Back to Journals » Cancer Management and Research » Volume 12
Combination of Brachytherapy with Iodine-125 Seeds and Systemic Chemotherapy versus Systemic Chemotherapy Alone for Synchronous Extracranial Oligometastatic Non-Small Cell Lung Cancer
Authors Li H , Duan Z, Zhao C, Fang W, Jia Y, Li X, Kong F, Zhao L
Received 14 June 2020
Accepted for publication 19 August 2020
Published 9 September 2020 Volume 2020:12 Pages 8209—8220
DOI https://doi.org/10.2147/CMAR.S267694
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Rudolph Navari
Huzi Li,1– 3 Zhendong Duan,2 Cheng Zhao,2 Wenyan Fang,2 Yingjie Jia,2 Xiaojiang Li,2 Fanming Kong,2 Lujun Zhao1
1Department of Radiology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, and Tianjin’s Clinical Research Center for Cancer, Tianjin, People’s Republic of China; 2Department of Oncology, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China; 3Graduate School of Tianjin Medical University, Tianjin, People’s Republic of China
Correspondence: Lujun Zhao
Department of Radiology, Tianjin Medical University Cancer Institute and Hospital, Huanhuxi Road, Hexi District, Tianjin, People’s Republic of China
, Email [email protected]
Background: A proportion of patients with synchronous oligometastatic non-small cell lung cancer (NSCLC) have poor survival, and currently no standard treatment is available, which poses a great challenge to physicians. This study aimed to assess and compare the efficacy and safety of the combination of brachytherapy with iodine-125 seeds and systemic chemotherapy versus systemic chemotherapy alone for synchronous extracranial oligometastatic NSCLC.
Materials and Methods: After a systematic retrospective review of the case database between 1st Mar 2014 and 30th Mar 2018, data were obtained on 69 NSCLC patients with extracranial oligometastatic NSCLC. Among them, 32 patients received brachytherapy with iodine-125 seeds combined with systemic chemotherapy (group A), and the remaining 37 patients received chemotherapy alone (group B). The primary endpoint was overall survival (OS), and the secondary endpoints included progression-free survival (PFS), objective response rate (ORR), and complications.
Results: The demographic and clinical characteristics were not significantly different between the groups (all p> 0.05). The overall 3-month ORR was significantly higher in group A (65.6% vs 37.8%, p=0.030) than in group B. With a median follow-up time of 23 months, the PFS and OS were 11.6 (95% CI: 7.0– 16.2) months vs 6.3 (95% CI: 3.4– 9.2) months (p=0.036) and 17.6 (95% CI: 13.9– 21.3) months vs 11.2 (95% CI: 7.7– 14.7) months (p=0.042) in groups A and B, respectively. Furthermore, in Cox regression analysis, local brachytherapy was an independent prognostic factor for both PFS (HR=0.416, 95% CI: 0.246– 0.702, p=0.001) and OS (HR=0.375, 95% CI: 0.216– 0.653, p=0.001). Severe complications were not observed in either of the groups.
Conclusion: The combination of brachytherapy with iodine-125 seeds and systemic chemotherapy is superior to chemotherapy alone for synchronous extracranial oligometastatic NSCLC.
Keywords: brachytherapy, iodine-125 seeds, NSCLC, oligometastatic, chemotherapy
Introduction
Approximately 75% of non-small cell lung cancers (NSCLCs) are diagnosed after the disease has reached an advanced and unresectable stage,1 and lose the opportunity of undergoing a curative treatment. Although chemotherapy has been shown to improve survival by approximately 2–3 months in randomized clinical trials,2 these patients have generally been considered incurable, with poor overall survival (OS) and progression-free survival (PFS).3 Even so, how to improve the survival rates remains a major challenge in the management of patients with metastatic NSCLC.
Patients with metastatic NSCLC comprise a heterogeneous group, and some studies have suggested that local consolidative treatment can be performed for selected patients with oligometastatic NSCLC.4 The oligometastatic state refers to a limited number of metastatic sites, and is considered as an intermediate status between the locally advanced phase and widely metastatic phase.5 It is also believed to have a better prognosis than widely metastatic NSCLC, with long-term overall survival after aggressive local ablative therapies to all visible disease sites, including both primary and all metastatic lesions,6 although systemic therapies still serve as the cornerstone of treatment for advanced NSCLC. Accumulated evidence from retrospective observational studies, prospective randomized trials,6–10 and a meta-analysis11 demonstrates survival benefits in patients with oligometastases compared with patients who have not received local therapy. This growing evidence has been recognized in the European Society For Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) guidelines, which recommend consideration of radical local treatment as an option for selected patients with oligometastatic disease.12,13 There is a biological rationale to administer local ablative therapy, as metastases can arise from both the primary tumor and the metastases, and local ablation therapy can prevent reseeding from the treated sites.14,15 These local treatments mainly consist of surgery, radiotherapy (RT), chemoradiotherapy (CRT), stereotactic body radiation therapy (SBRT) or stereotactic ablation radiotherapy (SABR), and radiofrequency ablation (RFA) or microwave ablation,16 while studies concerning stereotactic ablation brachytherapy have not been reported.
Implantation of radioactive seeds, serving as stereotactic ablation brachytherapy, is being paid more attention by physicians, with the development of a computerized three-dimensional planning system (TPS) for treating malignant tumors. This has provided a novel treatment modality for lung cancers17 and achieved satisfactory local control.18,19 Brachytherapy has the advantage of providing a high radiation dose in a single fraction to the target, allowing apoptosis over an extended period;20,21 it achieves better organ sparing and has a shorter overall treatment course than conventional radiation therapy. It may also provide a comparable dose within the planning target volume (PTV) compared with SBRT,22 because breathing mobility has very little effect on target precision and repeatability.23 So, the implanted iodine-125 seeds can generate a high dose (140–160 Gy) within target tumor volumes to continuously destroy tumor cells, while the surrounding non-neoplastic tissues only receive a very low dose and are subjected to little damage. In addition, the low radiation dose rate can induce the reoxygenation and increased blood flow of hypoxic tumor volumes, thus producing a radiation-induced bystander effect to kill tumor cells that can overcome the inhomogeneous distribution of the radiation dose.24,25
Long-term results of the SABR-COMET phase II randomized trial indicated that the 5-year survival rate was up to 42.3% for oligometastatic patients treated by SABR plus standard of care (SOC), compared to 17.7% in those patients who received SOC alone, without increased toxicity.26 At the same time, brachytherapy with iodine-125 seeds has the ability to generate a comparable dose within the PTV, while the surrounding tissue only receives a very low dose.22 So, it was postulated that brachytherapy as a local ablation treatment to oligometastatic sites may benefit patients and cure a small subset of stage IV NSCLCs, which previously were deemed incurable.
The purpose of this study was to compare stereotactic ablation brachytherapy with iodine-125 seeds plus systemic chemotherapy with systemic chemotherapy alone for synchronous extracranial oligometastatic NSCLC.
Materials and Methods
Study Design and Patients
This study was a single-institution retrospective study. All consecutive patients registered as having lung cancer with advanced stage in our institution’s database between 1st Mar 2014 and 30th Mar 2018 were retrospectively examined. The study’s eligibility criteria for patients were as follows: 1) had histologically or cytologically confirmed NSCLC; 2) clinical assessments were classified with NSCLC stage IV (The International Association for the Study of Lung Cancer: IASLC 8th edition of the TNM classification for lung cancer)27 by CT of the chest, abdomen, and pelvis, MRI of the brain, or positron emission tomography (PET); 3) had ≤5 synchronous metastases (intrathoracic nodal disease was counted as one site); 4) without oncogenic driver mutations; 5) platinum-based doublet chemotherapy administration had been initiated with a minimum of two cycles between 1st Mar 2014 and 30th Mar 2018; 6) showed good or moderate Eastern Cooperative Oncology Group (ECOG) performance status (PS) (PS≤2); 7) had not received any prior treatments for NSCLC; and 8) had neither refused nor had contraindications for brachytherapy and systemic chemotherapy. The exclusion criteria were as follows: 1) had received any targeted therapies or immune treatment; 2) had intracranial metastases or bone metastases involving the spinal cord; or 3) had incomplete medical records or imaging studies. The medical records and imaging studies were reviewed to collect data on patient demographics and clinical characteristics, complications, objective tumor response, and survival.
Implementation of Brachytherapy with Iodine-125
Brachytherapy with iodine-125 seeds was performed in a standard CT room, scanned on a 64-row CT scanner (GE, USA) with a slice thickness of 5 mm. Detailed images from contrast CT were used to confirm the percutaneous accessibility of the lesion and the security of trajectories from the skin to the target location, avoiding non-target tissue inference. The CT images were also imported to the treatment planning system (TPS) (Model HGGR-2000; Hokai Medical Instruments, Zhuhai, China). The gross tumor volume (GTV) was delineated, and the planning target volume (PTV) should cover the clinical target volume (CTV) and an extra 1 cm beyond the margin. The organs at risk (OARs) around the tumor were also delineated. The expected number of implanted iodine-125 seeds (sodium iodine, half-life period 59.41 days; Beijing Zhibo High-tech Biotechnology Co. Ltd, Beijing, China) was calculated automatically by the TPS and manual adjustment was performed if necessary. The primary planning goal was defined as adequate PTV coverage (V90 >90%) with the prescribed dose. After determination of final options, local anesthesia (2% lidocaine) was administered subcutaneously at the site of needle entry, and steel brachytherapy needles were implanted using the trajectory determined in the plan; needles were positioned at approximately 1–1.5-cm intervals and surrounded the entire lesion. The iodine-125 seeds were inserted through each needle using the implantation applicator. A final CT scan was conducted to verify that all seeds were correctly positioned and acquired for irradiation treatment, and the evaluation indicators included the tumor matched peripheral dose (MPD) and the dose that 90% of the target volume received (D90). Therapeutic focus was given to the primary tumor, regional mediastinal lymph nodes, and oligometastases in this study. Brachytherapy was performed concurrently with or before the first chemotherapy cycle.
Chemotherapy
All patients in this study received routine chemotherapy according to the NCCN guidelines. The chemotherapeutic drugs included cisplatin (50 mg/m2 on days 1 and 8; or 35 mg/m2 on days 1–3), paclitaxel (135 mg/m2 on day 1), gemcitabine (1000 mg/m2 on days 1 and 8), or pemetrexed (500 mg/m2 on day 1). The chemotherapy cycles were repeated every 3 weeks for all patients.
Ethics
The study protocol was approved by the Ethics Committee of the First Teaching Hospital of Tianjin University of TCM, Tianjin, China, and all patients gave their signed informed consent.
Follow-Up
A follow-up CT scan and routine laboratory blood examination were performed 3 months after intervention for patients in the two groups, and every 1–2 months thereafter until death or the follow-up deadline. The patients’ blood cell count, liver function, operation-induced complications, overall survival (OS), and progressive-free survival (PFS) were recorded in detail. The efficacy was evaluated following the guidelines for evaluating the response to treatment in solid tumors. In brief, according to the Response Evaluation Criteria in Solid Tumors (version 1.1) (RECIST 1.1), complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were recorded. The objective response rate (ORR) was defined as the sum of CR and PR.
Statistical Analysis
All statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). The significance of intergroup differences was determined by the chi-squared test, applied to compare categorical variables. The survival outcomes of this study were OS and PFS. OS was calculated from the date of diagnosis to the date of death, last contact, or the last follow-up time; and PFS was based on the time interval from the date of diagnosis to the date of progression, death, last contact, or the last follow-up time. Differences in OS and PFS between the two groups were estimated using the Kaplan–Meier method, and median time was reported with 95% confidence intervals (CIs). A Cox proportional hazards regression model was used to test the association of each factor with survival time, and hazard ratios (HRs) and their 95% CIs were calculated. Differences were considered statistically significant at p<0.05.
Results
Patients
The patient database from 1st Mar 2014 to 30th Mar 2018 was reviewed to define all eligible patients according to the inclusion and exclusion criteria listed in the subsection ‘Study Design and Patients’, above. A total of 69 patients with synchronous extracranial oligometastatic NSCLC were selected to enroll in the final study cohort. The baseline characteristics of the enrolled patients are shown in Table 1. Among them, 32 patients had achieved combination treatment of brachytherapy with iodine-125 seeds and systemic chemotherapy (group A), and the remaining 37 cases received systemic therapy alone (group B). Patient demographics and baseline characteristics were well balanced between both treatment arms in terms of age, gender, pathology, tumor stage, ECOG status, smoking status, primary tumor site, pathology, stage T, stage N, number of metastases, metastatic sites, and cycles of chemotherapy (all p>0.05) (Table 1).
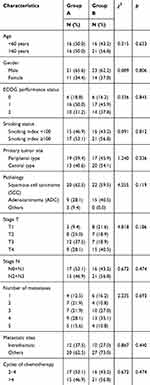 |
Table 1 General Characteristics of Patients in Both Groups |
Postoperative Verification of Quality
The information on brachytherapy dose and radioactive seeds from postoperative CT images was transferred to the TPS to verify the quality, and then exported as dose–volume histograms (DVHs). The 32 patients received a prescription dose of 110.0 Gy. The median number of seeds was 56 (range, 28–126). The median postoperative MPD was 108.0 Gy (range, 94.2–140.8 Gy) and the median D90 was 142.4 Gy (range, 107.0–174.2 Gy) (Table 2).
 |
Table 2 Parameters of Brachytherapy with Radioactive Iodine-125 Seeds |
Short-Term Therapeutic Efficacy
For comparison with pretreatment, CT images were obtained at the 3-month follow-up for short-term response evaluation according to RECIST 1.1 (Figure 1). The outcomes revealed that CR and PR were observed in 5/32 (15.6%) and 16/32 (50.0%) patients in group A and 0/37 (0.0%) and 14/37 (37.8%) in group B, and 4/50 patients with progressive disease (12.5%) in group A versus 12/37 patients (32.4%) in group B. The difference was statistically significant between the two groups (χ2=9.711; p=0.021) (Table 3).
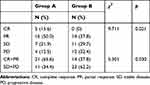 |
Table 3 Short-Term Evaluation of Overall Response |
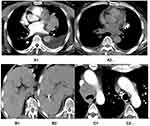 |
Figure 1 Preoperative and postoperative CT scan images of typical patients in group A. |
The ORR (CR+PR) of tumors of patients was higher in group A (65.6%) than in group B (37.8%), which was a significant difference (χ2=5.301; p=0.030) (Table 3).
Progression-Free Survival and Overall Survival Analysis
With a median follow-up of 23 months (range: 4–46 months), the median PFS was 11.6 months (95% CI: 7.0–16.2 months) in group A and 6.3 months (95% CI: 3.4–9.2 months) in group B (Figure 2). There was a statistically significant improvement in PFS for patients in group A compared to those in group B (p=0.036). The median OS for patients in group A (17.6 months, 95% CI: 13.9–21.3 months) was also longer than in group B (11.2 months, 95% CI: 7.7–14.7 months), with statistically significant difference (p=0.042) (Figure 3).
 |
Figure 2 Kaplan–Meier analysis of progression-free survival. |
 |
Figure 3 Kaplan–Meier analysis of overall survival. |
In univariate Cox regression analysis, number of metastases (HR=0.135, 95% CI: 0.071–0.260, p<0.001) and local brachytherapy (HR=0.596, 95% CI: 0.364–0.973, p=0.039) were closely related to PFS; and T stage (HR=0.571, 95% CI: 0.336–0.970, p=0.038), number of metastases (HR=0.159, 95% CI: 0.086–0.293, p<0.001), and local brachytherapy (HR=0.588, 95% CI: 0.349–0.989, p=0.045) were closely related to OS (Table 4).
 |
Table 4 Univariate and Multivariate Cox Regression Analysis of PFS and OS for Overall Patients |
Furthermore, in multivariate analysis, both the number of metastases (HR=0.100, 95% CI: 0.050–0.204, p<0.001) and local brachytherapy (HR=0.416, 95% CI: 0.246–0.702, p=0.001) were independent factors for PFS, and number of metastases=1–3 (HR=0.118, 95% CI: 0.060–0.231, p<0.001) and receiving local brachytherapy (HR=0.375, 95% CI: 0.216–0.653, p=0.001) were associated with significantly better OS (Table 4).
Complications
After brachytherapy in group A, three patients developed mild pneumothorax, which was accompanied by subcutaneous emphysema in one patient, and the thoracic cavity was closed for drainage for only one patient. Two patients experienced mild pulmonary hemorrhage; one of them had bloody sputum and the other had hemoptysis, and all symptoms disappeared within 1–3 days after receiving hemocoagulase or etamsylate injection. One patient experienced seed migration to the pleural cavity and one patient local radiation pneumonitis, and no significant related clinical symptoms were observed. None of the patients developed local skin erythema, hemoptysis, grade 4/5 complications, or operation-induced death (Table 5).
 |
Table 5 Complications in Patients After Brachytherapy with Radioactive Iodine-125 Seeds |
Hematological toxicity was defined as leukocyte decrease, anemia, and platelet decrease. No statistically significant difference was found in the hematological toxic events between group A and group B (p>0.05) (Table 6). Severe liver toxicity was not observed in either group, while mild elevated transaminase occurred in six of 32 patients (18.8%) in group A versus eight of 37 patients (21.6%) in group B (p=0.680) (Table 6). Severe complications of grade 4 or above did not occur in either group.
 |
Table 6 Adverse Events Observed in Both Groups Upon Follow-Up |
Discussion
The purpose of this trial was to evaluate the efficacy and safety of combination treatment of brachytherapy with iodine-125 and chemotherapy for patients with synchronous extracranial oligometastatic NSCLC compared to traditional chemotherapy alone. To the best of our knowledge, this has not been reported previously and is hence a novel treatment modality for these patients.
The results suggest that brachytherapy with iodine-125 seeds may be an alternative method with or without other treatments for all kinds of NSCLC patients. Brachytherapy has been proven to improve local control rates and decrease local recurrence, as postoperative adjuvant treatment with resection.28–31 Huo et al32 reported that iodine-125 brachytherapy combined with chemotherapy is an effective, minimally invasive method for the treatment of stage III NSCLC, with a median survival time of 24.76 months and a median local control time of 25.28 months. Yu et al33 explored the effectiveness of brachytherapy plus chemotherapy versus chemotherapy alone for recurrent stage III NSCLC (52 patients were equally divided into two groups), and the PFS was 8 months versus 5.5 months (p<0.05) with a median follow-up time of 11 months. Wang et al34 provided evidence that retroperitoneal metastatic lymph nodes can also be treated using CT-guided implantation of radioactive iodine-125 seeds, with the achievement of good pain relief. The findings of these studies suggest that intervention with brachytherapy with iodine-125 seeds could lead to a better prognosis for operable or inoperable or metastatic NSCLC patients, and it could be superior to chemotherapy alone when combined with chemotherapy, as proven by our findings in this study.
The primary results of our study were longer PFS (11.6 months vs 6.3 months, HR=0.416, 95% CI: 0.246–0.702, p=0.001) and OS (17.6 months vs 11.2 months, HR=0.375, 95% CI: 0.216–0.653, p=0.001) for patients receiving iodine-125 brachytherapy combined with chemotherapy compared with chemotherapy alone, as well as better ORR (65.6% vs 37.8%, p=0.030), which indicated that combination treatment could be a superior measure. These findings are in agreement with other experiences.35–37 In the study by Wu et al,36 the OS and PFS of patients with advanced NSCLC were 20 months (95% CI: 19.09–20.90 months) versus 15 months (95% CI: 14.48–15.51 months) (p<0.05) and 13 months (95% CI: 11.96–14.04 months) versus 8 months (95% CI: 7.63–8.37 months) (p<0.05), respectively, when a combination of brachytherapy with chemotherapy was compared to chemotherapy alone. Both PFS and OS were better than in our study, which may be attributed to the included standard and approximately 30% stage III patients being enrolled in Wu’s study. A meta-analysis37 of comparisons of iodine-125 brachytherapy combined with chemotherapy and chemotherapy alone showed that combination therapy was superior to chemotherapy alone in CR (risk ratio [RR]=3.66, 95% CI: 2.08–6.44, p<0.001), PR (RR=1.47, 95% CI: 1.16–1.86, p=0.001), ORR (RR=1.85, 95% CI: 1.54–2.22, p<0.001), disease control rate (RR=1.19, 95% CI: 1.10–1.29, p<0.001), one-year overall survival (RR=1.46, 95% CI: 1.12–1.92, p=0.006), and PD (RR=0.20, 95% CI: 0.09–0.43, p<0.001). Another meta-analysis,35 including 1188 cases, also indicated consistent results, with better ORR (RR=1.90, 95% CI: 1.67–2.16) and OS (RR=0.66, 95% CI: 0.50–0.86, p<0.001) in the combination treatment group.
Several potential mechanisms may explain the different from brachytherapy with seeds in terms of OS and PFS. First, the stereotactic ablation ability of radioactive seeds could reduce the burden of primary and metastatic malignant cells. In that case, chemotherapy would have a synergistic effect on the inhibition of progression. Second, local radiation therapy could render residual disease more sensitive to chemotherapy.6 A third possibility is that local treatment may delay polymetastatic conversion38 and slow the growth of distant micrometastatic disease.6
Emerging evidence suggests that local therapies are technically feasible for oligometastatic lesions, with favorable survival. However, brachytherapy as a novel local method has not been reported for oligometastatic NSCLC with or without systemic chemotherapy. In a multicenter, randomized, phase II trial6 enrolling patients with oligometastatic NSCLC, Gomez et al reported an observed benefit in PFS (14.2 months, 95% CI: 7.4–23.1 months vs 4.4 months, 95% CI: 2.2–8.3 months, p=0.022) and OS outcomes (41.2 months, 95% CI: 18.9 months to not reached vs 17.0 months, 95% CI: 10.1–39.8 months, p=0.017) for these patients. A prospective, single-arm phase II trial of local therapy in combination with systemic chemotherapy in 40 patients with one to five metastases showed a median OS and PFS of 13.5 months and 12.1 months, respectively.7 Iyengar’s single-institution randomized phase II study of SBRT followed by maintenance chemotherapy versus maintenance chemotherapy alone for patients with limited metastatic NSCLC showed a significant improvement in PFS (9.7 months vs 3.5 months, p=0.01) in the combination arm.10 In a retrospective study, Wei et al39 reported that the median PFS and OS were 14.0 and 47.8 months, respectively, after microwave ablation in the treatment of patients with oligometastatic NSCLC. Mitchell et al40 reported that comprehensive local treatment was independently associated with improved OS (HR=0.67, 95% CI: 0.46–0.97, p=0.034) in synchronous oligometastatic NSCLC. Collen et al9 designed a phase II study of stereotactic body radiotherapy to the primary tumor and metastatic locations in patients with oligometastatic NSCLC, and showed that median PFS and OS were 11.2 and 23 months, respectively. All of the evidence from previous trials exposed a similar PFS within a narrow range, in addition to the PFS data in our study, which suggest that the efficacy of brachytherapy may be considerable for other consolidative measures when combined with chemotherapy, at least for PFS. The data for OS were significantly different among these studies, which may attributable to several reasons, as follows: different definition of oligometastatic (≤3 metastases,40 or ≤5 metastases), different mean numbers of metastases, size of primary or metastatic lesions, and status of oligometastasis (synchronous or metachronous or both), selective bias, or other unreported information. Further randomized controlled trials should be implemented to determine whether stereotactic ablation brachytherapy has an advantage over other technologies for oligometastatic NSCLC.
Although complications developed during the perioperative period, no treatment-related deaths or severe complications were reported in the two groups, which is in agreement with previous trials,33,36 and all complications were managed accordingly. Pneumothorax is the most common implantation-induced complication,37 with an incidence rate of 9.4% in our study and 10.0–30.0% in a previous study.32 Mild pulmonary hemorrhage (6.3%), local radiation pneumonitis (3.1%), and seed migration (3.1%) were tolerable, with low incidence rates. Hematological toxicity and loss of liver function may be attributed to chemotherapy, and the incidence rate was not statistically different between the combination group and the chemotherapy-alone arm. In conclusion, this treatment was well tolerated by most patients because the unfavorable reactions of brachytherapy with radioactive iodine-125 seeds are mild and the incidence of serious adverse events is low.
Our study has several limitations. First, there is a selection bias, related to the monocenter and non-regional oncology clinics. Second, the retrospective nature of our study is also a limitation. Third, we could not take into account oncogenic driver mutations such as EGFR, RAS, HER2, and MET mutations, and ROS1, RET, and NTRK fusionss. Finally, patients with intracranial oligometastatic and metachronous oligometastatic NSCLC were excluded from our study, because fewer patients received brachytherapy in our department.
Conclusion
Our study demonstrated that brachytherapy combined with chemotherapy was effective and safe for synchronous extracranial oligometastatic NSCLC, leading to longer PFS and OS compared to chemotherapy alone, with fewer side effects. Future large-sample and higher-quality multicenter randomized control trials are needed to confirm the efficacy and safety of this treatment.
Acknowledgments
We would like to acknowledge all investigators, coordinators, and study site personnel, as well as the patients and their families, for their participation in this study.
Disclosure
Huzi Li reports grants from the Health and Family Planning Commission Key Scientific Research Project of Traditional Chinese Medicine of Tianjin and the National Natural Science Foundation of China for Youth, during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
1. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245–250. doi:10.1016/j.prro.2018.02.009
2. NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26(28):4617–4625.
3. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi:10.1038/nature25183
4. Liu K, Zheng D, Xu G, Du Z, Wu S. Local thoracic therapy improve prognosis for stage IV non-small cell lung cancer patients combined with chemotherapy: A Surveillance, Epidemiology, and End Results database analysis. PLoS One. 2017;12(11):e0187350. doi:10.1371/journal.pone.0187350
5. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8–10. doi:10.1200/JCO.1995.13.1.8
6. Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol. 2019;37(18):1558–1565. doi:10.1200/JCO.19.00201
7. De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol. 2012;7(10):1547–1555. doi:10.1097/JTO.0b013e318262caf6
8. Hasselle MD, Haraf DJ, Rusthoven KE, et al. Hypofractionated image-guided radiation therapy for patients with limited volume metastatic non-small cell lung cancer. J Thorac Oncol. 2012;7(2):376–381. doi:10.1097/JTO.0b013e31824166a5
9. Collen C, Christian N, Schallier D, et al. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol. 2014;25(10):1954–1959. doi:10.1093/annonc/mdu370
10. Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018;4(1):e173501. doi:10.1001/jamaoncol.2017.3501
11. Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer. 2014;15(5):346–355. doi:10.1016/j.cllc.2014.04.003
12. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi:10.1093/annonc/mdy275
13. Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(4):504–535. doi:10.6004/jnccn.2017.0050
14. Macintyre G, Van Loo P, Corcoran NM, Wedge DC, Markowetz F, Hovens CM. How Subclonal Modeling Is Changing the Metastatic Paradigm. Clin Cancer Res. 2017;23(3):630–635. doi:10.1158/1078-0432.CCR-16-0234
15. Newton PK, Mason J, Bethel K, et al. Spreaders and sponges define metastasis in lung cancer: a Markov chain Monte Carlo mathematical model. Cancer Res. 2013;73(9):2760–2769. doi:10.1158/0008-5472.CAN-12-4488
16. Ni Y, Ye X, Yang X, et al. Microwave ablation as local consolidative therapy for patients with extracranial oligometastatic EGFR-mutant non-small cell lung cancer without progression after first-line EGFR-TKIs treatment. J Cancer Res Clin Oncol. 2020;146(1):197–203. doi:10.1007/s00432-019-03043-6
17. Fleischman EH, Kagan AR, Streeter OE, et al. Iodine125 interstitial brachytherapy in the treatment of carcinoma of the lung. J Surg Oncol. 1992;49(1):25–28. doi:10.1002/jso.2930490107
18. Zhang T, Lu M, Peng S, et al. CT-guided implantation of radioactive 125I seed in advanced non-small-cell lung cancer after failure of first-line chemotherapy. J Cancer Res Clin Oncol. 2014;140(8):1383–1390. doi:10.1007/s00432-014-1655-x
19. Huo X, Wang H, Yang J, et al. Effectiveness and safety of CT-guided (125)I seed brachytherapy for postoperative locoregional recurrence in patients with non-small cell lung cancer. Brachytherapy. 2016;15(3):370–380. doi:10.1016/j.brachy.2016.02.001
20. Peters N, Wieners G, Pech M, et al. CT-guided interstitial brachytherapy of primary and secondary lung malignancies: results of a prospective phase II trial. Strahlenther Onkol. 2008;184(6):296–301. doi:10.1007/s00066-008-1718-5
21. Manning MA, Zwicker RD, Arthur DW, Arnfield M. Biologic treatment planning for high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2001;49(3):839–845. doi:10.1016/S0360-3016(00)01453-X
22. Li R, Zhang Y, Yuan Y, et al. Dosimetric comparison of CT-guided iodine-125 seed stereotactic brachytherapy and stereotactic body radiation therapy in the treatment of NSCLC. PLoS One. 2017;12(11):e0187390. doi:10.1371/journal.pone.0187390
23. Sharma DN, Rath GK, Thulkar S, Bahl A, Pandit S, Julka PK. Computerized tomography-guided percutaneous high-dose-rate interstitial brachytherapy for malignant lung lesions. J Cancer Res Ther. 2011;7(2):174–179. doi:10.4103/0973-1482.82914
24. Wang ZM, Lu J, Liu T, Chen KM, Huang G, Liu FJ. CT-guided interstitial brachytherapy of inoperable non-small cell lung cancer. Lung Cancer. 2011;74(2):253–257. doi:10.1016/j.lungcan.2011.03.006
25. Chen HH, Jia RF, Yu L, Zhao MJ, Shao CL, Cheng WY. Bystander effects induced by continuous low-dose-rate 125I seeds potentiate the killing action of irradiation on human lung cancer cells in vitro. Int J Radiat Oncol Biol Phys. 2008;72(5):1560–1566. doi:10.1016/j.ijrobp.2008.07.038
26. Palma DA, Olson R, Harrow S. et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol;2020. JCO2000818. doi:10.1200/JCO.20.00818
27. Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11(1):39–51. doi:10.1016/j.jtho.2015.09.009
28. Odell DD, Kent MS, Fernando HC. Sublobar resection with brachytherapy mesh for stage I non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2010;22(1):32–37. doi:10.1053/j.semtcvs.2010.04.003
29. Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a Phase III randomized trial for high-risk operable non-small-cell lung cancer. J Clin Oncol. 2014;32(23):2456–2462. doi:10.1200/JCO.2013.53.4115
30. Santos R, Colonias A, Parda D, et al. Comparison between sublobar resection and 125Iodine brachytherapy after sublobar resection in high-risk patients with Stage I non-small-cell lung cancer. Surgery. 2003;134(4):691–697. doi:10.1016/S0039-6060(03)00327-1
31. Fernando HC, Landreneau RJ, Mandrekar SJ, et al. The impact of adjuvant brachytherapy with sublobar resection on pulmonary function and dyspnea in high-risk patients with operable disease: preliminary results from the American College of Surgeons Oncology Group Z4032 trial. J Thorac Cardiovasc Surg. 2011;142(3):554–562. doi:10.1016/j.jtcvs.2010.10.061
32. Huo X, Huo B, Wang H, et al. Implantation of computed tomography-guided Iodine-125 seeds in combination with chemotherapy for the treatment of stage III non-small cell lung cancer. J Contemp Brachytherapy. 2017;9(6):527–534. doi:10.5114/jcb.2017.72605
33. Yu X, Li J, Zhong X, He J. Combination of Iodine-125 brachytherapy and chemotherapy for locally recurrent stage III non-small cell lung cancer after concurrent chemoradiotherapy. BMC Cancer. 2015;15(1):656. doi:10.1186/s12885-015-1657-3
34. Wang Z, Lu J, Gong J, et al. CT-guided radioactive 125I seed implantation therapy of symptomatic retroperitoneal lymph node metastases. Cardiovasc Intervent Radiol. 2014;37(1):125–131. doi:10.1007/s00270-013-0613-3
35. Zhang W, Li J, Li R, Zhang Y, Han M, Ma W. Efficacy and safety of iodine-125 radioactive seeds brachytherapy for advanced non-small cell lung cancer-A meta-analysis. Brachytherapy. 2018;17(2):439–448. doi:10.1016/j.brachy.2017.11.015
36. Wu C, Li B, Sun G, Peng C, Xiang D. Efficacy and safety of iodine-125 brachytherapy combined with chemotherapy in the treatment of advanced NSCLC in the elderly. Onco Targets Ther. 2018;11:6617–6624. doi:10.2147/OTT.S174457
37. Qiu H, Ji J, Shao Z, et al. The Efficacy and Safety of Iodine-125 Brachytherapy Combined with Chemotherapy in Treatment of Advanced Lung Cancer: A Meta-Analysis. J Coll Physicians Surg Pak. 2017;27(4):237–245. doi:2597
38. Nicosia L, Cuccia F, Mazzola R, et al. Stereotactic body radiotherapy (SBRT) can delay polymetastatic conversion in patients affected by liver oligometastases. J Cancer Res Clin Oncol. 2020;146(9):2351–2358. doi:10.1007/s00432-020-03223-9
39. Wei Z, Ye X, Yang X, et al. Efficacy and safety of microwave ablation in the treatment of patients with oligometastatic non-small-cell lung cancer: a retrospective study. Int J Hyperthermia. 2019;36(1):827–834. doi:10.1080/02656736.2019.1642522
40. Mitchell KG, Farooqi A, Ludmir EB, et al. Improved Overall Survival With Comprehensive Local Consolidative Therapy in Synchronous Oligometastatic Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2020;21(1):37–46. doi:10.1016/j.cllc.2019.07.007
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
