trastuzumab and pertuzumab plus 5-FU, epirubicin, and cyclophosphamide (FEC-T + Ptz) for 3 cycles followed by PTC + PTz for 6 cycles or PTC + Ptz for 9 cycles. The primary endpoint, pCR, occurred in 67% of patients in the FEC-T + Ptz-containing group compared to 68% in the PTC + Ptz (p=0·95). Adverse events were similar between arms including symptomatic left ventricular systolic dysfunction with the only major difference being grade 3 or greater febrile neutropenia (10% anthracycline arm vs 1% in non-anthracycline arm (p<0.0001)). Difference in cardiotoxicity risk became evident at a longer follow-up (median of 46 months) with 36% of those in the anthracycline arm showing either an LVEF decrease of greater than or equal to 10% or an LVEF decline to less than 50% compared to 22% in the non-anthracycline arm (p=0.0016).
Like the KATHERINE and KAITLIN studies wherein T-DM1 was used in the adjuvant setting for HER2-positive early-stage breast cancer, T-DM1 has also been investigated in the neoadjuvant setting. Hurvitz et al conducted the phase 3 multi-institutional study, KRISTINE, to determine if neoadjuvant chemotherapy could be spared in place of pertuzumab paired with T-DM1.68 The trial randomized 444 patients with operable HER2-positive breast cancer to either neoadjuvant T-DM1 with pertuzumab or TCHP. Patients in the T-DM1 arm went on to adjuvant T-DM1 plus pertuzumab and patients in the neoadjuvant TCHP arm received adjuvant trastuzumab and pertuzumab. The T-DM1 arm had a pCR rate of 44.4%, compared to 55.7% in the chemotherapy arm (p=0.016). At the planned 3-year follow-up, the EFS was 85.3% in the T-DM1 arm compared to 94.2% with TCHP, predominantly due to locoregional progression before surgery in the T-DM1 arm. IDFS was similar at three years (HR of 1.11). Overall, T–DM1 with pertuzumab was associated with less grade 3-4 toxicity compared to chemotherapy (9.9% vs 24.5%, respectively), but there were more treatment discontinuations in the T-DM1 arm and increased grade 3 adverse events in the adjuvant setting.68 While it was a negative study, it is hypothesis-generating as to which patients may be able to forego chemotherapy, receiving HER2 targeted therapy alone.
Chemotherapy Backbone to Minimize Cardiotoxicity
Minimizing cardiotoxicity is of prime importance due to the variety of available chemotherapy regimens. Two prominent studies, TRYPHAENA and BERENICE, were undertaken to specifically evaluate the cardiac tolerability of anthracycline-sparing regimens given with dual HER2-targeted therapy. The phase II trial TRYPHAENA evaluated three perioperative regimens, two of which had epirubicin and the third with carboplatin.65 Arms A and B used 5-fluorouracil, epirubicin, cyclophosphamide (FEC) followed by docetaxel with concurrent or sequential trastuzumab and pertuzumab, respectively. Arm C used docetaxel with carboplatin and concurrent trastuzumab and pertuzumab (TCHP). Each group received trastuzumab for a full year. The pCR rate was similar between arms, but greatest in those treated with TCHP at 66.2%. Long-term follow-up demonstrated a DFS HR of 0.27 for those who achieved total pathological complete response (tpCR) versus no tpCR.69 At long term follow-up, cardiac dysfunction (EF decline ≥10% points from baseline to <50%) was similar among groups, occurring among 16% of those in the FEC and sequential trastuzumab plus pertuzumab group, 11% in the group with FEC and concurrent trastuzumab and pertuzumab, and 12% in the TCHP group.
The phase II BERENICE study had similar endpoints, evaluating cardiac safety in taxane and anthracycline-based regimens.23 Patients received either dose-dense doxorubicin and cyclophosphamide (ddAC) followed by weekly paclitaxel or FEC followed by docetaxel. All patients received taxane therapy with concurrent pertuzumab and trastuzumab continuation for a full year of HER2-targeted therapy. The cardiac events were low, but more prevalent in the ddAC arm which experienced NYHA class III/IV heart failure events. Thirteen patients (6.5%) in the ddAC group and four (2.0%) in the FEC group experienced ≥10% LVEF decline. Most declines were reversible and often improved at the next assessment. The choice of chemotherapy backbone to use in the neoadjuvant setting remains a decision for the treating physician based upon the patient and their individual risks and comorbidities. Table 1 lists the trials described in this article conducted in early-stage HER2-positive breast cancer.
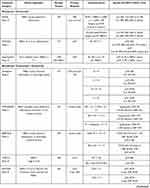 | 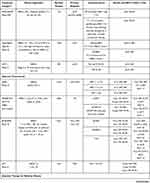 |  |
Table 1 Select Trials in HER2-Positive Early-Stage Breast Cancer |
New Formulation of Dual HER2 Targeting Therapy
Due to a desire to minimize health care resources, decrease patient treatment time and ease treatment administration, subcutaneous (SC) formulations of HER2 targeted therapy have been developed. The efficacy of subcutaneous trastuzumab in the neoadjuvant-adjuvant setting was found to be non-inferior to intravenous treatment in the phase III HannaH trial which enrolled 596 patients with early-stage HER2-positive breast cancer.70 Similar EFS and OS were reported in both treatment arms. In addition to SC trastuzumab, a new SC formulation of pertuzumab and trastuzumab with recombinant human hyaluronidase (permeation enhancer) has been developed. It allows administration of higher drug volumes, in a ready-to-use, fixed-dose combination vial (PH FDC SC). PH FDC SC was evaluated in an international, multicenter, randomized open-label two-arm phase III non-inferiority trial called FeDeriCa. The results of this study were presented at the San Antonio Breast Cancer Symposium in 2019 and subsequently published in the Lancet Oncology.71,72
PH FDC SC is injected into the thigh over 8 minutes for the loading dose and over 5 minutes for the maintenance dose. Dosing is not weight-based. The primary objective was to show non-inferiority of the pre-dose cycle 8 pertuzumab serum trough concentration (Ctrough) within the fixed-dose combination versus intravenous pertuzumab plus trastuzumab. The key secondary objectives were to show non-inferiority of pre-dose cycle 8 trastuzumab serum Ctrough within PH FDC SC, versus trastuzumab IV, total pCR in the breast and axilla (tpCR; ypT0/is, ypN0), and safety, including cardiac toxicity. For the primary and secondary pharmacokinetic endpoints, non-inferiority was concluded if the lower bound of the 90% confidence interval of the geometric mean ratio (GMR) was ≥0.8.
There were 500 patients aged 18 years and older, with an ECOG PS of 0 or 1, and HER2-positive, operable, locally advanced or inflammatory stage II–IIIC breast cancer randomly assigned to receive either chemotherapy with standard dose IV pertuzumab and IV trastuzumab (loading and maintenance) or PH FDC SC (1200 mg/600 mg loading dose and 30,000 units hyaluronidase, 600 mg/600 mg maintenance dose and 20,000 units hyaluronidase) every 3 weeks with adjuvant-neoadjuvant chemotherapy. Post-surgery patients continued adjuvant HER2-targeted treatment per the randomization. The chemotherapy backbone was either dose-dense doxorubicin and cyclophosphamide for 4 cycles followed by weekly paclitaxel for 12 weeks or doxorubicin and cyclophosphamide every 3 weeks for 4 cycles followed by docetaxel every 3 weeks for 4 cycles. The choice between chemotherapy regimen options was at the investigator’s discretion before randomization.
The GMR of the pre-dose cycle 8 pertuzumab Ctrough was 1.22 (90% CI 1.14–1.31). The GMR of pre-dose cycle 8 trastuzumab Ctrough was 1.33 (90% CI 1.24–1.43). The observed lower limits of the two-sided 90% CIs were above the pre-specified non-inferiority margin of 0.8, which meant the study met its primary endpoint. PH FDC SC was non-inferior to pertuzumab and trastuzumab IV, based on pre–dose cycle 8 pertuzumab and trastuzumab Ctrough concentrations. Pathologic CR was achieved by 150/252 patients (59.5%) in the intravenous group and 148/248 patients (59.7%) in the PH FDC SC arm and were similar to other pertuzumab, trastuzumab and chemotherapy trials.
No new or unexpected toxicities were observed. The most common grade 3–4 adverse events with PH FDC SC were neutropenia, and diarrhea, which were expected from this regimen. The incidence of primary and secondary cardiac events was low in both arms, and consistent with rates reported in previous studies with similar treatment regimens. About 13% of patients receiving PH FDC SC experienced grade 1-2 injection site reactions and the symptoms were pain, burning, and redness at the injection site. Overall, PH FDC SC offered a faster and less invasive method of dual HER2-directed antibody administration. On June 29, 2020, the US FDA approved PH FDC SC when used in combination with chemotherapy for the adjuvant treatment of HER2-positive early breast cancer or the neoadjuvant treatment of HER2-positive locally advanced, inflammatory, or early breast cancer. It was also approved for the treatment of HER2-positive metastatic breast cancer when combined with docetaxel. The National Comprehensive Cancer Network guidelines have a statement that for preoperative or adjuvant therapy for HER2-positive disease and systemic therapy for HER2-positive recurrent or stage IV disease that PH FDC SC may be substituted anywhere that the combination of IV trastuzumab and pertuzumab are given as part of systemic therapy.73
This formulation highlights the benefits of subcutaneous administration of a drug. It lessens the time that a patient spends in the treatment chair and improves the capacity of infusion centers. There are advantages to have this drug available during the SARS-CoV-2 pandemic. A shorter administration time would lessen the time patients spend in the infusion center, thereby decreasing their potential exposure to the virus. Additionally, the option to have a subcutaneous medication administered by a healthcare worker in a patient’s home is very appealing, because it saves a patient a trip to the infusion center and thereby minimizes exposure risk. However, this will require specific protocols to ensure the safety of this set-up and needs to be rigorously tested before fully implemented. This is being evaluated in a single-arm, multi-center study titled: An Expanded Access Study to Provide at Home Subcutaneous Administration of Pertuzumab and Trastuzumab Fixed-Dose Combination for Patients with HER2-Positive Breast Cancer During the COVID-19 Pandemic (NCT04395508). This study provides PH FDC SC to be administered at home by a home health nursing provider for patients with HER2-positive breast cancer who are currently receiving pertuzumab and trastuzumab IV to enable continuity of care during the pandemic. It will enroll approximately 200 patients with HER2-positive breast cancer who have completed concurrent chemotherapy with pertuzumab and trastuzumab IV and are currently receiving or will be receiving pertuzumab and trastuzumab.
Conclusions
Targeting HER2 is a model example of the importance of discovering and treating molecular drivers of disease. Since the incorporation of HER2-targeted therapies into clinical practice and treatment of patients with HER2-positive breast cancer, the clinical outcomes of this subtype have improved. Trastuzumab improves pCR rates in the neoadjuvant treatment of HER2-positive early-stage breast cancer and is beneficial in the adjuvant setting. The landscape has changed with the addition of pertuzumab to the treatment armamentarium with additional gains in pCR when used in the neoadjuvant setting and still of benefit in the adjuvant setting though more modest. Dual HER2-directed therapy in patients with high-risk disease has shown to be of benefit. The subcutaneous formulations of the HER2-targeting antibodies offer a faster mode of administration and obviates the necessity of a port for patients. The discovery and utilization of HER2-directed therapies has significantly changed the outcome of HER2-positive disease, and more progress is expected in the near future to further enhance treatment options for these patients.
Disclosure
Dr. Tan has conducted contracted research for Genentech/Roche and Daiichi-Sankyo. Dr. Jagosky has no disclosures to report.
References
1. Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. doi:10.1155/2014/852748
2. Capelan M, Pugliano L, De Azambuja E, et al. Pertuzumab: new hope for patients with HER2-positive breast cancer. Ann Oncol. 2013;24:273–282. doi:10.1093/annonc/mds328
3. Cronin KA, Harlan LC, Dodd KW, Abrams JS, Ballard-Barbash R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest. 2010;28:963–968. doi:10.3109/07357907.2010.496759
4. Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106:1–8. doi:10.1093/jnci/dju055
5. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi:10.1126/science.3798106
6. Bilous M, Ades C, Armes J, et al. Predicting the HER2 status of breast cancer from basic histopathology data: an analysis of 1500 breast cancers as part of the HER2000 International Study. Breast. 2003;12:92–98. doi:10.1016/S0960-9776(02)00273-4
7. Slamon D. Final Study Report (FSR) for H0407g: a Phase 1 safety and tolerance study of intravenous anti-p185 HER2 humanized monoclonal antibody (rhu MAb HER2) in patients with HER2 overexpressing tumors. US Food Drug Admin. 1996.
8. Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51.
9. Genentech, Inc. Herceptin (Trastuzumab) [Package Insert]. San Francisco, CA: Genentech, Inc; 2000.
10. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi:10.1056/NEJM200103153441101
11. Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23:2900–2902. doi:10.1200/JCO.2005.05.827
12. Lynce F, Barac A, Geng X, et al. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat. 2019;175:595–603. doi:10.1007/s10549-019-05191-2
13. Barron CC, Alhussein MM, Kaur U, et al. An evaluation of the safety of continuing trastuzumab despite overt left ventricular dysfunction. Curr Oncol. 2019;26:240–246. doi:10.3747/co.26.4631
14. Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi:10.1016/S1535-6108(04)00083-2
15. Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi:10.1158/0008-5472.CAN-03-3856
16. Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi:10.1158/0008-5472.CAN-08-4597
17. Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi:10.1056/NEJMoa1113216
18. Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi:10.1056/NEJMoa1413513
19. Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi:10.1056/NEJMra043186
20. Giordano SH, Temin S, Chandarlapaty S, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36:2736–2740. doi:10.1200/JCO.2018.79.2697
21. Moisan A, Michielin F, Jacob W, et al. Mechanistic investigations of diarrhea toxicity induced by anti-HER2/3 combination therapy. Mol Cancer Ther. 2018;17:1464–1474. doi:10.1158/1535-7163.MCT-17-1268
22. Swain SM, Schneeweiss A, Gianni L, et al. Incidence and management of diarrhea in patients with HER2-positive breast cancer treated with pertuzumab. Ann Oncol. 2017;28:761–768. doi:10.1093/annonc/mdw695
23. Swain SM, Ewer MS, Viale G, et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a Phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018;29:646–653. doi:10.1093/annonc/mdx773
24. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi:10.1056/NEJMoa0910383
25. Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi:10.1056/NEJMoa052122
26. Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi:10.1200/JCO.2014.55.5730
27. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi:10.1056/NEJMoa052306
28. Advani PP, Ballman KV, Dockter TJ, Colon-Otero G, Perez EA. Long-term cardiac safety analysis of NCCTG N9831 (Alliance) adjuvant trastuzumab trial. J Clin Oncol. 2016;34:581–587. doi:10.1200/JCO.2015.61.8413
29. de Azambuja E, Procter MJ, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01). J Clin Oncol. 2014;32:2159–2165. doi:10.1200/JCO.2013.53.9288
30. Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi:10.1016/S0140-6736(16)32616-2
31. Slamon DJ, Eiermann W, Robert NJ, et al.
32. Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–141. doi:10.1056/NEJMoa1406281
33. Tolaney SM, Guo H, Pernas S, et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2019;37:1868–1875. doi:10.1200/JCO.19.00066
34. von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi:10.1056/NEJMoa1814017
35. Denduluri N, Somerfield MR, Chavez-MacGregor M, et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO guideline update. J Clin Oncol. 2020;JCO2002510.
36. von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi:10.1056/NEJMoa1703643
37. Piccart M, Procter M, Fumagalli D, et al. Interim overall survival analysis of APHINITY (BIG 4-11): a randomized multicenter, double-blind, placebo-controlled trial comparing chemotherapy plus trastuzumab plus pertuzumab versus chemotherapy plus trastuzumab plus placebo as adjuvant therapy in patients with operable HER2-positive early breast cancer. In:
38. Piccart M, Procter M, Fumagalli D, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast Cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol. 2021;JCO2001204. doi:10.1200/JCO.20.01204
39. Harbeck N, Im S-A, Barrios CH, et al. Primary analysis of KAITLIN: a phase III study of trastuzumab emtansine (T-DM1) + pertuzumab versus trastuzumab + pertuzumab + taxane, after anthracyclines as adjuvant therapy for high-risk HER2-positive early breast cancer. J Clin Oncol. 2020;38(15_suppl):500. doi:10.1200/JCO.2020.38.15_suppl.500
40. Wuerstlein R, Harbeck N. Neoadjuvant therapy for HER2-positive breast cancer. Rev Recent Clin Trials. 2017;12:81–92. doi:10.2174/1574887112666170202165049
41. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi:10.1016/S0140-6736(13)62422-8
42. Broglio KR, Quintana M, Foster M, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016;2:751–760. doi:10.1001/jamaoncol.2015.6113
43. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–2159. doi:10.1056/NEJMoa1612645
44. Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi:10.1016/S0140-6736(09)61964-4
45. Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640–647. doi:10.1016/S1470-2045(14)70080-4
46. Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–3357. doi:10.1200/JCO.2010.31.4930
47. von Minckwitz G, Rezai M, Loibl S, et al. Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro study. J Clin Oncol. 2010;28:2015–2023. doi:10.1200/JCO.2009.23.8303
48. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, Phase 2 trial. Lancet Oncol. 2012;13:25–32. doi:10.1016/S1470-2045(11)70336-9
49. Semiglazov VF, Semiglazov VV, Dashyan GA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110:244–254. doi:10.1002/cncr.22789
50. Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi:10.1016/S1470-2045(16)00163-7
51. Bianchini G, Kiermaier A, Bianchi GV, et al. Biomarker analysis of the NeoSphere study: pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Res. 2017;19:16. doi:10.1186/s13058-017-0806-9
52. Seo Y, Park YH, Ahn JS, et al. PIK3CA mutations and neoadjuvant therapy outcome in patients with human epidermal growth factor receptor 2-positive breast cancer: a sequential analysis. J Breast Cancer. 2018;21:382–390. doi:10.4048/jbc.2018.21.e48
53. Hanker AB, Pfefferle AD, Balko JM, et al. Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proc Natl Acad Sci U S A. 2013;110:14372–14377. doi:10.1073/pnas.1303204110
54. Pogue-Geile KL, Song N, Jeong JH, et al. Intrinsic subtypes, PIK3CA mutation, and the degree of benefit from adjuvant trastuzumab in the NSABP B-31 trial. J Clin Oncol. 2015;33:1340–1347. doi:10.1200/JCO.2014.56.2439
55. Harbeck N, Gluz O, Christgen M, et al. De-escalation strategies in human epidermal growth factor receptor 2-positive early breast cancer (BC): final analysis of the West German Study Group Adjuvant Dynamic Marker-Adjusted Personalized Therapy Trial Optimizing Risk Assessment and Therapy response prediction in early BC HER2- and hormone receptor-positive phase II randomized trial-efficacy, safety, and predictive markers for 12 weeks of neoadjuvant trastuzumab emtansine with or without endocrine therapy (ET) versus trastuzumab plus ET. J Clin Oncol. 2017;35:3046–3054. doi:10.1200/JCO.2016.71.9815
56. Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17:345–356. doi:10.1016/S1470-2045(15)00542-2
57. Untch M, Jackisch C, Schneeweiss A, et al. NAB-paclitaxel improves disease-free survival in early breast cancer: GBG 69-GeparSepto. J Clin Oncol. 2019;37:2226–2234. doi:10.1200/JCO.18.01842
58. Buxton M, DeMichele A, Chia S, et al. Efficacy of pertuzumab/trastuzumab/paclitaxel over standard trastuzumab/paclitaxel therapy for HER2+ breast cancer: results from the neoadjuvant I-SPY 2 TRIAL. In:
59. DeMichele A, Moulder S, Buxton M, et al. Efficacy of T-DM1+pertuzumab over standard therapy for HER2+ breast cancer: results from the neoadjuvant I-SPY 2 TRIAL. In:
60. Chen W, He J, Song S, Wang M, Wu H, Wang X. Efficacy of TCH/TEC neoadjuvant chemotherapy for the treatment of HER-2-overexpressing breast cancer. Oncol Lett. 2015;9:1922–1926. doi:10.3892/ol.2015.2912
61. Lin C, Chen DR, Chang KJ, Chang TW, Wang HC. A phase II study of neoadjuvant chemotherapy with docetaxel, cisplatin and trastuzumab for T2 breast cancers. Cancer Chemother Pharmacol. 2012;69:1363–1368. doi:10.1007/s00280-012-1841-y
62. Sikov WM, Dizon DS, Strenger R, et al. Frequent pathologic complete responses in aggressive stages II to III breast cancers with every-4-week carboplatin and weekly paclitaxel with or without trastuzumab: a Brown University Oncology Group Study. J Clin Oncol. 2009;27:4693–4700. doi:10.1200/JCO.2008.21.4163
63. Shinde AM, Zhai J, Yu KW, et al. Pathologic complete response rates in triple-negative, HER2-positive, and hormone receptor-positive breast cancers after anthracycline-free neoadjuvant chemotherapy with carboplatin and paclitaxel with or without trastuzumab. Breast. 2015;24:18–23. doi:10.1016/j.breast.2014.10.008
64. Kolberg HC, Akpolat-Basci L, Stephanou M, Aktas B, Hannig CV, Liedtke C. Neoadjuvant chemotherapy with docetaxel, carboplatin and weekly trastuzumab is active in HER2-positive early breast cancer: results after a median follow-up of over 4 years. Breast Care (Basel). 2016;11:323–327. doi:10.1159/000452079
65. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24:2278–2284. doi:10.1093/annonc/mdt182
66. van Ramshorst MS, van der Voort A, van Werkhoven ED, et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1630–1640. doi:10.1016/S1470-2045(18)30570-9
67. Van der Voort A, van Ramshorst MS, van Werkhoven ED, et al. Three-year follow-up of neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2-blockade for HER2-positive breast cancer (TRAIN-2): a randomized phase III trial. J Clin Oncol. 2020;38(15_suppl):501. doi:10.1200/JCO.2020.38.15_suppl.501
68. Hurvitz SA, Martin M, Jung KH, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: three-year outcomes from the phase III KRISTINE study. J Clin Oncol. 2019;37:2206–2216. doi:10.1200/JCO.19.00882
69. Schneeweiss A, Chia S, Hickish T, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer. 2018;89:27–35. doi:10.1016/j.ejca.2017.10.021
70. Jackisch C, Stroyakovskiy D, Pivot X, et al. Subcutaneous vs intravenous trastuzumab for patients with ERBB2-positive early breast cancer: final analysis of the HannaH phase 3 randomized clinical trial. JAMA Oncol. 2019;5:e190339. doi:10.1001/jamaoncol.2019.0339
71. Tan AR, Im S-A, Mattar A, et al. Subcutaneous administration of the fixed-dose combination of trastuzumab and pertuzumab in combination with chemotherapy in HER2-positive early breast cancer: primary analysis of the phase III, multicenter, randomized, open-label, two-arm FeDeriCa study. In:
72. Tan AR, Im SA, Mattar A, et al. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): a randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2021;22:85–97. doi:10.1016/S1470-2045(20)30536-2
73. National Comprehensive Cancer Network. Breast cancer (Version 2); 2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
