Back to Journals » Drug Design, Development and Therapy » Volume 12
Combination of Ginsenoside Rg1 and Astragaloside IV reduces oxidative stress and inhibits TGF-β1/Smads signaling cascade on renal fibrosis in rats with diabetic nephropathy
Authors Du N , Xu Z, Gao M , Liu P, Sun B, Cao X
Received 16 April 2018
Accepted for publication 1 September 2018
Published 18 October 2018 Volume 2018:12 Pages 3517—3524
DOI https://doi.org/10.2147/DDDT.S171286
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Na Du, Zhiping Xu, Mingyue Gao, Peng Liu, Bo Sun, Xia Cao
Department of Pharmacology, Jilin University, Jilin, Changchun, China
Introduction: Anti-oxidative stress and inhibition of TGF-β1/Smads signaling cascade are essential therapeutic strategies for diabetic nephropathy (DN). In this study, we aimed to explore the effect of combination of Ginsenoside Rg1 and Astragaloside IV on oxidative stress and TGF-β1/Smads signaling in DN rats.
Materials and methods: Wistar rats were divided into five groups: N group, M group (streptozotocin [STZ], intraperitoneally), G group (STZ rats with Ginsenoside Rg1, intragastrically [ig]), A group (STZ rats with Astragaloside IV, ig) and C group (STZ rats with Ginsenoside Rg1 and Astragaloside IV, ig). The levels of methane dicarboxylic aldehyde (MDA), catalase (CAT), glutathione peroxidase (GSH-PX), total anti-oxidative capacity (T-AOC), blood urea nitrogen (BUN), β2-microglobulin (β2-MG), serum creatinine (SCr) and urinary creatinine (UCr) were detected in all the groups. The left kidneys of the rats were harvested to detect the expression of TGF-β1, Smad2/3, Smad7 and CTGF by immunohistochemical staining, while the right kidneys were used to detect the mRNA expression of TGF-β1, Smad7 and CTGF by real-time PCR.
Results: Rats in G group, A group and C group had lower level of MDA but higher levels of CAT, GSH-PX and T-AOC compared with rats in M group. Rats in C group showed the best anti-oxidative stress level. G group, A group and C group treatments significantly decreased the levels of BUN, SCr, β2-MG and UCr. In addition, C group treatment showed the best kidney protective effect. G group, A group and C group treatments significantly diminish ED both factor and mRNA overexpression of TGF-β1 and CTGF but increase Smad7 expression in kidney tissue.
Conclusion: The combination of Ginsenoside Rg1 and Astragaloside IV may potentially protect against DN by reducing oxidative stress and inhibiting TGF-β1/Smads signaling cascade.
Keywords: Ginsenoside Rg1, Astragaloside IV, oxidative stress, TGF-β1/Smads, diabetic nephropathy
Introduction
Diabetic nephropathy (DN) is one of the most common complications of diabetes mellitus (DM) which leads to end-stage renal failure in 30%–40% of DM patients.1–3 The incidence of DN will rapidly increase with increase in the incidence of DM in China in the decades to come.4,5 Oxidative stress injury and TGF-β/Smads signal transduction are vital to the development and progression of DN. The supererogatory advanced glycation end products (AGEs) and ROS act as pivotal mediators of microvascular injury when induced by DM and trigger various cell signaling pathways involved in the development of DN. Oxidative stress injury caused by ROS and AGEs can actuate TGF-β/Smads signaling which is involved in the development of fibrosis in renal tubular epithelial cells.6,7 Many results have shown that TGF-β expression is upregulated in animal renal fibrosis models as well as human counterparts.8,9 The transition of renal tubular epithelial cells to myofibroblasts which synthesize excessive amounts of extracellular matrix thus leading to renal fibrosis is modulated by TGF-β through TGF-β/Smads signaling pathway.2 Furthermore, oxidative stress and TGF-β/Smads signaling together with other disease processes interactively and rapidly promote DN. Therefore, agents with anti-oxidative or TGF-β1/Smads signaling prohibitive competence are likely to possess a therapeutic effect on DN.
Ginsenoside Rg1 is one of the main active ingredients of Panax ginseng C.A. Mey. Its proportion is the main criterion which determines the quality of P. ginseng.10 Studies have reported that Ginsenoside Rg1 has a strong anti-oxidative and anti-fibrotic effect.11–14 Astragaloside IV, a characteristic and active constituent of Radix Astragali, possesses many biological effects.15 Many studies have shown that Astragaloside IV has an excellent renal protective effect.16 Only a specific amount of P. ginseng and Radix Astragali and other frequently prescribed traditional Chinese medicines such as Shenqi Jiangtang Granules should be applied to avoid complications in DM patients. Some evidences show that a mixture of herbal medicines has a better effect than a single medicine in clinical practice. In this study, we aimed to determine whether the combination of Ginsenoside Rg1 and Astragaloside IV has a therapeutic effect on DN and its underlying mechanism.
Materials and methods
Chemicals
Ginsenoside Rg1 (purity ≥98%) and Astragaloside IV (purity ≥98%) were purchased from ChengDu ConBon Biotech Co., Ltd. (Chengdu, China). The methane dicarboxylic aldehyde (MDA), catalase (CAT), glutathione peroxidase (GSH-PX) and total anti-oxidative capacity (T-AOC) assay kits for rats were bought from Jiancheng Bioengineering Institute (Nanjing, China). Rat blood urea nitrogen (BUN), serum creatinine (SCr), β2-microglobulin (β2-MG) and urinary creatinine (UCr) ELISA kits were also purchased from Jiancheng Bioengineering Institute. Streptozotocin (STZ) was bought from Sigma (St Louis, MO, USA). Polyclonal antibodies used for immunohistochemical analysis included TGF-β1 (Santa Cruz Biotechnology, Dallas, TX, USA), Smad2/3 (Cell Signaling, Danvers, MA, China), Smad7 (Bioss, Woburn, MA, USA) and CTGF (Bioss). Rat SP immunohistochemistry kits were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The primer sequences of TGF-β1, Smad7, CTGF and ACTB (listed in Table 1) used for real-time PCR analysis were bought from Sangon Biotech Co., Ltd (Shanghai, China). Real-Time PCR Reagents & Kits and Total RNA Extractor (Trizol) were bought from Thermo Fisher Scientific. The other reagents used were of analytical grade.
  | Table 1 The primer sequences used for mRNA real-time PCR analysis |
Animals
A total of 50 male adult Wistar rats (180–220 g) were purchased from Laboratory Animal Center of Jilin University. All the experiments were approved by the Animal Care Committee of Jilin University Pharmaceutical College (in accordance with the Animal Experimental Ethical guidelines of Jilin University; permit number: 2017050802). The rats were kept at a temperature of 22°C±2°C on a 12-hour light/dark cycle and fed on a normal laboratory diet and water ad libitum.
In vivo experiments
The rats were marked after acclimating to the facilities for 7 days. Then, eight rats were randomly chosen and designated as N group, and the rest were intraperitoneally administered 60 mg/kg of STZ with 0.1 mol/L sodium citrate solution (pH 4.50).17–19 The blood glucose levels of all the rats, except N group, were measured at 72 hours after STZ injection (first experimental day). Only the rats with a blood glucose concentration higher than 13.8 mmol/L were chosen as model rats and used further in our study.
Model rats were randomly divided into four groups (n=8): M group, G group, A group and C group. Rats in N group and M group were intragastrically (ig) administered vehicle daily, rats in G group were ig administered 50 mg/kg/day of Ginsenoside Rg1, rats in A group were ig administered 16 mg/kg/day of Astragaloside IV and rats in C group were ig administered 50 mg/kg/day of Ginsenoside Rg1 and 16 mg/kg/day of Astragaloside IV on the first experimental day.
Detection of the levels of MDA, CAT, GSH-PX, T-AOC, BUN, SCr, β2-MG and UCr
At the end of 8 weeks (56th experimental day), the rats were housed in individual metabolic cages (ZS Dichuang Co., Ltd., Shanghai, China) to collect 24-hour urine samples. The plasma samples were used to detect MDA, CAT, GSH-PX, T-AOC, BUN and SCr levels, while urine samples were used to measure β2-MG and UCr levels using assay kits according to the manufacturer’s instructions.
Immunohistochemical staining and assessment in each group
After euthanization (57th experimental day), the kidneys (left) were harvested and rinsed free from blood with PBS. After fixing in 10% neutral formalin and embedding in paraffin, the antigen of kidney tissue slides was exposed by treatment with boiling citrate buffer (0.01 mol/L, pH 6.0). Then, the slides were respectively incubated with TGF-β1, Smad2/3, Smad7 and CTGF antibodies for immunohistochemical analysis and examined using a light microscope (Nikon Ti). The analyses were performed to test for the gray value of the immunohistochemical stainings using Motic Images Advanced 3.2.
Real-time PCR analysis in rats’ kidney tissue
The kidneys (right) were harvested and kept in liquid nitrogen for real-time PCR analysis. Total RNA was extracted from rats’ kidney tissue and reverse transcribed to cDNA according to the manufacturer’s protocol. Then, real-time PCR was proceeded with SYBR™ Select Master Mix, and expression was detected using iCycler iQ (Bio-Rad). ACTB was employed as the internal standard in our study. The ratio of TGF-β1, Smad7 and CTGF was normalized with ACTB. We took each factor of N group as 1.0000 to calculate and compare the homologous factors in the other groups.
Statistical analysis
Statistical analyses were performed utilizing SPSS 20.0 program. Results were expressed as mean ± SD and analyzed using one-way ANOVA and post hoc least square difference test. Statistical significance was determined at P<0.05.
Results
Combination of Ginsenoside Rg1 and Astragaloside IV increases DN rats’ anti-oxidative capacity
The results of oxidative stress levels in each group are shown in Figure 1. STZ injection obviously increased MDA level, but decreased the levels of CAT, GSH-PX and T-AOC as compared with N group (P<0.05). As shown in Figure 1A, G group and C group had lower level of MDA (P<0.05 vs M group). The rats in C group had the lowest MDA level, although there was no significant difference between G group and C group.
G group, A group and C group showed apparent increase in CAT level (P<0.05 vs M group; Figure 1B). Compared with G group, A group had lower CAT level (P<0.05) while C group had higher CAT level (P>0.05).
The treatment of G group and C group inhibited GSH-PX level decline in plasma (P<0.05 vs M group; Figure 1C). Compared with G group, C group had better GSH-PX level (P<0.05 vs G group).
In Figure 1D, we could find that rats in G group, A group and C group had improved T-AOC after STZ injection (P<0.05 vs M group). The rats in C group had better T-AOC than the other two groups.
Combination of Ginsenoside Rg1 and Astragaloside IV improves DN rats’ renal function
The tests showed that STZ injection induced significant increase in the levels of BUN, SCr, β2-MG and UCr as compared with N group (P<0.05; Figure 2). We also found that G group, A group and C group treatments significantly decreased the levels of BUN, SCr, β2-MG and UCr (P<0.05 vs M group; Figure 2).
Compared with G group and A group, rats in C group had lower BUN levels (P>0.05 vs G group and P<0.05 vs A group; Figure 2A). As shown in Figure 2B, C group had the lowest level of SCr among the three groups (P<0.05 vs G group and P<0.05 vs A group). The rats in G group had lower SCr level than rats in A group (P<0.05). As shown in Figure 2C, we found that C group had lower β2-MG level than the other two groups (P<0.05 vs G group and P>0.05 vs A group). However, there was no significant difference between G group and A group. As shown in Figure 2D, C group had the lowest UCr level after 8 weeks of treatment (P<0.05 vs G group or A group). However, there was no significant difference between G group and A group (P>0.05).
Combination of Ginsenoside Rg1 and Astragaloside IV inhibits TGF-β1/Smads signaling cascade
In order to explore the effect of combination of Ginsenoside Rg1 and Astragaloside IV on TGF-β1/Smads signaling in DN rats’ kidneys, immunohistochemical staining and real-time PCR were performed. Compared with N group, rats in M group showed severe immunopositivity for TGF-β1, Smad2/3 and CTGF, but no immunopositivity for Smad7. However, G group, A group and C group treatments diminished immunopositivity for TGF-β1, Smad2/3 and CTGF and elevated immunopositivity for Smad7 (Figure 3). More importantly, C group had the lowest levels of TGF-β1, Smad2/3 and CTGF and the highest level of Smad7 among the three groups as revealed by the gray value of immunohistochemical stainings tested using Motic Images Advanced 3.2 (Figure 4).
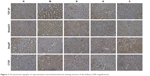  | Figure 3 The photomicrographs of representative immunohistochemical staining sections of the kidney (×200 magnification). |
Real-time PCR analysis showed that STZ injection induced overexpression of TGF-β1 (P<0.05) and CTGF (P<0.05), but decreased the expression of Smad7 (P<0.05) as compared with N group. The results demonstrated that G group, A group and C group had attenuated expression of TGF-β1 and CTGF but increased expression of Smad7 (P<0.05 vs M group). In addition, C group showed better effect than the other two groups (Figure 5).
  | Figure 5 The ratio of TGF-β1, Smad7 and CTGF mRNA expression in each group. |
Discussion
Hyperglycemic microenvironment, which leads to tubulointerstitial fibrosis and glomerulosclerosis, induces structural and functional abnormalities in the kidney at a very early stage of DM.20,21 Tubulointerstitial injury actually occurs earlier than glomerular injury in the development of DN.16,22
Oxidative stress injury and overactivation of TGF-β1/Smads signaling are the major culprits in DN.23–26 There is some causal link between oxidative stress and TGF-β1/Smads signal transduction in the development and progression of DN. As we know, renal cells exhibit enhanced glucose uptake in DM, but some cell populations such as glomerular mesangial cells cannot decrease glucose transport rates efficiently to maintain intracellular glucose homeostasis.27 As a result, cytosol and mitochondria in these cells will produce more ROS beyond local antioxidant capacity. Oxidative stress induced by the redundant ROS in renal cells such as podocytes and mesangial cells induces the development and progression of DN.28 AGEs are the other momentous cell metabolic products produced in DM patients. Oxidative stress may hasten AGEs formation.29 Superfluous ROS are also produced along with AGEs, inducing ROS/AGEs self-perpetuating cycle in DM.24 Since most AGEs are cleared by the kidneys, they are likely to accumulate and interact with renal cells. Studies have shown that AGEs correlate with the expression of TGF-β1 and CTGF.30 TGF-β is a polypeptide with three isoforms in mammals namely TGF-β1, TGF-β2 and TGF-β3. The isoforms share the same conserved regions in structure and signaling pathway, but differ in distribution. TGF-β1 is expressed in all kinds of renal cells, while TGF-β2 is expressed in cells of nervous system and TGF-β3 is expressed in rhabdomyosarcoma cells.31 Many experiments have found that the transition of tubular epithelial cells to myofibroblasts leading to renal fibrosis is modulated by TGF-β1/Smads signaling.32,33 In addition, CTGF, one of the main downstream products of TGF-β1/Smad signaling pathway, contributes to renal fibrosis and exerts positive feedback on the signaling. Smad7 as a negative factor can downregulate TGF-β1/Smads signal transduction. Hence, anti-oxidative stress and inhibition of TGF-β1/Smads signaling cascade may be vital targets for drugs used to treat DN.
P. ginseng and Radix Astragali are together compatible with other herbs used in traditional Chinese medicine for clinical treatment of diabetes. Ginsenoside Rg1 and Astragaloside IV are the two main bioactive components present in P. ginseng and Radix Astragali, respectively. Many experimental studies have indicated that both Ginsenoside Rg1 and Astragaloside IV exhibit some anti-oxidative ability and suppressive activity on TGF-β1/Smads signaling,34–40 but the application of combination of Ginsenoside Rg1 and Astragaloside IV and its benefits in DN have not been reported.
In our study, we found that the treatment groups (G group, A group and C group) showed different levels of improvement in DN. We found that rats in C group (treated with the combination of Ginsenoside Rg1 and Astragaloside IV) had the lowest MDA level but the highest CAT, GSH-PX and T-AOC levels among the three treatment groups. In addition, G group had lower MDA level but higher CAT, GSH-PX and T-AOC levels than A group. We can conclude that Ginsenoside Rg1 (50 mg/kg/day) has better anti-oxidative stress ability than Astragaloside IV (16 mg/kg/day). However, Astragaloside IV can strengthen the anti-oxidative stress effect of Ginsenoside Rg1 in DN. The rats in C group had better renal function than G group or A group rats with lower levels of BUN, SCr, β2-MG and UCr. There was almost no difference between G group and A group (merely in SCr, P<0.05). We may conclude that Ginsenoside Rg1 and Astragaloside IV interact with each other to improve renal function in DN. The inhibiting effect of the three treatments on TGF-β1/Smads signaling was detected in our experiments. G group, A group and C group treatments all significantly decreased both factor and mRNA overexpression of TGF-β1 and CTGF but increased Smad7 expression in the kidneys of DN rats. We also conclude that Ginsenoside Rg1 has a better inhibiting effect on TGF-β1 overexpression, while Astragaloside IV has a better activating effect on Smad7 expression. Thus, combination of Ginsenoside Rg1 and Astragaloside IV may exert excellent inhibiting effect on TGF-β1/Smads signaling cascade.
Conclusion
Our findings provide some evidence for the protective effect of the combination of Ginsenoside Rg1 and Astragaloside IV on DN rats. We conclude that combination of Ginsenoside Rg1 and Astragaloside IV can benefit DN patients by attenuating oxidative stress, improving renal function and inhibiting TGF-β1/Smads signaling cascade. Therefore, our study provides laboratory evidence for the beneficial effect of the combination of Ginsenoside Rg1 and Astragaloside IV on the treatment of DN. However, more investigations are required to study the underlying mechanism in detail.
Acknowledgment
This work was supported by Special Funds for Pharmaceutical Industry Development of Jilin province (20130727024YY).
Disclosure
The authors report no conflicts of interest in this work.
References
Wang X, Li W, Kong D. Cyclocarya paliurus extract alleviates diabetic nephropathy by inhibiting oxidative stress and aldose reductase. Ren Fail. 2016;38(5):678–685. | ||
Du N, Liu S, Cui C, et al. DMP-1 attenuates oxidative stress and inhibits TGF-β activation in rats with diabetic kidney disease. Ren Fail. 2017;39(1):229–235. | ||
Nieto-Ríos JF, Serna-Higuita LM, Builes-Rodriguez SA, et al. Clinical outcomes of kidney transplants on patients with end-stage renal disease secondary to lupus nephritis, polycystic kidney disease and diabetic nephropathy. Colomb Med. 2016;47(1):51–58. | ||
Guo K, Zhang L, Zhao F, et al. Prevalence of chronic kidney disease and associated factors in Chinese individuals with type 2 diabetes: Cross-sectional study. J Diabetes Complications. 2016;30(5):803–810. | ||
Lu Y, Tang L, Li Y, He Q. High glucose-induced fibronectin upregulation in cultured mesangial cells involves caveolin-1-dependent RhoA-GTP activation via Src kinase. Mol Med Rep. 2016;14(1):963–968. | ||
Fang Y, Tian X, Bai S, et al. Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med. 2012;30(1):85–92. | ||
Chung AC, Zhang H, Kong YZ, et al. Advanced glycation end-products induce tubular CTGF via TGF-beta-independent Smad3 signaling. J Am Soc Nephrol. 2010;21(2):249–260. | ||
Tang F, Hao Y, Zhang X, Qin J. Effect of echinacoside on kidney fibrosis by inhibition of TGF-β1/Smads signaling pathway in the db/db mice model of diabetic nephropathy. Drug Des Devel Ther. 2017;11:2813–2826. | ||
Chang B, Chen W, Zhang Y, Yang P, Liu L. Tripterygium wilfordii mitigates hyperglycemia-induced upregulated Wnt/β-catenin expression and kidney injury in diabetic rats. Exp Ther Med. 2018;15(4):3874–3882. | ||
Guan S, Liu Q, Han F, et al. Ginsenoside Rg1 Ameliorates Cigarette Smoke-Induced Airway Fibrosis by Suppressing the TGF-β1/Smad Pathway In Vivo and In Vitro. Biomed Res Int. 2017;2017:6510198–12. | ||
Li J, Cai D, Yao X, et al. Protective Effect of Ginsenoside Rg1 on Hematopoietic Stem/Progenitor Cells through Attenuating Oxidative Stress and the Wnt/β-Catenin Signaling Pathway in a Mouse Model of d-Galactose-induced Aging. Int J Mol Sci. 2016;17(6):849. | ||
Zhang YJ, Zhang XL, Li MH, et al. The ginsenoside Rg1 prevents transverse aortic constriction-induced left ventricular hypertrophy and cardiac dysfunction by inhibiting fibrosis and enhancing angiogenesis. J Cardiovasc Pharmacol. 2013;62(1):50–57. | ||
Xie XS, Liu HC, Wang FP, et al. Ginsenoside Rg1 modulation on thrombospondin-1 and vascular endothelial growth factor expression in early renal fibrogenesis in unilateral obstruction. Phytother Res. 2010;24(11):1581–1587. | ||
Li JP, Gao Y, Chu SF, et al. Nrf2 pathway activation contributes to anti-fibrosis effects of ginsenoside Rg1 in a rat model of alcohol- and CCl4-induced hepatic fibrosis. Acta Pharmacol Sin. 2014;35(8):1031–1044. | ||
Gu Y, Wang G, Pan G, et al. Transport and bioavailability studies of astragaloside IV, an active ingredient in Radix Astragali. Basic Clin Pharmacol Toxicol. 2004;95(6):295–298. | ||
Wang Y, Lin C, Ren Q, Liu Y, Yang X. Astragaloside effect on TGF-β1, SMAD2/3, and α-SMA expression in the kidney tissues of diabetic KKAy mice. Int J Clin Exp Pathol. 2015;8(6):6828–6834. | ||
Xu L, Shen P, Bi Y, et al. Danshen injection ameliorates STZ-induced diabetic nephropathy in association with suppression of oxidative stress, pro-inflammatory factors and fibrosis. Int Immunopharmacol. 2016;38:385–394. | ||
Solmaz V, Çinar BP, Yiğittürk G, et al. Neuroprotective effects of octreotide on diabetic neuropathy in rats. Biomed Pharmacother. 2017;89:468–472. | ||
He WY, Zhang B, Xiong QM, et al. Intrathecal administration of rapamycin inhibits the phosphorylation of DRG Nav1.8 and attenuates STZ-induced painful diabetic neuropathy in rats. Neurosci Lett. 2016;619:21–28. | ||
Jindal A, Garcia-Touza M, Jindal N, Whaley-Connell A, Sowers JR. Diabetic kidney disease and the cardiorenal syndrome: old disease, new perspectives. Endocrinol Metab Clin North Am. 2013;42(4):789–808. | ||
Sowers JR. Metabolic risk factors and renal disease. Kidney Int. 2007;71(8):719–720. | ||
Wang JY, Gao YB, Zhang N, et al. miR-21 overexpression enhances TGF-β1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy. Mol Cell Endocrinol. 2014;392(1–2):163–172. | ||
Zhang L, Zhou F, García de Vinuesa A, et al. TRAF4 promotes TGF-β receptor signaling and drives breast cancer metastasis. Mol Cell. 2013;51(5):559–572. | ||
Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57(6):1446–1454. | ||
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. | ||
Horbelt D, Denkis A, Knaus P. A portrait of Transforming Growth Factor β superfamily signalling: Background matters. Int J Biochem Cell Biol. 2012;44(3):469–474. | ||
Coward RJ, Welsh GI, Yang J, et al. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54(11):3095–3102. | ||
Zhang L, Liu J, Zhou F, Wang W, Chen N. PGC-1α ameliorates kidney fibrosis in mice with diabetic kidney disease through an antioxidative mechanism. Mol Med Rep. 2018;17(3):4490–4498. | ||
Fu MX, Wells-Knecht KJ, Blackledge JA, et al. Glycation, glycoxidation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes. 1994;43(5):676–683. | ||
Burns WC, Twigg SM, Forbes JM, et al. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol. 2006;17(9):2484–2494. | ||
Meng XM, Tang PM, Li J, Lan HY. TGF-β/Smad signaling in renal fibrosis. Front Physiol. 2015;6:82. | ||
Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21(11):1819–1834. | ||
Sun L, Zhang D, Liu F, et al. Low-dose paclitaxel ameliorates fibrosis in the remnant kidney model by down-regulating miR-192. J Pathol. 2011;225(3):364–377. | ||
Du JX, Sun MY, Du GL, et al. Ingredients of Huangqi decoction slow biliary fibrosis progression by inhibiting the activation of the transforming growth factor-beta signaling pathway. BMC Complement Altern Med. 2012;12:33. | ||
Zhao J, Wang L, Cao AL, et al. HuangQi Decoction Ameliorates Renal Fibrosis via TGF-β/Smad Signaling Pathway In Vivo and In Vitro. Cell Physiol Biochem. 2016;38(5):1761–1774. | ||
Han H, Cao A, Wang L, et al. Huangqi Decoction Ameliorates Streptozotocin-Induced Rat Diabetic Nephropathy through Antioxidant and Regulation of the TGF-β/MAPK/PPAR-γ Signaling. Cell Physiol Biochem. 2017;42(5):1934–1944. | ||
Guan S, Xu W, Han F, et al. Ginsenoside Rg1 Attenuates Cigarette Smoke-Induced Pulmonary Epithelial-Mesenchymal Transition via Inhibition of the TGF-β1/Smad Pathway. Biomed Res Int. 2017;2017:1–12. | ||
Yu H, Zhen J, Yang Y, et al. Ginsenoside Rg1 ameliorates diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress-induced apoptosis in a streptozotocin-induced diabetes rat model. J Cell Mol Med. 2016;20(4):623–631. | ||
Shen J, Zhao Z, Shang W, et al. Ginsenoside Rg1 nanoparticle penetrating the blood-brain barrier to improve the cerebral function of diabetic rats complicated with cerebral infarction. Int J Nanomedicine. 2017;12:6477–6486. | ||
Meng LQ, Tang JW, Wang Y, et al. Astragaloside IV synergizes with ferulic acid to inhibit renal tubulointerstitial fibrosis in rats with obstructive nephropathy. Br J Pharmacol. 2011;162(8):1805–1818. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



