Back to Journals » Drug Design, Development and Therapy » Volume 14
Combination Chemotherapy of Lung Cancer – Co-Delivery of Docetaxel Prodrug and Cisplatin Using Aptamer-Decorated Lipid–Polymer Hybrid Nanoparticles
Authors Wu R, Zhang Z, Wang B, Chen G, Zhang Y, Deng H, Tang Z, Mao J, Wang L
Received 19 January 2020
Accepted for publication 15 April 2020
Published 9 June 2020 Volume 2020:14 Pages 2249—2261
DOI https://doi.org/10.2147/DDDT.S246574
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Ruifeng Wu,1,* Zhiqiang Zhang,1,* Baohua Wang,2 Ge Chen,3 Yaozhong Zhang,3 Haowen Deng,3 Zilong Tang,3 Junjie Mao,3 Lei Wang3
1Department of Thoracic Surgery, Baoding No.1 Central Hospital, Baoding, Hebei Province, People’s Republic of China; 2Department of Thoracic Surgery, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, People’s Republic of China; 3Department of Thoracic Surgery, Fourth Hospital of Hebei Medical University, Tumor Hospital of Hebei Province, Shijiazhuang, Hebei Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Wang Email [email protected]
Purpose: Lung cancer is the leading cause of cancer mortality worldwide. Drug resistance is the major barrier for the treatment of non-small cell lung cancer (NSCLC). The aim of this research is to develop an aptamer-decorated hybrid nanoparticle for the co-delivery of docetaxel prodrug (DTXp) and cisplatin (DDP) and to treat lung cancer.
Materials and Methods: Aptamer-conjugated lipid–polymer ligands and redox-sensitive docetaxel prodrug were synthesized. DTXp and DDP were loaded into the lipid–polymer hybrid nanoparticles (LPHNs). The targeted efficiency of aptamer-decorated, DTXp and DDP co-encapsulated LPHNs (APT-DTXp/DDP-LPHNs) was determined by performing a cell uptake assay by flow cytometry-based analysis. In vivo biodistribution and anticancer efficiency of APT-DTXp/DDP-LPHNs were evaluated on NSCLC-bearing mice xenograft.
Results: APT-DTXp/DDP-LPHNs had a particle size of 213.5 ± 5.3 nm, with a zeta potential of 15.9 ± 1.9 mV. APT-DTXp/DDP-LPHNs exhibited a significantly enhanced cytotoxicity (drug concentration causing 50% inhibition was 0.71 ± 0.09 μg/mL), synergy antitumor effect (combination index was 0.62), and profound tumor inhibition ability (tumor inhibition ratio of 81.4%) compared with the non-aptamer-decorated LPHNs and single drug-loaded LPHNs.
Conclusion: Since the synergistic effect of the drugs was found in this system, it would have great potential to inhibit lung tumor cells and in vivo tumor growth.
Keywords: lung cancer, combination therapy, docetaxel prodrug, cisplatin, aptamer-decorated, lipid–polymer hybrid nanoparticles
Introduction
The leading cause of cancer death is non-small cell lung cancer (NSCLC).1 Conventional cancer chemotherapy encounters drastic limitations in terms of nonspecific delivery of antitumor drugs and severe side-effects.2 Clinical applications of chemotherapeutic drugs faced many challenges, including degradation in serum, rapid blood clearance, stimulation of immune response, off-target effects, and poor cellular uptake.3 Active targeting towards malignant cells by chemotherapy drugs is a widely studied approach that allows for high selectivity of anti-tumor drugs, thereby reducing the dose of drugs needed for effective treatment while minimizing the side effects of conventional chemotherapy.4 Aptamers are oligonucleic acids or peptides that have high target ability and robust selectivity toward several types of target molecules, including proteins, peptides, small molecules and cells.5 Aptamers have several distinctive advantages due to their unique three-dimensional structure, high structural flexibility, non-immunogenicity, non-toxicity and smaller size than antibodies.6 Aptamers are playing an increasingly important role in the treatment and diagnosis of cancers. Overexpressed receptors in cancer cells are the main targets of aptamers in therapy. To date, aptamers for cancer cells have been developed in large numbers.7
The targeted nano-drug delivery system may overcome the non-specific toxicity of chemotherapy because nanoparticles can not only accumulate in tumor sites through enhanced permeability and retention, but can also be surface-conjugated using targeting ligands to improve their tumor targeting and cellular internalization.8 Lipid–polymer hybrid nanoparticles (LPHNs) of biodegradable polymers and lipids represent superior candidate drug delivery systems, as they combine the advantages of liposomes and polymer nanoparticles,9 including superior biocompatibility, high drug loading, sustained release, and easy modification of targeting molecules including aptamers.10 Modified LPHNs have been widely developed and used for targeted lung cancer therapy.11–13
Combination therapy with multiple chemotherapeutics for refractory cancers is a successful strategy for its synergistic effects, lower toxicity and drug resistance.14 Platinum agents remain the leading therapy for advanced NSCLC,15 which were often used in combination with paclitaxel, docetaxel (DTX), gemcitabine or irinotecan, in concurrence with radiotherapy.16 However, the combination therapy is challenged by distinct physicochemical properties and in vivo pharmacokinetics/pharmacodynamics of the individual pharmaceuticals, which make the optimization of dosing and administration schedule challenging.17 Prodrugs based nano-system represents an effective alternative strategy for the delivery of anti-cancer drugs.18 Hypoxia-activated prodrugs are effective for targeting the hypoxic tumor microenvironment.19 Here a redox-sensitive docetaxel prodrug (DTXp) was designed and used along with cisplatin (DDP).
In the present study, aptamer-conjugated lipid–polymer ligands were synthesized and used for the decoration of LPHNs. DTXp was synthesized and co-loaded with DDP into the aptamer-modified LPHNs. The anticancer efficiency of this system was evaluated on lung cancer cells and in vivo xenograft, in comparison with the un-modified LPHNs, single DDP- or DTXp-loaded LPHNs, and DTX-loaded LPHNs.
Materials and Methods
Materials
A549 cell-binding aptamer (S6, sequence: GTGGCCAGTCACTCAATTGGGTGTAGGGGTGGGGATTGTGGGTTG) with a sulfhydryl group at the 5ʹ-end was synthesized by RiboBio Co., Ltd. (Guangzhou, China). Poly(L-lactide) (5000)-poly(ethylene glycol) (2000)-maleimide (PLA-PEG-MAL) and fluorescein isothiocyanate-poly(L-lactide) (5000)-poly(ethylene glycol) (2000)-maleimide (FITC-PLA-PEG-MAL) were purchased from Xi’an ruixi Biological Technology Co., Ltd. Docetaxel, glyceryl monostearate, thiodiglycolic anhydride, lecithin, N-(3-dimethylaminopropyl)-N0-ethylcarbodiimide hydrochloride (EDC) 1-hydroxybenzotriazole monohydrate (HOBt) were purchased from Aladdin Industrial Corporation (Shanghai, China). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin, and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) were purchased from Invitrogen Corporation (Carlsbad, CA). A549 cells and human lung (bronchial) epithelial cells (BEAS2B cells) were obtained from American Type Culture Collections (ATCC, Manassas, VA).
Synthesis of Aptamer-Conjugated Lipid–Polymer Ligands
Aptamer-conjugated lipid–polymer ligands were synthesized by conjugating the aptamer to PLA-PEG-Mal (Figure 1).20 PLA-PEG-MAL (100 mg) was dissolved in PBS (5 mL, pH 7.4). Then, S6 aptamers (APT, 10 mg) were mixed with the solution for 6 h, the product was then dialyzed against distilled water using dialysis membrane (MWCO 7500 Da) for 12 h and lyophilized to get aptamer-conjugated PLA-PEG (PLA-PEG-APT) and stored at 4°C until further use.
Synthesis of Redox-Sensitive Docetaxel Prodrug (DTXp)
Redox-sensitive docetaxel prodrug (DTXp) was synthesized by conjugating the DTX with glyceryl monostearate (GM) using redox-responsive thiodiglycolic anhydride (TA) (Figure 1).21,22 GM (1 equivalent) was dissolved in chloroform, EDC (1 equivalent), and HOBt (1 equivalent) were then added and stirred for 30 minutes to activate the carboxyl groups of TA. After that, TA (1 equivalent) was added and the mixture was reacted at room temperature under stirring for 24 hours to get GM-TA. DTX (1 equivalent), EDC (1 equivalent), and HOBt (1 equivalent) were added to GM-TA (1 equivalent). Then, the temperature of the solution was declined to 0°C and stirred for 30 min under nitrogen. After that, the mixture was reacted at room temperature under stirring for 24 hours to obtain DTX-GM-TA. Finally, after being freeze-dried, the resulting product was obtained and stored at 4°C until further use.
Preparation of DTXp and DDP-Co-Loaded Aptamer-Modified LPHNs
DTXp and DDP-co-loaded aptamer-modified LPHNs (APT-DTXp/DDP-LPHNs) were using the thin-film hydration and ultrasonic dispersion method.23 PLA-PEG-APT, DTX-GM-TA, DDP, lecithin (weight ratio 50:10:10:30, total weight 200 mg) were dissolved in chloroform in an eggplant-shaped flask. The flask was then connected to a rotary evaporator and water bath with temperature maintained at 35°C under the aspirate vacuum. The thin-film layer formed was flushed with nitrogen gas for 5 minutes and maintained overnight under vacuum to remove chloroform. Then, the solid film layer was preheated in a warm water bath at 65°C to obtain transparent gel samples. The aqueous phase of purified water heat to the same temperature as the gel sample was added to the gel sample and hydrated at 65°C for 60 minutes. The mixture was then ultrasonically treated on an ice bath for 10 minutes until the solution was clear. Then, the solution was centrifuged at 10,000×g for 30 minutes, wash for 3 times, to remove the unloaded drugs.
DTXp and DDP-co-loaded unmodified LPHNs (DTXp/DDP-LPHNs) were prepared using the same method, changing PLA-PEG-APT to PLA-PEG. Single DTXp, DTX or DDP-loaded aptamer-modified LPHNs (APT-DTXp-LPHNs, APT-DTX-LPHNs or APT-DDP-LPHNs) were prepared using the same method, using a single DTXp, DTX or DDP only. Blank aptamer-modified LPHNs (APT-LPHNs) were prepared without adding drugs.
Characterization and Serum Stability of LPHNs
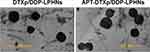
Particle size and zeta potential of LPHNs were measured by dynamic light scattering using a Zetasizer (Nano-ZS, Malvern, UK).24 Morphology and size of APT-DTXp/DDP-LPHNs and DTXp/DDP-LPHNs were recorded by a transmission electron microscopy (TEM, JEM-1200EX, JEOL, Japan):25 A drop of nanoparticle suspension was placed onto a copper grid and air drying, followed by negative staining with one drop of 3% aqueous solution of sodium phosphotungstate for contrast enhancement.
The DTX and DDP loading efficiency (LE) and loading content (LC) were determined spectrophotometrically following centrifugation using a filter (0.1 μm pore size). The amount of DTX in LPHNs was measured using high-performance liquid chromatography (HPLC).26 The analysis was performed at 230 nm using an Extend-C18 column (4.6 mm × 250 mm). The amount of DDP in LPHNs was determined with a UV-Vis spectrophotometer at 706 nm.27 LE (%) = The quality of drugs in nanoparticles/Total quality of drugs used×100%; LC (%) = The quality of drugs in nanoparticles/The quality of nanoparticles×100%.
The stability of LPHNs in serum was investigated by mixing the LPHNs (20 mg) with FBS (55% in volume,10 mL) at pH 7.4 and incubated at 37°C for 4 days.28 The changes in particle size, zeta potential, and LE LPHNs were analyzed by the same methods.
In vitro Drug Release of LPHNs
In vitro drug release of LPHNs was conducted by dialysis bag diffusion method in both hypoxic and normal conditions.29,30 The PBS solution (pH 7.4, 10 mL) was degassed with nitrogen in the presence of nicotinamide adenine dinucleotide phosphate (NADPH, 100 mM) for the entire period of the release experiment to maintain the hypoxic condition and the normal (nonhypoxic) condition was prepared using PBS containing 100 mM NADPH without degassing. APT-DTXp/DDP-LPHNs and other LPHNs suspensions (1 mL) were sealed in dialysis bags (Molecular weight cut-off 10 KDa) and the bags were placed in hypoxic or normal condition under 100 rpm constantly shaken. The release medium (0.5 mL) was taken out at determined time intervals the amount of DTX and DDP was calculated using the method of the above section.
Cell Uptake of LPHNs
To evaluate cell uptake efficiency of LPHNs, fluorescent dye (FITC) was applied to the LPHNs systems.31 During the preparation process, FITC-PLA-PEG-MAL was used instead of PLA-PEG-MAL. A549 cells and BEAS2B cells (1×105 cells/well) were seeded in 12-well culture plates; then, serum-free RPMI 1640 medium containing LPHNs were added to each well. After incubated for 2 h, the cells were washed three times with cold PBS. The fluorescence intensity of the cells was determined by flow cytometer (BD Biosciences, Franklin Lakes, NJ).
Cell Viability Assays of LPHNs
A549 cells (1×104 cells/well) were seeded in 96-well plates, and cultured in RPMI 1640 medium (supplemented with 10% fetal bovine serum, 100 U/mL of penicillin and 100 mg/mL streptomycin) and maintained at 37°C in a 5% CO2 atmosphere.32 After the overnight incubation, the medium was replaced with medium containing drug-loaded LPHNs at concentrations (from 0.1 to 100 mg/mL). Then, the cells were incubated for an additional 72 h. The cell viability was measured using the MTT method: MTT reagent (5 mg/mL, 20 µL) was added to each well and incubated (37 °C, 4 h). DMSO (150 µL) was then added after the medium was discarded. The absorbance was measured at 570 nm.
Synergistic Effect of Drugs Combinations
To evaluate the synergistic effect of APT-DTXp/DDP-LPHNs, combination index (CI) analysis was undertaken.33 CI values were calculated through the Chou–Talalay method.34 For each level of the fraction of affected cells (Fa), the CI provides a quantitative value for synergy and is given by CIDTX+DDP = DDTX(DTX+DDP)/DDTX + DDDP(DTX+DDP)/DDDP, where DDTX(DTX+DDP) and DDDP(DTX+DDP) are the concentrations of DTX or DDP g in the combination, DDTX and DDDP are the concentrations of the drugs alone.35 CI values less than 1.0 indicate synergy, with values closer to zero representing increasing synergy.
Lung Cancer Xenograft
Female BALB/c mice (Six-week-old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China), xenograft mouse models were produced by injecting A549 cells (7×106, 0.2 mL per mice) subcutaneously into the flanks of nude mice. All animal experiments were performed according to protocols approved by the Animal Use and Care Administrative Committee of Fourth Hospital of Hebei Medical University, Tumor Hospital of Hebei Province and followed the National Institutes of Health guide for the care and use of laboratory animals.
In vivo Biodistribution
Lung cancer xenograft mice were randomized into 4 groups. APT-DTXp/DDP-LPHNs, DTXp/DDP-LPHNs, APT-DTX-LPHNs, and free DTX/DDP were injected into the tail vein at a single equivalent dose of DTX or CDDP 5 mg/kg.36 At 48 h post-injection, major tissues (tumor, heart, liver, spleen, lung and kidney) and were harvested from the mice. The organs were weighed and dissolved in concentrated nitric acid by heating up and evaporated to dryness, and then re-dissolved in 0.1 N HCl for tissue biodistribution analysis by the method of section 2.5.
In vivo Antitumor Effect
Lung cancer xenograft mice were randomized into 7 groups (6 mice per group). APT-DTXp/DDP-LPHNs, DTXp/DDP-LPHNs, APT-DTXp-LPHNs, APT-DTX-LPHNs, APT-DDP-LPHNs, free DTX/DDP, and normal saline were injected into the tail vein every 3 days at an equivalent dose of DTX or CDDP 5 mg/kg.37 Tumor volumes of mice were measured every 3 days. Tumor volume was calculated by the following formula: Volume = length × width2/2. After the last injection, the whole blood was collected and centrifuged (4000 rpm, 5 min) to obtain the serum.15 Blood urea nitrogen (BUN), serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were assayed as indicators of renal and hepatic function.
Results
Characterization of PCL-PEG-APT and DTX-GM-TA
PCL-PEG-APT and DTX-GM-TA were characterized by the 1H NMR spectra (Figure 1). PCL-PEG-APT: the characteristic peaks of PCL were identified at 1.39 and 4.34 ppm; the peaks in the range of 3.36–3.63 ppm belong to PEG; APT was confirmed by the peaks at 0.96, 2.01 and 2.51 ppm. DTX-GM-TA: the peaks assigned to DTX were 1.11, 1.51, and 2.09; GM has the peaks at 3.41; the signals at 1.72, 2.25, and 4.41 ppm attributed to TA; peak at 4.08 proved the formation of an ester linkage.
Characterization of APT-DTXp/DDP-LPHNs
The particle size of APT-DTXp/DDP-LPHNs was 213.5 ± 5.3 nm, which was similar to DTXp/DDP-LPHNs and other LPHNs (Table 1). APT-DTXp/DDP-LPHNs had a zeta potential of 15.9 ± 1.9 mV, which is positive compared with negatively charged DTXp/DDP-LPHNs (−13.7 ± 1.6 mV). The DTX LE of APT-DTXp/DDP-LPHNs was 81.2 ± 3.1%, which is familiar with DTXp/DDP-LPHNs (80.9 ± 2.1%). TEM images compared the morphology and size of APT-DTXp/DDP-LPHNs and DTXp/DDP-LPHNs (Figure 2). Besides spherical in shape and uniform in size, APT-DTXp/DDP-LPHNs showed coats on the particles’ surface which do not exist in DTXp/DDP-LPHNs. The stability of LPHNs in the presence of serum was evaluated by testing the particle size, zeta potential, and LE variations. Figure 3 shows that no obvious change of size (A), zeta potential (B), DTX LE (C) and DDP LE (D) was found during the administration time (3 days).
 |
Table 1 Characterization of LPHNs (Mean ± SD, n=3) |
In vitro Drug Release and Cell Uptake of LPHNs
Sustained-release behaviors were observed for DTXp-, DTX- and/or DDP-loaded LPHNs (Figure 4). DDP release profiles of APT-DTXp/DDP-LPHNs, DTXp/DDP-LPHNs and APT-DDP-LPHNs were similar in hypoxic and normal condition (Figure 4B). However, DTX release manners of APT-DTXp/DDP-LPHNs, DTXp/DDP-LPHNs and APT-DTXp-LPHNs were different, faster and more in hypoxic compared with the normal condition (Figure 4A). In contrast, DTX release from APT-DTX-LPHNs was not affected by the condition of the release medium.
Cell uptake of aptamer contained LPHNs (APT-DTXp/DDP-LPHNs) was higher than that of non-modified DTXp/DDP-LPHNs on A549 cells, in contrast, there were no differences on BEAS2B cells. These results are presented in Figure 5A and calculated in Figure 5B.
Cell Viability and Synergistic Effect of LPHNs
Cell viability of LPHNs was evaluated at various drug concentrations using MTT assay. Blank APT-LPHNs was nontoxic to A549 cells, while drug-loaded LPHNs and free drugs exhibited dose-dependent cytotoxicity (Figure 6). Drug-loaded LPHNs showed significantly higher cytotoxicity than free drugs, especially in lower concentrations. Aptamer-decorated APT-DTXp/DDP-LPHNs exhibited better cell inhibition ability than that of APT-DTXp-LPHNs, APT-DTX-LPHNs, APT-DDP-LPHNs, and non-decorated DTXp/DDP-LPHNs (P<0.05). Prodrug encapsulated APT-DTXp-LPHNs was more cytotoxic than APT-DTX-LPHNs (P<0.05). Dual drug-loaded APT-DTXp/DDP-LPHNs exhibited better cell inhibition ability than that of APT-DTXp-LPHNs, APT-DTX-LPHNs, and APT-DDP-LPHNs, which may attribute to the synergistic effect of two drugs co-encapsulated in LPHNs. To further quantify the synergistic efficiency thus to determine the drug ratio used for the preparation of LPHNs, CI values were evaluated using Chou–Talalay method and summarized in Table 2. When compared to the IC50 values of APT-DTXp/DDP-LPHNs, APT-DTXp-LPHNs and APT-DDP-LPHNs in different DTXp to DDP weight ratios, we concluded that DTXp to DDP between 5:1 and 1:5 showed synergy effects, among which 1:1 gain the best synergistic effect (0.62, closer to zero).
 |
Table 2 CI Values Were Evaluated According to the IC50 Values (Mean ± SD, n=3) |
In vivo Biodistribution and Antitumor Effect
The highest accumulation was found in APT-DTXp/DDP-LPHNs (Figure 7) as well as the best tumor inhibition efficiency (Figure 8). Figure 7 shows that free drugs mainly accumulated in kidney, and heart compared with tumor tissue. On the contrary, LPHNs distribution in the tumor was significantly higher than free drugs (P < 0.05). APT-DTXp/DDP-LPHNs showed higher tumor distribution than non-decorated DTXp/DDP-LPHNs (P < 0.05), which was in accordance with the antitumor efficiency comparison. Single drug contained APT-DTXp-LPHNs and APT-DDP-LPHNs exhibited weaker tumor inhibition efficiency than APT-DTXp/DDP-LPHNs (P < 0.05) (Figure 8). Prodrug-based APT-DTXp-LPHNs gained smaller tumor size than that of APT-DTX-LPHNs at the end of the study (P < 0.05). Hematological parameters BUN, AST, and ALT were all within the normal range for LPHNs groups (Table 3).
 |
Table 3 BUN, AST, and ALT Levels (Mean ± SD, n=3) |
Discussion
The aim of this research is to develop an aptamer decorated, DTX prodrug based, dual drug-co-loaded LPHNs. On one hand, PLA-PEG-APT was synthesized and applied as ligands for the decoration of the nano-systems. On the other hand, redox-sensitive DTX-GM-TA was designed and co-loaded with DDP to produce dual drugs contained LPHNs.
Aptamer-decorated nanocarriers were wildly developed for the cancer cell targeting and in vivo application in tumor mice by many researchers.38–40 APT-DTXp/DDP-LPHNs constructed in this study had a size of 213.5 nm. This was in accordance with the data gained by Li et al, who prepared aptamer-functionalized nanoparticles with a size of 203.75 nm for targeted therapy in colorectal cancer.41 They drew a conclusion that these nanoparticles have several advantages including high drug loading capacity. In the characterization of LPHNs section, we also observed over 80% of drug encapsulation efficiency, which could be the evidence of the advantage of this system. The stability of LPHNs in the presence of serum was evaluated by testing the particle size, zeta potential, LE, and LC variations, just in line with the experiment carried out by Yang et al, to produce a hyaluronic acid and cisplatin prodrug-co-loaded nanoparticulate system.42 They argued that the size and LE remained unchanged during the serum stability test means the particles did not aggregate in the serum and also did not adsorb the serum proteins on the surfaces. We agreed with their point of view and the results showed that no obvious change of size (Figure 3A), zeta potential (Figure 3B), DTX LE (Figure 3C) and DDP LE (Figure 3D), which proved the stability of the system in the serum.
The drug was released from LPHNs in sustained behaviors, which was also reported in the research of aptamer-decorated nanoparticles constructed by Engelberg et al.43 Zeng et al also summarized that both hypoxia-activated prodrugs and redox-responsive drug delivery nanocarriers are effective for targeting the hypoxic microenvironment.19 Different from their study, we introduced two different release media: hypoxic and normal media. The redox-sensitive ligands in DTX prodrug were easier to decompose and release the drugs faster and more sufficient.44 In the release study section, significantly higher drug release was observed in hypoxic conditions, which indicated that the cumulative drug release was redox-sensitive attributed to the rupture of redox-responsive bonds.12 The aptamer-decorated platforms were reported to display selective binding and internalization to A549 cells,4 which was also proved in the cell uptake section: Cell uptake of aptamer contained APT-DTXp/DDP-LPHNs was higher than that of non-modified DTXp/DDP-LPHNs.
Cell viability assays showed that was no significant cytotoxicity of blank APT-LPHNs, which may be the proof of the low toxicity of the materials used in the preparation.45 Yan Nan also reported that the drug-carrying particles are absorbed by cells mainly through endocytosis, and play anti-tumor activity after drug molecule release. Therefore, different drug dose and proportion may have different effects, some of which have synergistic effects, while others have antagonistic effects.46 Combination index (CI) was calculated to validate the synergistic effect of drug-co-loaded LPHNs using the isobologram equation of Chou and Talalay.34 When the DTXp to DDP ratio was 1:1, the lowest IC50 value was observed among all the data, confirming the synergistic effect and indicating the amount of the drugs that should be used in the LPHNs preparation.
The in vivo enhancement effect of aptamer-conjugated nanotubes was assessed in the transplanted tumor model by Gu et al, and they found that higher accumulation was found in the tumor, heart, and kidney.47 The same phenomenon was observed in this study that APT-DTXp/DDP-LPHNs showed the highest accumulation in the tumor and lower in heart and kidney. APT-DTXp/DDP-LPHNs showed better antitumor efficiency than non-decorated DTXp/DDP-LPHNs, which may prove the targeted ability of the aptamer.48 APT-DTXp/DDP-LPHNs exhibited stronger tumor inhibition efficiency than single drug contained APT-DTXp-LPHNs and APT-DDP-LPHNs, which could illustrate the synergistic effect of the two drugs.49 Drug-loaded LPHNs exhibited better antitumor ability that free drugs which may be explained by the structure of lipid shell have a high affinity to the lipid cell surface, promote the fusion of the carriers to the cells and deliver the drugs into the tumor cells. Hematological parameters BUN, AST, and ALT were all within the normal range for LPHNs groups, which demonstrated that LPHNs have successfully mitigated the prominent toxicities of the drug treatment while maintaining significant synergistic therapeutic efficacy.15
Conclusion
Aptamer-conjugated lipid–polymer ligands and redox-sensitive docetaxel prodrug were synthesized. DTXp and DDP were loaded into the LPHNs. APT-DTXp/DDP-LPHNs exhibited a significantly enhanced cytotoxicity (drug concentration causing 50% inhibition was 0.71 ± 0.09 μg/mL), synergy antitumor effect (combination index was 0.62), profound tumor inhibition ability (tumor inhibition ratio of 81.4%) compared with the non-aptamer-decorated LPHNs and single drug-loaded LPHNs. APT-DTXp/DDP-LPHNs have great potential to inhibit lung tumor cells and in vivo tumor growth.
Disclosure
Ruifeng Wu and Zhiqiang Zhang are co-first authors. The authors report no conflicts of interest in this work.
References
1. Sacko K, Thangavel K, Shoyele SA. Codelivery of genistein and miRNA-29b to A549 cells using aptamer-hybrid nanoparticle bioconjugates. Nanomaterials. 2019;9(7):1052. doi:10.3390/nano9071052
2. Shen Y, Li M, Liu T, et al. A dual-functional HER2 aptamer-conjugated, pH-activated mesoporous silica nanocarrier-based drug delivery system provides in vitro synergistic cytotoxicity in HER2-positive breast cancer cells. Int J Nanomedicine. 2019;14:4029–4044. doi:10.2147/IJN.S201688
3. Perepelyuk M, Maher C, Lakshmikuttyamma A, Shoyele SA. Aptamer-hybrid nanoparticle bioconjugate efficiently delivers miRNA-29b to non-small-cell lung cancer cells and inhibits growth by downregulating essential oncoproteins. Int J Nanomedicine. 2016;11:3533–3544. doi:10.2147/IJN.S110488
4. Engelberg S, Modrejewski J, Walter JG, Livney YD, Assaraf YG. Cancer cell-selective, clathrin-mediated endocytosis of aptamer decorated nanoparticles. Oncotarget. 2018;9(30):20993–21006. doi:10.18632/oncotarget.24772
5. Jo H, Ban C. Aptamer-nanoparticle complexes as powerful diagnostic and therapeutic tools. Exp Mol Med. 2016;6(48):e230. doi:10.1038/emm.2016.44
6. Nguyen NV, Jen CP. Selective detection of human lung adenocarcinoma cells based on the aptamer-conjugated self-assembled monolayer of gold nanoparticles. Micromachines. 2019;10(3):195.
7. Poturnayová A, Dzubinová Ľ, Buríková M, Bízik J, Hianik T. Detection of breast cancer cells using acoustics aptasensor specific to HER2 receptors. Biosensors. 2019;9(2):72.
8. Duan T, Xu Z, Sun F, et al. HPA aptamer functionalized paclitaxel-loaded PLGA nanoparticles for enhanced anticancer therapy through targeted effects and microenvironment modulation. Biomed Pharmacother. 2019;117:109121. doi:10.1016/j.biopha.2019.109121
9. Zhang Y, Zhao J, Sun J, Huang L, Li Q. Targeting lung cancer initiating cells by all-trans retinoic acid-loaded lipid-PLGA nanoparticles with CD133 aptamers. Exp Ther Med. 2018;16(6):4639–4649. doi:10.3892/etm.2018.6762
10. Gao J, Feng SS, Guo Y. Antibody engineering promotes nanomedicine for cancer treatment. Nanomedicine (Lond). 2010;5(8):1141–1145. doi:10.2217/nnm.10.94
11. Mandal B, Mittal NK, Balabathula P, Thoma LA, Wood GC. Development and in vitro evaluation of core-shell type lipid-polymer hybrid nanoparticles for the delivery of erlotinib in non-small cell lung cancer. Eur J Pharm Sci. 2016;81:162–171. doi:10.1016/j.ejps.2015.10.021
12. Wang J, Su G, Yin X, et al. Non-small cell lung cancer-targeted, redox-sensitive lipid-polymer hybrid nanoparticles for the delivery of a second-generation irreversible epidermal growth factor inhibitor-Afatinib: in vitro and in vivo evaluation. Biomed Pharmacother. 2019;120:109493. doi:10.1016/j.biopha.2019.109493
13. Wang G, Wang Z, Li C, et al. RGD peptide-modified, paclitaxel prodrug-based, dual-drugs loaded, and redox-sensitive lipid-polymer nanoparticles for the enhanced lung cancer therapy. Biomed Pharmacother. 2018;106:275–284. doi:10.1016/j.biopha.2018.06.137
14. Lu Z, Su J, Li Z, Zhan Y, Ye D. Hyaluronic acid-coated, prodrug-based nanostructured lipid carriers for enhanced pancreatic cancer therapy. Drug Dev Ind Pharm. 2017;43(1):160–170. doi:10.1080/03639045.2016.1226337
15. Xiong Y, Zhao Y, Miao L, Lin CM, Huang L. Co-delivery of polymeric metformin and cisplatin by self-assembled core-membrane nanoparticles to treat non-small cell lung cancer. J Control Release. 2016;244(Pt A):63–73. doi:10.1016/j.jconrel.2016.11.005
16. Guo S, Zhang Y, Wu Z, et al. Synergistic combination therapy of lung cancer: cetuximab functionalized nanostructured lipid carriers for the co-delivery of paclitaxel and 5-Demethylnobiletin. Biomed Pharmacother. 2019;118:109225. doi:10.1016/j.biopha.2019.109225
17. Wu L, Leng D, Cun D, Foged C, Yang M. Advances in combination therapy of lung cancer: rationales, delivery technologies and dosage regimens. J Control Release. 2017;28(260):78–91. doi:10.1016/j.jconrel.2017.05.023
18. Li F, Huang Z, Chen H, et al. Redox-sensitive lipophilic prodrugs: delivering unstable chemotherapeutant for improved cancer therapy. Drug Deliv. 2019;26(1):1068–1079. doi:10.1080/10717544.2019.1678696
19. Zeng Y, Ma J, Zhan Y, et al. Hypoxia-activated prodrugs and redox-responsive nanocarriers. Int J Nanomedicine. 2018;18(13):6551–6574. doi:10.2147/IJN.S173431
20. Wang H, Zhao X, Guo C, et al. Aptamer-dendrimer bioconjugates for targeted delivery of miR-34a expressing plasmid and antitumor effects in non-small cell lung cancer cells. PLoS One. 2015;10(9):e0139136. doi:10.1371/journal.pone.0139136
21. Song Y, Cai H, Yin T, et al. Paclitaxel-loaded redox-sensitive nanoparticles based on hyaluronic acid-vitamin E succinate conjugates for improved lung cancer treatment. Int J Nanomedicine. 2018;15(13):1585–1600. doi:10.2147/IJN.S155383
22. Li M, Zhao L, Zhang T, et al. Redox-sensitive prodrug nanoassemblies based on linoleic acid-modified docetaxel to resist breast cancers. Acta Pharm Sin B. 2019;9(2):421–432. doi:10.1016/j.apsb.2018.08.008
23. Zhang L, Zhu D, Dong X, et al. Folate-modified lipid-polymer hybrid nanoparticles for targeted paclitaxel delivery. Int J Nanomedicine. 2015;16(10):2101–2114.
24. Zhang RX, Cai P, Zhang T, et al. Polymer-lipid hybrid nanoparticles synchronize pharmacokinetics of co-encapsulated doxorubicin-mitomycin C and enable their spatiotemporal co-delivery and local bioavailability in breast tumor. Nanomedicine. 2016;12(5):1279–1290. doi:10.1016/j.nano.2015.12.383
25. Zhang Y, Zhou Z, Chen M. The Length of hydrophobic chain in amphiphilic polypeptides regulates the efficiency of gene delivery. Polymers (Basel). 2018;10(4):379.
26. Zhang X, Liu J, Li X, et al. Trastuzumab-coated nanoparticles loaded with docetaxel for breast cancer therapy. Dose Response. 2019;17(3):1559325819872583. doi:10.1177/1559325819872583
27. Catanzaro D, Nicolosi S, Cocetta V, et al. Cisplatin liposome and 6-amino nicotinamide combination to overcome drug resistance in ovarian cancer cells. Oncotarget. 2018;9(24):16847–16860. doi:10.18632/oncotarget.24708
28. Wang Z, Wei Y, Fang G, et al. Colorectal cancer combination therapy using drug and gene co-delivered, targeted poly(ethylene glycol)-ε-poly(caprolactone) nanocarriers. Drug Des Devel Ther. 2018;24(12):3171–3180. doi:10.2147/DDDT.S175614
29. Zhang Y, Angelidaki I. Submersible microbial fuel cell sensor for monitoring microbial activity and BOD in groundwater: focusing on impact of anodic biofilm on sensor applicability. Biotechnol Bioeng. 2011;108(10):2339e47. doi:10.1002/bit.23204
30. Yu D, Li W, Zhang Y, Zhang B. Anti-tumor efficiency of paclitaxel and DNA when co-delivered by pH responsive ligand modified nanocarriers for breast cancer treatment. Biomed Pharmacother. 2016;83:1428–1435. doi:10.1016/j.biopha.2016.08.061
31. Wang C, Su L, Wu C, Wu J, Zhu C, Yuan G. RGD peptide targeted lipid-coated nanoparticles for combinatorial delivery of sorafenib and quercetin against hepatocellular carcinoma. Drug Dev Ind Pharm. 2016;42(12):1938–1944. doi:10.1080/03639045.2016.1185435
32. Zhang G, Liu F, Jia E, Jia L, Zhang Y. Folate-modified, cisplatin-loaded lipid carriers for cervical cancer chemotherapy. Drug Deliv. 2016;23(4):1393–1397. doi:10.3109/10717544.2015.1054052
33. Liu J, Cheng H, Han L, et al. Synergistic combination therapy of lung cancer using paclitaxel- and triptolide-coloaded lipid-polymer hybrid nanoparticles. Drug Des Devel Ther. 2018;25(12):3199–3209. doi:10.2147/DDDT.S172199
34. Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi:10.1016/0065-2571(84)90007-4
35. Park BJ, Whichard ZL, Corey SJ. Dasatinib synergizes with both cytotoxic and signal transduction inhibitors in heterogeneous breast cancer cell lines—lessons for design of combination targeted therapy. Cancer Lett. 2012;320(1):104–110. doi:10.1016/j.canlet.2012.01.039
36. Cai L, Xu G, Shi C, Guo D, Wang X, Luo J. Telodendrimer nanocarrier for co-delivery of paclitaxel and cisplatin: A synergistic combination nanotherapy for ovarian cancer treatment. Biomaterials. 2015;37:456–468. doi:10.1016/j.biomaterials.2014.10.044
37. Ni XL, Chen LX, Zhang H, et al. In vitro and in vivo antitumor effect of gefitinib nanoparticles on human lung cancer. Drug Deliv. 2017;24(1):1501–1512. doi:10.1080/10717544.2017.1384862
38. Zhang P, Zhao Z, Li C, et al. Aptamer-decorated self-assembled aggregation-induced emission organic dots for cancer cell targeting and imaging. Anal Chem. 2018;90(2):1063–1067. doi:10.1021/acs.analchem.7b03933
39. Vandghanooni S, Eskandani M, Barar J, Omidi Y. AS1411 aptamer-decorated cisplatin-loaded poly(lactic-co-glycolic acid) nanoparticles for targeted therapy of miR-21-inhibited ovarian cancer cells. Nanomedicine (Lond). 2018;13(21):2729–2758. doi:10.2217/nnm-2018-0205
40. Zhao Y, Xu J, Le VM, et al. EpCAM aptamer-functionalized cationic liposome-based nanoparticles loaded with miR-139-5p for targeted therapy in colorectal cancer. Mol Pharm. 2019;16(11):4696–4710. doi:10.1021/acs.molpharmaceut.9b00867
41. Li Y, Duo Y, Bao S, et al. EpCAM aptamer-functionalized polydopamine-coated mesoporous silica nanoparticles loaded with DM1 for targeted therapy in colorectal cancer. Int J Nanomedicine. 2017;12:6239–6257. doi:10.2147/IJN.S143293
42. Yang F, Li A, Liu H, Zhang H. Gastric cancer combination therapy: synthesis of a hyaluronic acid and cisplatin containing lipid prodrug coloaded with sorafenib in a nanoparticulate system to exhibit enhanced anticancer efficacy and reduced toxicity. Drug Des Devel Ther. 2018;4(12):3321–3333. doi:10.2147/DDDT.S176879
43. Engelberg S, Netzer E, Assaraf YG, Livney YD. Selective eradication of human non-small cell lung cancer cells using aptamer-decorated nanoparticles harboring a cytotoxic drug cargo. Cell Death Dis. 2019;10(10):702. doi:10.1038/s41419-019-1870-0
44. Zhao J, Yang Y, Han X, et al. Redox-sensitive nanoscale coordination polymers for drug delivery and cancer theranostics. ACS Appl Mater Interfaces. 2017;9(28):23555–23563. doi:10.1021/acsami.7b07535
45. Yan J, Wang Y, Zhang X, Liu S, Tian C, Wang H. Targeted nanomedicine for prostate cancer therapy: docetaxel and curcumin co-encapsulated lipid-polymer hybrid nanoparticles for the enhanced anti-tumor activity in vitro and in vivo. Drug Deliv. 2016;23(5):1757–1762. doi:10.3109/10717544.2015.1069423
46. Nan Y. Lung carcinoma therapy using epidermal growth factor receptor-targeted lipid polymeric nanoparticles co-loaded with cisplatin and doxorubicin. Oncol Rep. 2019;42(5):2087–2096. doi:10.3892/or.2019.7323
47. Gu F, Hu C, Xia Q, Gong C, Gao S, Chen Z. Aptamer-conjugated multi-walled carbon nanotubes as a new targeted ultrasound contrast agent for the diagnosis of prostate cancer. J Nanopart Res. 2018;20(11):303. doi:10.1007/s11051-018-4407-z
48. Morita Y, Leslie M, Kameyama H, Volk DE, Tanaka T. Aptamer Therapeutics in Cancer: current and Future. Cancers (Basel). 2018;10(3):80. doi:10.3390/cancers10030080
49. Colombo S, Cun D, Remaut K, et al. Mechanistic profiling of the siRNA delivery dynamics of lipid-polymer hybrid nanoparticles. J Control Release. 2015;201:22–31. doi:10.1016/j.jconrel.2014.12.026
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.