Back to Journals » OncoTargets and Therapy » Volume 13
Combination of Immune Checkpoint Inhibitors with Chemotherapy in Lung Cancer
Authors Liu W, Zhang L , Xiu Z, Guo J, Wang L, Zhou Y, Jiao Y, Sun M , Cai J
Received 25 March 2020
Accepted for publication 26 June 2020
Published 27 July 2020 Volume 2020:13 Pages 7229—7241
DOI https://doi.org/10.2147/OTT.S255491
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Wei Liu,1,2 Lei Zhang,1,2 Zhiming Xiu,2 Jian Guo,1 Liye Wang,3 Yue Zhou,4 Yang Jiao,1 Meiyan Sun,1 Jianhui Cai1,2
1College of Laboratory Medicine, Jilin Medical University, Jilin 132013, People’s Republic of China; 2Jilin Collaborative Innovation Center for Antibody Engineering, Jilin Medical University, Jilin 132013, People’s Republic of China; 3Department of Pharmacological and Pharmaceutical Science, College of Pharmacy, University of Houston, Houston, TX 77030, USA; 4Department of Statistics, North Dakota State University, Fargo, ND 58102, USA
Correspondence: Jianhui Cai; Meiyan Sun Email [email protected]; [email protected]
Abstract: Tremendous progress has been achieved in the field of immune checkpoint inhibitors (ICIs) therapy in lung cancer in recent years. To generate robust, long-lasting anti-tumor immune responses in lung cancer patients, combinational ICI therapies have been explored deeply. Conventionally, chemotherapy was considered as immunosuppressive. It is now recognized that chemotherapy could also reinstate cancer cell immune-surveillance and enable the perception of cancer cells as dangerous. That is to say that chemotherapeutic drugs are not only a source of direct cytotoxic effects but also an adjuvant for anti-tumor immunity. Recently, multiple clinical studies of ICIs combined with chemotherapeutic drugs have been explored and proved effective. However, there are still crucial questions that are not well addressed, such as the optimal dose and schedule for a given combination may differ across disease indications, and the appropriate strategy of selecting patient population that can benefit from ICIs remains unclear. To facilitate more rational lung cancer ICIs therapy development, this review summarizes the immune-regulatory effects and related mechanisms of chemotherapeutic drugs and the clinical progress of ICIs and their combination with chemotherapies in lung cancer treatment.
Keywords: ICIs therapy, chemotherapy, lung cancer, immunomodulation
Introduction
Lung cancer is the leading cause of cancer death worldwide in men and women. More than 2 million people are diagnosed with lung cancer every year, of which nearly 1.8 million died from the disease.1 Lung cancer is subdivided into two major types: non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer while small cell lung cancer (SCLC) accounts for 15%.2 Traditional treatment approaches including surgery, chemotherapy, radiotherapy, and targeted therapy are unsatisfactory. The 5-year survival rate of lung cancer remains merely 16%.3 With the discovery of immune checkpoint molecules such as programmed death protein-1 (PD-1), programmed cell death-Ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), immune checkpoint inhibitors (ICIs) have recently revolutionized treatment of multiple types of cancers, including lung cancer. PD-1 targeted antibodies were approved for second-line treatment of metastatic NSCLC and non-squamous NSCLC in 2015.4,5 Subsequently, a variety of ICIs have been approved for the treatment of lung cancer because of the consistently observed clinical benefits.
However, only a small subset of lung cancer patients can benefit from ICIs.6,7 This limitation has pushed immunotherapy researchers toward the exploration of immunotherapy in combination with other treatment regimens, such as chemotherapy, radiotherapy, and targeted therapy. For a long time, platinum-based chemotherapy has been the main option for first-line treatment for lung cancer patients. Chemotherapeutic drugs take effect by not only kill tumor cells but also regulate anti-tumor T cell response through increasing tumor antigenicity, inducing immunogenic cell death, disrupting immune suppressive pathways, and enhancing effector T-cell response.8,9 A series of combinational strategies for chemotherapy with ICIs have been explored, and the clinical outcomes were promising.10,11 The goal of this study was to review the immune-regulatory effects of chemotherapeutic drugs and their clinical applications in combination with ICIs.
Mechanism of ICIs Therapy
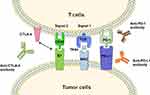
T cells play a central role in cell-mediated immunity against cancers.12 The activation of specific anti-tumor T cells requires dual signals, the first is the combination of T-cell receptor (TCR) with major histocompatibility complex (MHC)-tumor-associated antigens (TAAs) complex, the second is the combination of costimulatory molecules (CD80/86, also known as B7-1 and B7-2) expressed by antigen-presenting cells (APCs) or tumor cells with the ligand (CD28) on the surface of T cells.13 Co-inhibitory molecules can hinder T cell signal transduction processes and restrain T cell functions.14 These molecules are called immune checkpoints.15 Immune checkpoint is an important inhibitory pathway in the immune system, which can inhibit the excessive activation of the immune cells, so as to avoid damage to the physiological function of normal tissue. However, the suppression caused by immune checkpoints would make the infiltrating T cells in the tumor tend to be exhausted and unresponsive.16,17 Multiple studies have shown that immune checkpoint molecules are overexpressed in a variety of cancers and positively correlated with cancer progression and poor prognosis.18–24
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) is a member of the immunoglobulin superfamily, which is expressed on the surface of T cells. CTLA-4 competes with CD-28 to bind with B7 and causes immune evasion of tumor cells via inhibitory immune checkpoint pathway.25 CTLA-4 targeted antibody can block the CTLA-4-mediated co-inhibitory signal pathway, and subsequently induce the activation and proliferation of T cells to recover the function of killing tumor cells26,27 (Figure 1).
Programmed cell death-1 (PD-1), another inhibitory regulatory molecule of T cells, which is mainly expressed in activated T cells, dendritic cells (DCs), and monocytes.28 Programmed cell death receptor ligand 1 (PD-L1) expresses on the surface of tumor cells. The PD-L1/PD-1 interaction leads to inactivation and silencing of activated T cells, and eventually enable cancer cells to escape immune surveillance.29,30 Antibodies of PD-1 or PD-L1 could promote the proliferation of effector T cells and re-activate the exhausted T cells via blocking the inhibitory signal pathway.31,32
Except for T cells, natural killer cells (NK cells) are another powerful weapon for eliminating cancer cells. Many studies demonstrated that the infiltration degree of NK cells in tumor tissue is positively correlated with the prognosis of patients. NK cells may play an important role in controlling tumor progression.33 NK cells, as the most well-characterized cytotoxic innate lymphoid cells (ILCs), are considered as the first line of defense in cancer cells before the specific anti-tumor immune response is triggered. There are both activatory receptors (such as NKG2D) in charge of prompting “kill” signal and inhibitory receptors (such as PD-1, T-cell immunoreceptor with Ig and ITIM domains protein-TIGIT, natural killer group 2 member A-NKG2A) in charge of overriding the “kill” signal on the surface of NK cells. The enhanced expression of activatory receptors of tumor cells would activate NK cells, which would perform an immune surveillance role to trigger the release of perforin and granzyme, and also activate other immune cells by secreting cytokines.34 Therefore, we can enhance the killing effect of NK cells by blocking the inhibitory signal produced by immune checkpoint molecules. Studies have shown that anti-PD-1 therapy could activate NK cells against the tumor, especially in the case of T cell-mediated tumor resistance.35 Zhang et al found that blockade of TIGIT prevented NK cell exhaustion and promoted NK cell-dependent tumor immunity in several tumor-bearing mouse models.36
Efficacy and Strategy of ICIs in Lung Cancer
In 2011, US FDA approved a monoclonal antibody to CTLA-4 for the treatment of metastatic melanoma, marking the beginning of the new era for cancer immunotherapy.37 The preclinical and clinical success of ICIs soon promoted the development of immunotherapy in lung cancer treatment. ICI monotherapy has made remarkable achievements in the treatment of lung cancer.
In 2015, based on the result of KEYNOTE-001 trial, PD-1 targeted antibody (pembrolizumab) was approved by US FDA for second-line treatment of advanced NSCLC (including non-squamous cell carcinoma and lung squamous cell carcinoma).4 The subsequent Phase II/III KEYNOTE-010 study evaluated the efficacy of pembrolizumab compared with docetaxel in the second-line treatment of PD-L1-positive (TPS≥1%) advanced NSCLC. This was also the first trial to evaluate the relationship between the level of PD-L1 expression and benefit from PD-1 targeted antibody of lung cancer patients.38 Subsequently, anti-PD-1 antibody nivolumab and anti-PD-L1 antibody atezolizumab were also approved as the second-line treatment of advanced NSCLC (including non-squamous cell carcinoma and lung squamous cell carcinoma) based on the results of Checkmate-017/057 and POPLAR trials.39,40 The objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) of the above ICIs in the second-line treatment of advanced NSCLC were 14-20%, 3.4-4.2 months, and 9.2-13.8 months, respectively. In 2018, the FDA granted anti-PD-L1 antibody durvalumab a breakthrough therapy designation to treat patients with locally advanced, unresectable NSCLC whose disease has not progressed following platinum-based chemoradiation. Compared with the placebo group, the PFS was prolonged for 11 months (16.8 months vs. 5.6 months), and the ORR was significantly improved (28.4% vs. 16%) in the durvalumab group.41
Due to the success of second-line treatment, ICIs started entering to the field of first-line treatment of advanced NSCLC. In 2016, based on the result of the KEYNOTE-024 trial, FDA first approved PD-1 inhibitor monotherapy for first-line treatment of advanced NSCLC with PD-L1 expression on at least 50% of tumor cells and no sensitizing mutation of epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK). The trial results showed that the median PFS was 10.3 months vs 6.0 months, median OS was 30.0 months vs. 14.2 months, ORR was 44.8% vs. 27.8% in the pembrolizumab group and in the chemotherapy group, respectively.42 PD-1 inhibitor monotherapy was proved effective in advanced NSCLC patients with PD-L1≥50%, whether it be benefited in a wider range of lung cancer patients? Clinical result of KEYNOTE-042 was presented at the 2018 American Society of Clinical Oncology (ASCO) annual meeting. The study included 1274 diagnosed NSCLC patients with PD-L1≥1% without EGFR/ALK mutation. The results showed that the significant benefit of OS observed in PD-1 targeted antibody (pembrolizumab) group compared with chemotherapy in patients with PD-L1≥1%, ≥20%, or ≥50%. However, the results of sub-studies analysis demonstrated that among the patients with PD-L1 expression between 1-49%, pembrolizumab group did not benefit significantly compared with chemotherapy, while the patients with PD-L1≥50% benefited more.43 Although the feasibility of PD-1 inhibitor monotherapy as the first-line standard treatment for patients with PD-L1-positive tumors is confirmed again, it does not change the status that the drug is only recommended for first-line treatment in patients with high PD-L1 expression.
In the case of SCLC, Platinum-based doublets chemotherapy was the standard treatment, and the OS has not been improved significantly for decades. ICIs monotherapy had made a breakthrough in SCLC second-line therapy. In 2018, US FDA approved PD-1 targeted antibody nivolumab for the treatment of metastatic SCLC treated with platinum chemotherapy and at least one other therapy (CheckMate-032). This was the first approved novel treatment of SCLC in nearly 20 years. In 2019, the FDA approved another PD-1 targeted antibody pembrolizumab as monotherapy for patients with SCLC with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy (KEYNOTE-158 and KEYNOTE-028).44,45
Despite the impressive results obtained with ICIs, only a small subset of patients have a durable clinical benefit from such therapies. Moreover, ICIs therapy could also trigger hyper-progression disease (6%-29%) and pseudo-progression (4.5%).46 Numerous clinical trials have shown that combinational therapies might enhance and augment the anti-tumor activity of ICIs. A number of combinations with ICIs therapy are currently being investigated including chemotherapy, radiation therapy, and targeted therapy: (a) ICIs therapy combined with chemotherapy gradually changed the mode of first-line treatment of lung cancer. Phase II of KEYNOTE-021, Phase III of KEYNOTE-189, and phase III of KEYNOTE-407 trials performed in NSCLC patients and IMpower133 and phase III of CASPIAN trials in SCLC patients suggested that ICIs combined with chemotherapeutic drugs could significantly improve PFS, ORR, and OS. Chemotherapy combined with ICIs therapy as a first-line treatment strategy for driver oncogenes-negative advanced NSCLC has been included in the upgraded NCCN guidelines;47–51 (b) The combination of radiotherapy and ICIs therapy provides a new model for the treatment of lung cancer. Radiotherapy induces tumor cells to release antigens and trigger local or systemic anti-tumor immune responses.52 The phase III of PACIFIC evaluated the efficacy of PD-L1 targeted antibody (durvalumab) consolidation therapy or placebo therapy in patients with stage III NSCLC after chemoradiotherapy showed that combination therapy could significantly improve the median PFS, OS, and ORR. Based on this, durvalumab was approved by the US FDA for patients with unresectable stage III NSCLC who did not progress after radiotherapy and chemotherapy;41 (c) Double ICIs therapy has also been explored in the treatment of lung cancer. Phase III of CheckMate-227 showed that compared with platinum double chemotherapy, PD-L1 targeted antibody (nivolumab) combined with CTLA-4 targeted antibody (ipilimumab) first-line treatment of advanced NSCLC significantly prolonged the PFS of patients with high tumor mutation load (≥10 mut/mb), which was not related to the expression level of PD-L1.53 In May 2020, US FDA approved nivolumab combined with ipilimumab as a first-line treatment for metastatic NSCLC with PD-L1 expression (≥1%) and no EGFR or ALK mutation, the results showed that median OS of the combination group was significantly improved compared with the platinum-doublet chemotherapy group (17.1 months vs. 14.9 months). The breakthrough of the treatment is that both squamous lung cancer and non-squamous lung cancer could benefit from the treatment;54 (d) Anti-EGFR agents are standard treatments for patients with EGFR-mutant advanced NSCLC. In the advanced NSCLC population, although more than half of patients carry driving mutations that would respond to targeted therapy,55 most of the patients will develop acquired resistance, but immunotherapy generally maintained the curative effect. KEYNOTE-021 revealed that PD-1 targeted antibody (pembrolizumab) combined with EGFR inhibitor (erlotinib) could nearly double the median PFS of patients with untreated stage IIIB/IV EGFR-mutant NSCLC compared with single targeted agent, and the patients were well tolerated.56 In addition, it was observed that nivolumab combined with erlotinib strategy (CHECKMATE-021) produced positive effects and long-term benefits as a second-line treatment.57 Clinical studies have shown that the combination of ICIs therapy and chemotherapy can alleviate immune tolerance and enhance the efficacy (Table 1).
 |
Table 1 Summary of US FDA-Approved ICIs Combined Immunotherapeutic Drugs for Lung Cancer |
Immunomodulation Mechanisms of Chemotherapy
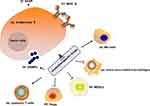
Chemotherapy is one of the most common approaches for improving the survival rate of cancer patients. Chemotherapeutic drugs can eliminate cancer cells by provoking inter- or intra-strand DNA crosslinks that destabilize DNA (alkylating agents), inhibition of DNA replication (antimetabolites), chemical damaging of DNA, inhibition of the function of crucial enzymes required for DNA synthesis (topoisomerase inhibitors), or prevention of mitosis (microtubular poisons).58 Chemotherapy has conventionally been believed to directly kill tumor cells through their cytotoxic effects and trigger tumor cell death. Chemotherapeutic drugs can affect the immunogenicity of tumor cells in a variety of ways. After the death of tumor cells induced by chemotherapeutic drugs, immunogenic components in tumor cells are released, which activate the antigen-presenting cell (APC)-mediated antigen presentation process, leading to the anti-tumor immune response. A study found that a large number of genes were significantly up-regulated after tumor cells were treated with cisplatin, and the APC stimulated by these substances can significantly enhance the proliferation and functional level of CD8+ T cells, suggesting that the antigen release induced by chemotherapeutic drugs can significantly activate the anti-tumor immune response through antigen presentation.59 Immunogenic cell death (ICD) is a process where apoptotic cells elicit an immunogenic response through the induction of damage-associated molecular patterns (DAMPs), which can be recognized by various immune cells.60 ICD has the following characteristics: (1) “eat me” signal-calreticulin membrane translocation. Calreticulin is the molecular chaperone in endoplasmic reticulum, acting as a phagocytic signal molecule when it translocates on the cell surface.61 Calreticulin interacts with a variety of receptors on the surface of innate immune cells, including pattern recognition receptors (Toll-like receptors, TLRs), endocytosis receptors (CD91), and purine receptors (nod-like receptors, NLRs), thereby stimulating the phagocytosis of immune cells against tumor cells;62 (2) “Active” signal-high mobility group box 1 (HMGB1) release. Among the DAMPs released by tumor cells after death, HMGB1 is considered to be closely related to ICD and plays an important role in the activation of antigen-presenting cells. Several studies reported that HMGB1 is up-regulated in tumor tissues of breast cancer patients receiving chemotherapy.63 The released HMGB1 works as cytokines, which can activate the TLR4 and advanced glycation end-products (RAGE) receptors of macrophages and DC cells, and then activate the immune response of APC and induce the release of TNF-α.64 Chemotherapeutic drugs can remodel the immunogenicity of tumor cells by up-regulating the expression of MHC I. Other studies have found that cisplatin can up-regulate MHC I, enhance tumor antigen presentation, and promote the recognition and activation of CTL against head and neck cancer cells.65,66 Similar results were confirmed in vivo, where plasmacytoma cells pretreated with cisplatin in vitro were inoculated subcutaneously, and the tumor cells were not conducive to tumor formation compared with the control group.67 When the cell damage reaches a certain limit, the cell stops proliferating and shows the phenotype of senescence, which is an important mechanism preventing the proliferation of damaged cells.68 Although the proliferation has stopped, the senescent cells are active in the expression of a series of inflammatory cytokines (such as IL-1 and IL-6), which is called senescence-associated secretory phenotype (SASP).69 Studies have found that the senescence of tumor cells caused by DNA damage treated by cisplatin can further trigger SASP, inducing the release of inflammatory factors.70 Some studies have noted that CCL2 in SASP can directly recruit macrophages and phagocytose senescent tumor cells.71 Chemotherapeutic drugs can inhibit the immune system by up-regulating the immune checkpoint ligands of tumor cells.
To summarize, chemotherapeutic drugs affect the immunogenicity of tumor cells including (1) enhancing the antigenicity of the tumors through the increase in mutation burden and neoantigen load, (2) increase the release of DAMPs from tumor cells and ICD, (3) up-regulate the expression of MHC molecules, and (4) trigger SASP (Figure 2).
A number of chemotherapeutic compounds may also directly affect the immune system. Clinical studies have found that anthracyclines, paclitaxel, and fludarabine can reduce the quantity or proportion of Treg cells in breast cancer, lymphoma, and other cancer, accompanied by increasing the activity cytotoxic T cells and NK cells.72,73 In vitro studies have shown that cyclophosphamide and oxaliplatin can directly inhibit the differentiation of Treg cells.63 Kan et al isolated peripheral blood mononuclear cells from patients with tumors and administered different chemotherapeutic drugs. The results showed that gemcitabine could significantly inhibit Treg cells in peripheral blood of patients.74 Studies reported that chemotherapeutic drugs doxorubicin, methotrexate, mitomycin, and paclitaxel can up-regulate the expression of MHCII and co-stimulatory molecules CD80/CD86 and the ability of antigen presentation of DCs.75 Several chemotherapeutic drugs can cause a decrease in MDSCs and M2 macrophages, and even inhibit their activity, promoting immune surveillance and clearance of the tumor immune microenvironment. Taxanes, gemcitabine, and 5-fluorouracil (5-FU) were found to reduce MDSCs in tumor-bearing mice, thereby reducing the secretion of immunosuppressive cytokines and enhancing the activity cytotoxic T cells and NK cells.76–78
Clinical Study of Combined ICIs Therapy and Chemotherapy in Lung Cancer Treatment
The efficacy of ICIs therapy in the treatment of advanced tumors has been universally confirmed. It is generally accepted that successful anti-tumor immune responses following ICIs require reactivation and proliferation of anti-tumor T cells in the tumor microenvironment. However, the ORR of ICIs represented by anti-CTLA-4 or PD-1/PD-L1 antibody is still low, with majority of patients do not respond to ICIs therapy. The minority of patients with good initial response to drugs would possibly develop resistance, which debased the long-term clinical benefit of ICIs therapy. ICIs resistance is divided into primary or adaptive resistance and acquired resistance.79 The mechanisms of primary or adaptive resistance including (a) Alteration of signaling pathways: MAPK, PI3K, WNT/β-catenin, and IFN, which suppressed the process of tumor antigen expression, tumor antigen presentation, and T cell infiltration;80–83 (b) Lack of antigenic mutations;84 (c) loss of tumor antigen expression; (d) loss of HLA expression;85 (e) up-regulation of constitutive PD-L1 expression.86 The mechanisms of acquired resistance including (a) loss of target antigen;87 (b) loss of T cell function;87 (c) inhibitory immune checkpoints in T cells (eg, VISTA, LAG-3, TIM-3);88,89 (d) T cell exhaustion;90 (e) immunosuppressive cells (eg, TAMs, Tregs and MDSCs);91,92 (d) intestinal dysbacteriosis;93 (f) release of cytokines and metabolites in tumor microenvironment (eg, TGF-β, CSF-1, and adenylate).94,95
The combination of PD-1 targeted antibody with chemotherapy has been evaluated in KEYNOTE-021, a Phase 2 clinical trial initiated by Merck in chemotherapy-naive, stage IIIB or IV NSCLC patients without targetable EGFR or ALK mutations. There were 123 patients enrolled: 60 were treated with pembrolizumab plus pemetrexed and carboplatin, 63 were treated with pemetrexed and carboplatin alone. A significantly higher ORR and PFS was observed in the combination group than in the chemotherapy alone group (55% vs. 29%, 13.0 months vs. 8.9 months). In 2017, US FDA approved of pembrolizumab combined with chemotherapy for first-line treatment of non-squamous NSCLC, regardless of the expression of PD-L1.47 A double-blind, phase III randomized trial of pembrolizumab–platinum–pemetrexed compared with placebo–platinum–pemetrexed was conducted in patients with metastatic non-squamous NSCLC (KEYNOTE-189). In 2019, ASCO updated the results, the median PFS was 9.0 months in the pembrolizumab-combination group versus 4.9 months in the placebo-combination group, while the median OS was 22.0 months versus 10.7 months, respectively.51 Can PD-1 targeted antibody plus chemotherapy have a better effect on squamous NSCLC (regardless of the level of PD-L1 expression)? The phase III of KEYNOTE-407 trial has given the answer. In a phase III clinical trial of carboplatin and paclitaxel or nab-paclitaxel with or without pembrolizumab in metastatic squamous NSCLC patients (KEYNOTE-407), the median PFS was 6.4 months in the pembrolizumab combination group and 4.8 months in the placebo-combination group. The OS was 15.9 months in the pembrolizumab-combination group and 11.3 months in the placebo-combination group. In addition, compared with the placebo combination group, the pembrolizumab combination group had a higher response rate (57.9% vs. 38.4%). Except for immune-related toxicity, the adverse events of the two groups were similar.
Phase III clinical trial IMpower131 evaluated whether chemotherapy combined with PD-L1 targeted antibody (atezolizumab) could become another new first-line treatment for squamous NSCLC (regardless of the expression of PD-L1). Different from the KEYNOTE-407 trial design, patients were randomly assigned to three groups: atezolizumab+ carboplatin/paclitaxel (Arm A), atezolizumab+ carboplatin/nab-paclitaxel (Arm B), and carboplatin/nab-paclitaxel (Arm C). In the first round assessment, the benefit of chemotherapy combined with atezolizumab on PFS was similar to KEYNOTE-407 trial, and the benefit of median PFS was observed in different subgroups with different levels of PD-L1 expression. However, IMpower131 did not show a statistical difference in OS between Arm B and Arm C (14.0 months vs. 13.9 months). Although the OS of Arm B tended to show benefit in the high PD-L1 expression (PD-L1≥50%) subgroup (median OS: 23.6 months vs. 14.1 months), in the low PD-L1 expression subgroup (1%≤PD-L1≤49%), the median OS of chemotherapy combined with atezolizumab was unexpectedly significantly worse (12.4 months vs. 16.6 months). Compared with chemotherapy alone, atezolizumab combined with chemotherapy for first-line treatment of lung squamous cell carcinoma reduced the risk of disease progression by 29%, the risk of disease progression by 44% and the risk of death by 36%.96 Nevertheless, the final phase III result of IMpower131 showed that the median overall survival of atezolizumab combined with carboplatin plus nab-paclitaxel in the treatment of advanced SCLC was not significantly higher than that in the control group (14.2 months vs. 13.5 months). The efficacy of combination group in the treatment of squamous NSCLC was inferior to that of pembrolizumab. KEYNOTE-407 clinical trial data showed that the median overall survival of pembrolizumab combined chemotherapy was 15.9 months, which was 4.5 months longer than chemotherapy alone. Comparatively, the median PFS of atezolizumab combined with carboplatin plus nab-paclitaxel group in IMpower131 compared with chemotherapy alone was significantly improved (5.6 months vs. 6.3 months), while reducing the risk of recurrence and metastasis by 29%. Although the median PFS of IMpower131 is gratifying, the failure of the main end point-OS could not be concealed. Compared with the double success of PFS and OS in KEYNOTE-407 of pembrolizumab, atezolizumab temporarily lost the opportunity to enter the field of first-line treatment in lung squamous cell carcinoma. However, the results of atezolizuma plus chemotherapy for patients with high expression of PD-L1 are promising. Therefore, atezolizumab plus chemotherapy is expected to be approved in specific PD-L1 expression subtypes in the future.97
In addition, the latest results of CheckMate-9LA trial demonstrate that ipilimumab in combination with nivolumab in NSCLC treatment was effective. The combination of nivolumab and ipilimumab with limited chemotherapy demonstrated a median OS of 14.1 months versus 10.7 months with chemotherapy alone. Recently, US FDA has approved the combination of nivolumab and ipilimumab with limited platinum-doublet chemotherapy for the first-line treatment of patients with metastatic or recurrent NSCLC who do not have EGFR or ALK genomic tumor aberrations (PD-L1≥1%).98
Since 2018, immunotherapy has made a breakthrough in SCLC. Following the approval of PD-1 targeted antibody (nivolumab and pembrolizumab) for the third-line treatment of SCLC, PD-L1 targeted antibody has made remarkable achievements as the first-line therapy of SCLC. In 2019, FDA approved atezolizumab in combination with carboplatin plus etoposide for first-line treatment of extensive-stage SCLC (ES-SCLC) based on IMpower133. The results showed that atezolizumab combined with chemotherapy could significantly prolong survival compared with chemotherapy alone (median OS: 12.3 months vs. 10.3 months). Compared with chemotherapy alone, atezolizumab combination regimen also significantly reduced the risk of disease deterioration or death (PFS: 5.2 months vs. 4.3 months). Atezolizumab combination group reduced the risk of death by 30% compared with placebo combination group. Neutropenia and anemia are common grade 3 adverse events (AEs). The immune-related AE (irAEs) was 39.9% in the atezolizumab combination group and 24.5% in the placebo combination group. In general, patients with atezolizumab combined with chemotherapy were well tolerated in SCLC treatment.49 Durvalumab has been approved by US FDA as a first-line treatment for adult patients with ES-SCLC in combination with standard-of-care (SoC) chemotherapies, etoposide plus either carboplatin or cisplatin (platinum-etoposide). The approval was based on positive results from the Phase III of CASPIAN trial showing durvalumab in combination with SoC platinum-etoposide had a statistically significant and clinically meaningful improvement (OS: 13.0 months vs. 10.3 months). The risk of death was reduced by 27% compared with SoC alone.50
However, it appeared that fewer patients with lung cancer were able to benefit from CTLA-4 targeted antibody plus chemotherapy. A clinical trial enrolled 1132 patients with newly diagnosed extensive-stage disease SCLC shows that the addition of ipilimumab to chemotherapy did not prolong OS versus chemotherapy alone in patients (11.0 months vs. 10.9 months).99 A randomized, double-blind phase III clinical trial focused on paclitaxel plus carboplatin with or without ipilimumab in patients with advanced lung squamous cell carcinoma show that the median OS in the ipilimumab plus chemotherapeutic drugs group had no significant extension than placebo plus chemotherapeutic drugs group (13.4 months vs. 12.4 months), with the median PFS being 5.6 months in both groups.100
Considerations and Challenges
Clinical studies of ICIs therapy combined with chemotherapy have commenced optimistically. Some of these studies resulted in survival prolongation or inhibition of cancer progression. Meta-analysis has proved that combined ICIs and chemotherapy therapy is basically safe with manageable toxicity profiles in first-line treatment of advanced NSCLC.101 However, the limited understanding of the interplay between chemotherapeutic drugs and ICIs hurdles the design of the optimal combination strategy. The benefit has been limited to significant portion of patients with lung cancer. In order to benefit more lung cancer patients, we need to address the following challenges in the design of clinical combination regimens in the future. First, what is the genetic standard for patient screening? From the clinical data, pembrolizumab is obviously the leader in the first-line immunotherapeutic drug of NSCLC. In squamous cell carcinoma or non-squamous cell carcinoma patients with high expression of PD-L1 (PD-L1≥50%) and no EGFR or ALK mutation, pembrolizumab monotherapy is significantly more effective than chemotherapy alone, and clinical trials significantly prolong the ORR and reduce the risk of death. Comparatively, if the expression of PD-L1 in patients is low or even negative, pembrolizumab monotherapy is not the best choice but should be considered in combination with chemotherapy. It is worth emphasizing that the chemotherapeutic drugs used in combination with pembrolizumab are different for squamous cell carcinoma (carboplatin plus paclitaxel/nab-paclitaxel) and non-squamous cell carcinoma (pemetrexed plus carboplatin/cisplatin). Different chemotherapeutic drugs work on different immunomodulatory cells. For example, cisplatin and fludarabine can decrease the number or function of Tregs, bleomycin and melphalan can promote T helper type 1 immunity, and mitomycin-C can enhance DCs function.102 Second, what is the optimal dosage and schedule of chemotherapeutic drugs while used in combination with ICIs? In the majority of clinical trials, chemotherapy and ICB are administered at regular doses. Recently, US FDA has accepted its supplemental Biologics License Application (sBLA) for nivolumab plus ipilimumab, administered concomitantly with a limited course of chemotherapy, for the first-line treatment of patients with metastatic or recurrent NSCLC. In particular, the scheme of chemotherapy is “limited” (only two cycles) in combination group compared to chemotherapy alone (up to four cycles followed by optional pemetrexed maintenance therapy if eligible). During combination treatment, chemotherapy is now often administered at the maximum tolerated dose, which would lead to altered immune cell function. For chemo-immunotherapy to be effective, lower doses of chemotherapies might show better anti-tumor effects which suggest that the balance between immune activation and immunosuppression of chemotherapeutic drugs may be crucial for the final outcome. Mpekris et al found that metronomic chemotherapy can induce tumor vascular normalization and promote tumor perfusion. Increased tumor perfusion can reduce tumor hypoxia, which can promote the reconstruction of the tumor immune microenvironment, activate the immune response of the body, and improve the delivery and therapeutic effect of chemotherapeutic drugs.103 In brief, reducing the inhibition of chemotherapeutic drugs on the immune system while maximizing the effect of immune activation should be the focus in immunotherapy combined chemotherapy.
Last, the scheduling and timing of chemotherapy in ICIs therapy are also critical for achieving clinical success. As the agonist of immunotherapy, chemotherapeutic drugs tend to be utilized prior to ICIs treatment. In a phase II study in lung cancer assessed the activity of ipilimumab plus paclitaxel and carboplatin, 3 arms were involved, including chemotherapeutic drugs before ipilimumab treatment, concurrent ipilimumab treatment and chemotherapeutic drugs plus placebo, a median PFS of 5.1, 4.1, and 4.2 months, respectively, suggesting that there is indeed a need for a chemotherapy induction phase.104 Moreover, the interval between chemotherapy and ICIs therapy should not be too long. Immunotherapy is a type of cancer treatment that helps immune system fight cancer. The healthier the immune cells are, the more effective the ICIs would be. If the patient has undergone multiple rounds of chemotherapy, the function of immune cells decreased, and the efficiency of ICIs therapy may be reduced.
In addition, according to KEYNOTE-024 and KEYNOTE-189 trial, lung squamous cell carcinoma subgroup data showed that pembrolizumab alone reduced the risk of death by 65%, which was significantly lower than the risk of death in the pembrolizumab combination chemotherapy group. Therefore, the risk of overtreatment by ICIs therapy and chemotherapy combination should not be ignored.105
Conclusions
ICIs therapy has shed light on lung cancer treatment in clinical settings with promising anticancer effects. However, ICIs monotherapy only works on a small subset of patients, which limits its clinical application. Clinical and preclinical data strongly suggest that chemotherapeutic drugs could regulate the anti-tumor immune response. A number of clinical trials showed that ICIs therapy combined with chemotherapy increases survival rate of lung cancer patients. ICIs therapy combined with chemotherapy bring ICIs into the first-line treatment of lung cancer. However, in-depth research on the immunosuppressive activity of chemotherapeutic drugs is lacking. Whether chemotherapeutic drugs exert immunosuppressive function through other mechanisms, and whether the immunosuppressive effect can be avoided by changing the dose, frequency, and sequence of administration, require further study. Understanding the cellular and molecular mechanisms underlying the interplay of ICIs therapy and chemotherapy could help guide the design of safer and more efficient cancer therapeutic strategies. Optimizing the treatment regimens in lung cancer ICIs therapy combined with chemotherapy is a task worthy of pursuit.
Disclosure
The authors declare no competing interests.
References
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi:10.1002/ijc.31937
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
3. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomark Prev. 2016;25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
4. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi:10.1056/NEJMoa1501824
5. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643
6. Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thor Oncol. 2017;12(2):403–407. doi:10.1016/j.jtho.2016.10.007
7. Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. doi:10.1158/1078-0432.CCR-15-3101
8. Pusuluri A, Wu D, Mitragotri S. Immunological consequences of chemotherapy: single drugs, combination therapies and nanoparticle-based treatments. J Controlled Rel. 2019;305:
9. Joshi S, Durden DL. Combinatorial approach to improve cancer immunotherapy: rational drug design strategy to simultaneously hit multiple targets to kill tumor cells and to activate the immune system. J Oncol. 2019;2019:5245034.
10. Diker O. Pembrolizumab plus chemotherapy in lung cancer. N Engl J Med. 2018;379(11):e18.
11. Lazzari C, Karachaliou N, Bulotta A, et al. Combination of immunotherapy with chemotherapy and radiotherapy in lung cancer: is this the beginning of the end for cancer? Ther Adv Med Oncol. 2018;10:1758835918762094.
12. Bethune MT, Joglekar AV. Personalized T cell-mediated cancer immunotherapy: progress and challenges. Curr Opin Biotechnol. 2017;48:
13. Wieder T, Eigentler T, Brenner E, Rocken M. Immune checkpoint blockade therapy. J Allergy Clin Immunol. 2018;142(5):1403–1414. doi:10.1016/j.jaci.2018.02.042
14. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11. doi:10.1038/s12276-018-0191-1
15. Ljunggren HG, Jonsson R, Hoglund P. Seminal immunologic discoveries with direct clinical implications: the 2018 Nobel Prize in physiology or medicine honours discoveries in cancer immunotherapy. Scand J Immunol. 2018;88(6):e12731. doi:10.1111/sji.12731
16. Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24(1):26. doi:10.1186/s12929-017-0329-9
17. Dong P, Xiong Y, Yue J, Hanley SJB, Watari H. Tumor-intrinsic PD-L1 signaling in cancer initiation, development and treatment: beyond immune evasion. Front Oncol. 2018;8:386.
18. Yang MW, Fu XL, Jiang YS, et al. Clinical significance of programmed death 1/programmed death ligand 1 pathway in gastric neuroendocrine carcinomas. World j Gastroenterol. 2019;25(14):1684–1696. doi:10.3748/wjg.v25.i14.1684
19. Fan P, Zhao J, Meng Z, et al. Overexpressed histone acetyltransferase 1 regulates cancer immunity by increasing programmed death-ligand 1 expression in pancreatic cancer. J Exp Clin Cancer Res. 2019;38(1):47. doi:10.1186/s13046-019-1044-z
20. Guan X, Lin L, Chen J, et al. Efficient PD-L1 gene silence promoted by hyaluronidase for cancer immunotherapy. J Controlled Rel. 2019;293:
21. Foy JP, Bertolus C, Michallet MC, et al. The immune microenvironment of HPV-negative oral squamous cell carcinoma from never-smokers and never-drinkers patients suggests higher clinical benefit of IDO1 and PD1/PD-L1 blockade. Ann Oncol. 2017;28(8):1934–1941. doi:10.1093/annonc/mdx210
22. D’Alterio C, Nasti G, Polimeno M, et al. CXCR4-CXCL12-CXCR7, TLR2-TLR4, and PD-1/PD-L1 in colorectal cancer liver metastases from neoadjuvant-treated patients. Oncoimmunology. 2016;5(12):e1254313. doi:10.1080/2162402X.2016.1254313
23. Engelhardt JJ, Sullivan TJ, Allison JP. CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol. 2006;177(2):1052–1061. doi:10.4049/jimmunol.177.2.1052
24. Paulsen -E-E, Kilvaer TK, Rakaee M, et al. CTLA-4 expression in the non-small cell lung cancer patient tumor microenvironment: diverging prognostic impact in primary tumors and lymph node metastases. Cancer Immunol Immunother. 2017;66(11):1449–1461. doi:10.1007/s00262-017-2039-2
25. Egen JG, Ouyang W, Wu LC. Human anti-tumor immunity: insights from immunotherapy clinical trials. Immunity. 2020;52(1):36–54. doi:10.1016/j.immuni.2019.12.010
26. Hernandez J, Ko A, Sherman LA. CTLA-4 blockade enhances the CTL responses to the p53 self-tumor antigen. J Immunol. 2001;166(6):3908–3914. doi:10.4049/jimmunol.166.6.3908
27. Liu Y, Zheng P. Preserving the CTLA-4 checkpoint for safer and more effective cancer immunotherapy. Trends Pharmacol Sci. 2020;41(1):4–12. doi:10.1016/j.tips.2019.11.003
28. Salmaninejad A, Khoramshahi V, Azani A, et al. PD-1 and cancer: molecular mechanisms and polymorphisms. Immunogenetics. 2018;70(2):73–86. doi:10.1007/s00251-017-1015-5
29. Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561.
30. Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi:10.1038/nm730
31. Beltra JC, Manne S, Abdel-Hakeem MS, et al. Developmental relationships of four exhausted CD8(+) T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity. 2020;52(5):825–841 e828. doi:10.1016/j.immuni.2020.04.014
32. Fu D, Wu J, Lai J, et al. T cell recruitment triggered by optimal dose platinum compounds contributes to the therapeutic efficacy of sequential PD-1 blockade in a mouse model of colon cancer. Am J Cancer Res. 2020;10(2):473–490.
33. Liu Y, Cheng Y, Xu Y, et al. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene. 2017;36(44):6143–6153. doi:10.1038/onc.2017.209
34. Bodduluru LN, Kasala ER, Madhana RM, Sriram CS. Natural killer cells: the journey from puzzles in biology to treatment of cancer. Cancer Lett. 2015;357(2):454–467. doi:10.1016/j.canlet.2014.12.020
35. Pesce S, Greppi M, Tabellini G, et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J Allergy Clin Immunol. 2017;139(1):335–346 e333. doi:10.1016/j.jaci.2016.04.025
36. Zhang Q, Bi J, Zheng X, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19(7):723–732. doi:10.1038/s41590-018-0132-0
37. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi:10.1056/NEJMoa1003466
38. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-7
39. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. doi:10.1056/NEJMoa1504627
40. Fehrenbacher L, von Pawel J, Park K, et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase iii study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thor Oncol. 2018;13(8):1156–1170. doi:10.1016/j.jtho.2018.04.039
41. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in Stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi:10.1056/NEJMoa1709937
42. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
43. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, Phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi:10.1016/S0140-6736(18)32409-7
44. Chung HC, Ros W, Delord JP, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the Phase II KEYNOTE-158 Study. J clin oncol. 2019;37(17):1470–1478. doi:10.1200/JCO.18.01265
45. Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the Phase Ib KEYNOTE-028 study. J clin oncol. 2017;35(34):3823–3829. doi:10.1200/JCO.2017.72.5069
46. Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA oncol. 2018;4(11):1543–1552. doi:10.1001/jamaoncol.2018.3676
47. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi:10.1016/S1470-2045(16)30498-3
48. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi:10.1056/NEJMoa1810865
49. Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi:10.1056/NEJMoa1809064
50. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi:10.1016/S0140-6736(19)32222-6
51. Gadgeel S, Rodriguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J clin oncol. 2020;38(14):1505–1517. doi:10.1200/JCO.19.03136
52. Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the game in immunotherapy. Trends Cancer. 2016;2(6):286–294. doi:10.1016/j.trecan.2016.05.002
53. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi:10.1056/NEJMoa1801946
54. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi:10.1056/NEJMoa1910231
55. Tsao AS, Scagliotti GV, Bunn PA
56. Yang JC, Gadgeel SM, Sequist LV, et al. Pembrolizumab in combination with erlotinib or gefitinib as first-line therapy for advanced NSCLC with sensitizing EGFR mutation. J Thor Oncol. 2019;14(3):553–559. doi:10.1016/j.jtho.2018.11.028
57. Gettinger S, Hellmann MD, Chow LQM, et al. Nivolumab plus erlotinib in patients with EGFR-mutant advanced NSCLC. J Thor Oncol. 2018;13(9):1363–1372.
58. DeVita VT
59. Beyranvand Nejad E, van der Sluis TC, van Duikeren S, et al. Tumor eradication by cisplatin is sustained by CD80/86-mediated costimulation of CD8+ T Cells. Cancer Res. 2016;76(20):6017–6029. doi:10.1158/0008-5472.CAN-16-0881
60. Li X. The inducers of immunogenic cell death for tumor immunotherapy. Tumori. 2018;104(1):1–8. doi:10.5301/tj.5000675
61. Raghavan M, Wijeyesakere SJ, Peters LR, Del Cid N. Calreticulin in the immune system: ins and outs. Trends Immunol. 2013;34(1):13–21. doi:10.1016/j.it.2012.08.002
62. Gold LI, Eggleton P, Sweetwyne MT, et al. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010;24(3):665–683. doi:10.1096/fj.09-145482
63. Aoto K, Mimura K, Okayama H, et al. Immunogenic tumor cell death induced by chemotherapy in patients with breast cancer and esophageal squamous cell carcinoma. Oncol Rep. 2018;39(1):151–159. doi:10.3892/or.2017.6097
64. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi:10.1038/nature00858
65. Gameiro SR, Caballero JA, Hodge JW. Defining the molecular signature of chemotherapy-mediated lung tumor phenotype modulation and increased susceptibility to T-cell killing. Cancer Biother Radiopharm. 2012;27(1):23–35. doi:10.1089/cbr.2012.1203
66. Tran L, Allen CT, Xiao R, et al. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol Res. 2017;5(12):1141–1151. doi:10.1158/2326-6066.CIR-17-0235
67. Nio Y, Hirahara N, Minari Y, et al. Induction of tumor-specific antitumor immunity after chemotherapy with cisplatin in mice bearing MOPC-104E plasmacytoma by modulation of MHC expression on tumor surface. Anticancer Res. 2000;20(5A):3293–3299.
68. Sieben CJ, Sturmlechner I, van de Sluis B, van Deursen JM. Two-step senescence-focused cancer therapies. Trends Cell Biol. 2018;28(9):723–737. doi:10.1016/j.tcb.2018.04.006
69. Capece D, Verzella D, Tessitore A, Alesse E, Capalbo C, Zazzeroni F. Cancer secretome and inflammation: the bright and the dark sides of NF-kappaB. Semin Cell Dev Biol. 2018;78:
70. Sun X, Shi B, Zheng H, et al. Senescence-associated secretory factors induced by cisplatin in melanoma cells promote non-senescent melanoma cell growth through activation of the ERK1/2-RSK1 pathway. Cell Death Dis. 2018;9(3):260. doi:10.1038/s41419-018-0303-9
71. Eggert T, Wolter K, Ji J, et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell. 2016;30(4):533–547. doi:10.1016/j.ccell.2016.09.003
72. Senovilla L, Vitale I, Martins I, et al. An anticancer therapy-elicited immunosurveillance system that eliminates tetraploid cells. Oncoimmunology. 2013;2(1):e22409. doi:10.4161/onci.22409
73. Ladoire S, Mignot G, Dabakuyo S, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol. 2011;224(3):389–400. doi:10.1002/path.2866
74. Kan S, Hazama S, Maeda K, et al. Suppressive effects of cyclophosphamide and gemcitabine on regulatory T-cell induction in vitro. Anticancer Res. 2012;32(12):5363–5369.
75. Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183(1):137–144. doi:10.4049/jimmunol.0900734
76. Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–6721. doi:10.1158/1078-0432.CCR-05-0883
77. Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16(18):4583–4594. doi:10.1158/1078-0432.CCR-10-0733
78. Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–3061. doi:10.1158/0008-5472.CAN-09-3690
79. Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118(1):9–16. doi:10.1038/bjc.2017.434
80. Benci JL, Xu B, Qiu Y, et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167(6):1540–1554 e1512. doi:10.1016/j.cell.2016.11.022
81. Shin MH, Kim J, Lim SA, Kim J, Lee KM. Current insights into combination therapies with MAPK inhibitors and immune checkpoint blockade. Int J Mol Sci. 2020;21(7).
82. Chen K, Shang Z, Dai AL, Dai PL. Novel PI3K/Akt/mTOR pathway inhibitors plus radiotherapy: strategy for non-small cell lung cancer with mutant RAS gene. Life Sci. 2020;255:117816.
83. Hong CF, Chen WY, Wu CW. Upregulation of Wnt signaling under hypoxia promotes lung cancer progression. Oncol Rep. 2017;38(3):1706–1714. doi:10.3892/or.2017.5807
84. Lu YC, Yao X, Crystal JS, et al. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin Cancer Res. 2014;20(13):3401–3410. doi:10.1158/1078-0432.CCR-14-0433
85. Gettinger S, Choi J, Hastings K, et al. Impaired HLA Class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017;7(12):1420–1435. doi:10.1158/2159-8290.CD-17-0593
86. Ishii H, Azuma K, Kawahara A, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thor Oncol. 2015;10(3):426–430. doi:10.1097/JTO.0000000000000414
87. Shi H, Lan J, Yang J. Mechanisms of resistance to checkpoint blockade therapy. Adv Exp Med Biol. 2020;1248:
88. Ma K, Jin Q, Wang M, Li X, Zhang Y. Research progress and clinical application of predictive biomarker for immune checkpoint inhibitors. Expert Rev Mol Diagn. 2019;19(6):517–529. doi:10.1080/14737159.2019.1617702
89. Sun F, Guo ZS, Gregory AD, Shapiro SD, Xiao G, Qu Z. Dual but not single PD-1 or TIM-3 blockade enhances oncolytic virotherapy in refractory lung cancer. J Immunother Cancer. 2020;8(1).
90. Xia L, Liu Y, Wang Y. PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: current status and future directions. The Oncologist. 2019;24(Suppl 1):S31–S41. doi:10.1634/theoncologist.2019-IO-S1-s05
91. Tian T, Gu X, Zhang B, et al. Increased circulating CD14(+)HLA-DR-/low myeloid-derived suppressor cells are associated with poor prognosis in patients with small-cell lung cancer. Cancer Biomark. 2015;15(4):425–432. doi:10.3233/CBM-150473
92. Bhat TA, Panzica L, Kalathil SG, Thanavala Y. Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12(Suppl 2):S169–175. doi:10.1513/AnnalsATS.201503-126AW
93. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi:10.1126/science.aan3706
94. Horn LA, Riskin J, Hempel HA, et al. Simultaneous inhibition of CXCR1/2, TGF-beta, and PD-L1 remodels the tumor and its microenvironment to drive antitumor immunity. J Immunother Cancer. 2020;8(1).
95. Priceman SJ, Sung JL, Shaposhnik Z, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115(7):1461–1471. doi:10.1182/blood-2009-08-237412
96. Zhang Y, Zhou H, Zhang L. Which is the optimal immunotherapy for advanced squamous non-small-cell lung cancer in combination with chemotherapy: anti-PD-1 or anti-PD-L1? J Immunother Cancer. 2018;6(1):135. doi:10.1186/s40425-018-0427-6
97. Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized Phase III trial. J Thor Oncol. 2020. doi:10.1016/j.jtho.2020.03.028
98. U.S. Food and Drug Administration Approves Opdivo (Nivolumab) + Yervoy (Ipilimumab) Combined with Limited Chemotherapy as First-Line Treatment of Metastatic or Recurrent Non-Small Cell Lung Cancer [News Release]. Princeton, NJ: Bristol Myers Squibb; 2020 May 26. https://bwnews.pr/2znmG9j
99. Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J clin oncol. 2016;34(31):3740–3748. doi:10.1200/JCO.2016.67.6601
100. Govindan R, Szczesna A, Ahn MJ, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J clin oncol. 2017;35(30):3449–3457. doi:10.1200/JCO.2016.71.7629
101. Tun AM, Thein KZ, Thein WL, Guevara E. Checkpoint inhibitors plus chemotherapy for first-line treatment of advanced non-small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Future Sci OA. 2019;5(9):FSO421. doi:10.2144/fsoa-2019-0081
102. Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother. 2013;62(2):203–216. doi:10.1007/s00262-012-1388-0
103. Mpekris F, Baish JW, Stylianopoulos T, Jain RK. Role of vascular normalization in benefit from metronomic chemotherapy. Proc Natl Acad Sci U S A. 2017;114(8):1994–1999. doi:10.1073/pnas.1700340114
104. Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J clin oncol. 2012;30(17):2046–2054. doi:10.1200/JCO.2011.38.4032
105. Liu J, Li C, Seery S, Yu J, Meng X. Identifying optimal first-line interventions for advanced non-small cell lung carcinoma according to PD-L1 expression: a systematic review and network meta-analysis. Oncoimmunology. 2020;9(1):1746112. doi:10.1080/2162402X.2020.1746112
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.