Back to Journals » Journal of Hepatocellular Carcinoma » Volume 7
Combination Antiangiogenic and Immunotherapy for Advanced Hepatocellular Carcinoma: Evidence to Date
Authors Raybould AL, Sanoff H
Received 24 April 2020
Accepted for publication 30 July 2020
Published 15 September 2020 Volume 2020:7 Pages 133—142
DOI https://doi.org/10.2147/JHC.S224938
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Alison L Raybould, Hanna Sanoff
Department of Medicine, Division of Hematology-Oncology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
Correspondence: Alison L Raybould Division of Hematology/Oncology, Physicians Office Building, 3rd Floor, 170 Manning Drive, CB #7305, Chapel Hill, NC 27599, USA
Tel +1-919-966-4431
Fax +1-919-966-6735
Email [email protected]
Abstract: For over a decade, sorafenib remained the only systemic agent with proven clinical efficacy for patients with advanced hepatocellular carcinoma (HCC). Recent years have seen a proliferation of agents. In the first line, lenvatinib was found to be non-inferior to sorafenib in terms of overall survival (OS), with significantly better progression-free survival and objective response rate. Meanwhile, encouraging efficacy signals were observed in phase I/II studies of immune checkpoint inhibitors as monotherapy in HCC. Although subsequent phase III trials failed to demonstrate statistically significant benefit in OS, other clinically meaningful outcomes were observed, including long-term disease control with a favorable toxicity profile. In addition, a synergistic response has been postulated based on the interplay between antiangiogenic molecular targeted agents and immunotherapy. On this basis, interest has turned toward combination strategies of immunotherapy with these standard-of-care medications in the hope of improving treatment efficacy for advanced HCC, while maintaining tolerable safety profiles. Indeed, preliminary results from phase I studies of lenvatinib plus pembrolizumab and atezolizumab plus bevacizumab have proved favorable, prompting phase III investigations in the frontline setting, and for atezolizumab plus bevacizumab, these positive findings have been substantiated by recent reporting of phase III data from IMbrave150. In this review, we will present the currently available data on combination therapy atezolizumab plus bevacizumab in advanced HCC, and compare these findings to other promising combination treatments, most notably that of lenvatinib plus pembrolizumab.
Keywords: advanced hepatocellular carcinoma, combination therapy, antiangiogenic treatment, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and a major leading cause of cancer death, being responsible for more than 700,000 deaths annually.1 In the USA, HCC represents the fastest rising cause of cancer-related death.2,3 The prognosis of HCC remains dismal, with a 5-year survival of 18.1% across all stages and 2.3% for advanced disease.4 For patients with advanced HCC, for whom options are limited to systemic therapy, the 1-year survival rate has been less than 50% following diagnosis.5 Without major driver mutations in HCC, treating with medications that impair hepatic functional reserve has proven quite difficult.
Prior to 2007, there were no effective treatment options for patients diagnosed at an advanced stage or those who progressed after locoregional therapy.6 The advent of sorafenib, a multikinase inhibitor that targets the vascular endothelial growth factor receptor (VEGFR), among others, provided the first breakthrough in treatment of advanced HCC following the results of two large phase III randomized control trials (SHARP trial7 and Asia-Pacific trial8). The SHARP trial demonstrated that sorafenib resulted in a nearly 3-month survival benefit compared to placebo in patients with advanced HCC, leading to FDA approval in the first-line setting (10.7 vs 7.9 months; HR 0.69; p<0.0001).7 This survival benefit was similarly borne out in a trial evaluating patients with advanced HCC from the Asia-Pacific region, which showed that the median improvement in overall survival (OS) compared to placebo was 2.3 months (6.5 vs 4.2 months; HR 0.68; p=0.014).8 Both trials restricted enrollment to patients with Child–Pugh class A cirrhosis and Eastern Cooperative Oncology Group (ECOG) performance status 2 or less. Post-marketing data, including the prospective non-interventional GIDEON study, have demonstrated an acceptable safety profile in patients with Child–Pugh class B7 cirrhosis.9
In the 10 years that followed, several global phase III trials failed to prove non-inferiority10 or superiority in terms of OS in the first-line setting.11–18 Without therapeutic alternatives or second-line agents available, sorafenib remained the only FDA-approved therapy for a decade. The 2-year period from 2017 to 2018 brought on a dramatic boon in available therapies, with approval of multitarget inhibitors regorafenib, lenvatinib, and cabozantinib; single-target ramucirumab; and immune checkpoint inhibitors nivolumab and pembrolizumab, which were granted FDA approval based on phase I/II data despite negative phase III studies.
Oral multikinase inhibitor regorafenib was the first systemic treatment shown to provide survival benefit in HCC patients who had progressed on sorafenib. In the phase III RESORCE trial, treatment with regorafenib led to improved OS for patients with disease progression on sorafenib compared to placebo (median OS 10.6 vs 7.8 months; HR 0.63 [95% CI 0.50–0.79]).19
Shortly thereafter, the phase III CELESTIAL trial showed improved survival with oral molecular kinase inhibitor cabozantinib versus placebo in advanced HCC patients previously treated with sorafenib (median OS 10.2 vs 8.0 months; HR 0.76 [95% CI 0.36–0.52]; p<0.001). The study included patients who had received up to two lines of systemic therapy and progressed after at least one. In the subgroup of patients who had received sorafenib as their only prior systemic treatment, median OS was 11.3 months with cabozantinib versus 7.2 months with placebo (HR 0.70 [95% CI 0.55–0.88]).20
The phase III REACH-2 trial evaluated the efficacy of ramucirumab, a human monoclonal antibody VEGFR-2 antagonist, versus placebo in the second-line setting for patients with advanced HCC and baseline elevated alpha-fetoprotein (AFP) levels ≥400 ng/mL. This population was selected based on positive results from the REACH trial,21 which showed OS benefit in this prespecified subgroup. In REACH-2, treatment with ramucirumab significantly improved OS compared to placebo (median OS 8.5 vs 7.3 months; HR 0.710 [95% CI 0.531–0.949]; p=0.0199), while also having a tolerable toxicity profile.22 While these advances provided more treatment options in the second-line setting, sorafenib remained the only FDA-approved first-line therapy until lenvatinib entered the scene.
Lenvatinib is an oral multikinase inhibitor that inhibits VEGFR-1–3, fibroblast growth factor receptors (FGFR)-1–4, platelet-derived growth factor receptor (PDGFR)-α, KIT, and RET, thereby suppressing the activity of factors important in angiogenesis and tumor growth. Based on phase II efficacy signal,23 the multicenter, randomized, open-label, phase III REFLECT trial investigated the efficacy and safety of lenvatinib versus standard-of-care sorafenib as first-line treatment in 954 patients with advanced HCC.24 The REFLECT trial met its primary endpoint, demonstrating non-inferiority of lenvatinib to sorafenib in OS (13.6 months on lenvatinib vs 12.3 months on sorafenib; HR 0.92 [95% CI 0.79–1.06]). In addition, lenvatinib showed a statistically significant improvement versus sorafenib for all secondary efficacy endpoints, including progression-free survival (PFS) (7.4 vs 3.7 months; HR 0.66 [95% CI 0.57–0.77]), time to progression (TTP) (8.9 vs 3.7 months; HR 0.63 [95% CI 0.53–0.73]), and objective response rate (ORR) by mRECIST (24.1% vs 9.2%; OR 3.1 [95% CI 2.2–4.6]). The results of the REFLECT trial led to FDA approval of lenvatinib as first-line therapy for patients with advanced HCC in August 2018.25
Until very recently, sorafenib and lenvatinib represented the two available first-line options for advanced HCC, but these have now been joined by combination atezolizumab plus bevacizumab, which was granted FDA approval on May 29, 2020, based on positive phase III data from IMbrave150.26 These phase III data substantiate promising early phase data of durable objective responses in a subset of patients treated with anti-programmed cell death-1 (PD-1) therapy.27,28
Immune Checkpoint Inhibition
Immuno-oncology has drastically changed the treatment landscape of a number of malignancies, and its potential has been investigated in the treatment of advanced HCC. Two immune checkpoint inhibitors, nivolumab and pembrolizumab, received conditional accelerated approval from the FDA for use in the second-line setting of advanced HCC based on phase I/II data demonstrating durable objective responses observed in nearly 20% of patients.29,30 Although subsequent phase III data failed to demonstrate statistically significant improvement in OS, clinically meaningful outcomes were observed, and it remains to be seen whether the FDA will withdraw its conditional approval.
In CheckMate-040, a phase I/II, open-label, non-comparative, dose-escalation and expansion trial, 262 patients with advanced HCC who were sorafenib naïve, sorafenib intolerant, or sorafenib refractory were treated with anti-PD-1 monoclonal antibody nivolumab at doses of 0.1–10 mg/kg once every 2 weeks (dose-escalating cohort) or at a dose of 3 mg/kg once every 2 weeks (expansion cohort).27 This trial yielded a manageable safety profile with promising efficacy, including an ORR of 20% (95% CI 15–26) in the dose-expansion phase, with three complete responses (CRs) and 39 partial responses (PRs); 91% of responders had responses lasting for 6 months or longer.
Based on these encouraging results, the follow-up randomized, multicenter, phase III study CheckMate-459 investigated nivolumab against sorafenib in the frontline setting for advanced HCC patients. Nivolumab failed to meet its primary endpoint of improved OS (median OS 16.4 months for nivolumab vs 14.7 months for sorafenib; HR 0.85 [95% CI 0.72–1.02]; p=0.0752).31 Although the predetermined statistically significant OS was not met, clinically meaningful improvements in ORR were observed, with 14 CRs (4%) and 43 PRs (12%) in the nivolumab arm compared to five CRs (1%) and 21 PRs (6%) in the sorafenib arm. A detailed analysis of the study results is not yet available, which may further help guide the role of nivolumab monotherapy in advanced HCC therapy.
A later stage of CheckMate-040 evaluated the role of nivolumab in combination with ipilimumab at various dose levels in 148 patients with advanced HCC who had previously been treated with sorafenib.32 A total of 49 patients received nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses, followed by nivolumab 240 mg every 2 weeks. After 28 months’ follow-up, the ORR was 33% (95% CI 20–48) in this arm, with four CRs and 12 PRs. The median duration of response (DOR) was 17.5 months (range 4.6–30.5+ months), with 56% lasting for at least 12 months and 31% for at least 24 months. This combination was well tolerated, with rash and pruritis as the most common any-grade treatment-related adverse events (TRAEs). Grade 3–4 TRAEs occurred in 38% of patients, with aspartate aminotransferase (AST) elevation being the most common (33%); among the grade 3–4 TRAEs, 5% led to discontinuation of treatment. Based on this study, the FDA granted accelerated approval of combination therapy of nivolumab plus ipilimumab in March 2020.33
The utility of the anti-PD-1 monoclonal antibody pembrolizumab has also been explored for the treatment of patients with advanced HCC. KEYNOTE-224, a non-randomized, single-arm, multi-center, open-label, phase II study, evaluated the efficacy and safety of pembrolizumab in 104 patients with advanced HCC who had progressed on or been intolerant to sorafenib, with preserved ECOG and Child–Pugh class A liver disease.28 Exclusion criteria included those with autoimmune disease or an immunosuppressed state. Eighteen of 104 patients (17% [95% CI 11–26]) achieved the primary endpoint of an objective response, with one CR (1%) and 17 PRs (16%). Response was independent of causation of HCC and appeared to be durable, with duration ranging from 3.1 to 16.7 months; 89% of responders had a response duration of 6 months or greater and 56% of responders had a response duration of 12 months or greater. TRAEs were observed in 76 patients (73%), with grade 3 toxicities in 25 patients (24%) and immune-mediated events in 15 patients (14%), including three cases (3%) of immune-mediated hepatitis. There were no cases of viral hepatitis flares. These results led to accelerated FDA approval for pembrolizumab in the second-line setting, with continued approval potentially contingent upon further confirmatory trials of clinic efficacy. This has been subsequently explored in KEYNOTE-240, a phase III, double-blind, placebo-controlled trial.
In KEYNOTE-240, 413 patients with advanced HCC who had been previously treated with sorafenib were randomized in a 2:1 ratio to receive 200 mg of pembrolizumab every 3 weeks plus best supportive care (BSC) or placebo with BSC.34 Those who had received prior immunotherapy or any systemic therapy for HCC other than sorafenib were excluded. The primary endpoints were OS and PFS. Median OS was 13.9 months (95% CI 11.6–16 months) in the pembrolizumab group and 10.6 months (95% CI 8.3–13.5 months) in the placebo group. Median PFS was 3 months (95% CI 2.8–4.1 months) versus 2.8 months (95% CI 2.5–4.1 months), respectively.
Although the pembrolizumab arm had longer OS (HR 0.78 [95% CI 0.61–0.99]) and longer PFS (HR 0.78 [95% CI 0.61–0.99]), it did not reach statistical significance per the planned analysis. Failure to meet primary co-endpoints was postulated to be due to a better OS in the placebo group than predicted, likely related to unanticipated availability of other effective post-study second-line therapies, including regorafenib and nivolumab. Nevertheless, similarly to KEYNOTE-224, there were observed improvements in ORR in those treated with pembrolizumab compared to placebo (18.3% [95% CI 14–23.4] vs 4.4% [95% CI 1.6–9.4]), and a subset of patients derived long-term benefit, with more than 19% of pembrolizumab-treated patients remaining without progression for more than 1 year.
The toxicity profile remained similar to KEYNOTE-224, with grade 3 adverse events occurring in 147 patients (52.7%) in the pembrolizumab-treated arm versus 62 patients (46.3%) in the placebo arm. Immune-mediated adverse events occurred in 51 patients (18.3%) in the pembrolizumab group and 11 patients (9.2%) in the placebo group. Among these, 10 patients (3.6%) experienced immune-mediated hepatitis in the pembrolizumab group versus no events of immune-mediated hepatitis observed in the placebo group. Similarly, there were no identified cases of viral hepatitis flares. Despite not meeting either primary endpoint, pembrolizumab remains FDA approved in the second-line setting for those who have failed sorafenib therapy.35 Its efficacy continues to be investigated in KEYNOTE-394, a phase III trial in Asia, as second-line therapy for patients with advanced HCC (NCT03062358). The results of that study were not available at time of publication but are expected to be reported in the coming months.36 Even though neither nivolumab nor pembrolizumab monotherapy demonstrated statistically significant improvement in OS compared with either sorafenib in the first-line or placebo in the second-line setting, it is clear that a subset of patients with advanced HCC experience considerable clinical benefit from PD-1 inhibition, prompting further investigation of immunotherapy in the treatment arsenal for advanced HCC via combination therapy.
Atezolizumab and Bevacizumab Combination Therapy
Multikinase inhibitors with antiangiogenic properties have been the mainstay of standard first-line therapy for advanced HCC. Lenvantinib, along with the other three multikinase inhibitors for HCC (sorafenib, regorafenib, and cabozantinib), also has immunomodulatory effects on the tumor microenvironment. These immunomodulating effects include the promotion of dendritic cell maturation, upregulation of T-cell trafficking and function, and reversal of immunosuppression cell expression caused by tissue hypoxia.37–39 In preclinical HCC models, single-cell RNA sequencing demonstrated that treatment of lenvatinib with or without anti-PD-1 antibody led to decreased monocyte and macrophage populations and increased CD8+ T-cell populations. Further, immunocompetent mice treated with lenvatinib plus anti-PD-1 antibody had greater tumor regression and a higher response rate compared with either treatment alone.40 These observations suggest a potentially synergistic effect of combining immunotherapy with standard-of-care therapies known to exhibit immunomodulatory activity. In advanced HCC, the most extensively studied combination regimen consists of anti-PD-1 or anti-PD-L1 plus antiangiogenic agents.41 One such regimen involving an anti-PD-L1 monoclonal antibody, atezolizumab, plus a VEGF inhibitor, bevacizumab, has emerged as a practice-changing treatment option, with recent FDA approval in the frontline setting based on positive findings from the phase III study IMbrave150.42
This combination first showed promise in a phase Ib study of patients with advanced HCC and preserved liver function (Child–Pugh class A) treated with atezolizumab 1200 mg IV every 3 weeks and bevacizumab 15 mg/kg IV every 3 weeks. This study demonstrated durable responses, with 12 of 23 responses (52%) lasting for 6 months or more and 6 of 23 responses (26%) lasting for 12 months or more.43 Responses were observed across all clinically relevant subgroups, irrespective of etiology, geographic region, baseline AFP, or tumor burden. The ORR was 32% per investigator-assessed mRECIST. Median PFS was 14.9 months (range 0.5–23.9+). Among 103 safety-evaluable patients, TRAEs occurred in 82%, with decreased appetite (28%), fatigue, rash, and pyrexia (20% each) being the most common. Five deaths were reported, two of which were considered treatment related (one grade 4 drug-induced liver injury with hepatic decompensation and one pneumonitis). Overall, adverse events were felt to be consistent with the known safety profile of each drug, and no new safety signals were identified.
These favorable results have been further substantiated by the multicenter, open-label, randomized, phase III study IMbrave150, leading to recent FDA approval of atezolizumab plus bevacizumab as first-line therapy. This trial randomized 501 treatment-nave patients with advanced HCC and preserved liver function (Child–Pugh class A) in a 2:1 ratio to receive experimental atezolizumab 1200 mg IV infusion every 3 weeks plus bevacizumab 15 mg/kg every 3 weeks (n=336) versus standard-of-care sorafenib 400 mg orally twice a day (n=165).4,44 Co-primary endpoints were PFS and OS, as assessed by an independent review facility (IRF) per RECIST 1.1 criteria. This study showed a significantly longer median PFS in the combination arm compared to the sorafenib arm (6.8 vs 4.3 months; HR 0.59 [95% CI 0.47−0.76]; p<0.0001). Similarly, the median OS was not reached in the combination arm compared to 13.2 months in the sorafenib arm (HR 0.58 [95% CI 0.42−0.79]; p=0.0006). This makes it the first therapy in over a decade to demonstrate improved survival for patients with advanced HCC who have not yet received treatment. The ORR was more than double in the combination group versus the sorafenib group per RECIST 1.1 criteria (27% vs 12%; p<0.0001) and nearly threefold increased per IRF-assessed mRECIST criteria (33% vs 13%; p<0.0001). The median duration of treatment was 7.4 months with the combination and 3 months for sorafenib. Grade 3−4 adverse events occurred at similar rates across the two groups (57% combined vs 55% sorafenib), as did grade 5 adverse events (5% combined vs 6% sorafenib). The investigators noted that atezolizumab plus bevacizumab also delayed deterioration of quality of life compared to sorafenib. The combination of atezolizumab plus bevacizumab appeared to have a tolerable safety profile and yielded both statistically significant and clinically meaningful improvement in OS and PFS for untreated patients with unresectable HCC. In May 2020, the FDA approved atezolizumab plus bevacizumab as first-line therapy for patients with advanced HCC.42 It is currently the only combination regimen with positive phase III data and is expected become the new standard of care in the first-line setting. Table 1 demonstrates the efficacy and safety results of atezolizumab plus bevacizumab from IMbrave150 and provides a comparison to data from other available combination regimens.
 |
Table 1 Results of Phase I Trials of Antiangiogenics Plus Immune Checkpoint Inhibitors for HCC |
Lenvatinib and Pembrolizumab Combination Therapy
Lenvatinib plus pembrolizumab is another combination therapy for advanced HCC with promising early phase data. KEYNOTE-524, an open-label, multicenter, phase Ib trial, evaluated the tolerability and safety of lenvatinib (12 mg/day if weight >60 kg or 8 mg/day if weight ≤60 kg, orally) plus pembrolizumab (200 mg IV every 3 weeks) in 104 patients with BCLC stage B or C HCC, Child–Pugh class A, ECOG 0 or 1, and no prior systemic therapies.45 No dose-limiting toxicities (DLTs) were observed, and enrollment was expanded to 104 patients (DLT, n=6; escalation, n=98). At median follow-up of 10.6 months, 37 patients remained on treatment (lenvatinib only, n=3; combination, n=34). ORR was 36% (95% CI 26.6–46.2), including one CR (1%) and 35 PRs (35%) by RECIST v1.1. These results compare favorably to the lenvatinib arm of the REFLECT trial, in which the ORR was 24.1% with CR in six patients (1%) and PR in 109 patients (23%).19 Median PFS was 8.6 months (95% CI 7.1–9.7), and median DOR was 12.6 months (95% CI 6.9–not estimable).
Updated safety data will be forthcoming, but available safety analysis reported that any-grade treatment-emergent adverse events (TEAEs) occurred in 99 patients (99%), with 85% representing grade ≥3 TEAEs. Among these, hypertension was the most common, seen in 18% of patients. Three treatment-related deaths were reported (one acute respiratory distress syndrome, one intestinal perforation, one abnormal hepatic function). Based on these results of tolerable toxicities and promising efficacy, the FDA granted a breakthrough therapy designation for the combination of lenvatinib and pembrolizumab in the treatment of patients with newly diagnosed, advanced HCC in July 2019.46
A multicenter, double-blinded, phase III trial, LEAP-002 (NCT03713593), is currently underway to examine this combination in the frontline setting for patients with advanced HCC and well-preserved liver function (Child–Pugh class A).47 Approximately 750 patients will be randomized to receive either standard-of-care lenvatinib (12 mg if weight ≥60 kg or 8 mg if weight <60 kg) orally daily plus pembrolizumab 200 mg IV every 3 weeks in the experimental arm or lenvatinib plus placebo in the control arm. The co-primary endpoints include OS and PFS, and secondary endpoints include ORR, DOR, disease control rate (DCR), TTP per RECIST 1.1, adverse events, and pharmacokinetics.
In addition, a single-arm phase IIb trial is investigating the safety and efficacy of lenvatinib and pembrolizumab in the second-line setting for patients with advanced hepatobiliary malignancies, including HCC, while also exploring potential biomarkers of therapeutic response (NCT03895970).48 Eligible patients must have preserved liver function (Child–Pugh class A or mild class B), BCLC stage B–C, and ECOG 0–2. Primary outcomes include ORR, DCR, and PFS, while secondary outcomes include OS, DOR, stable disease, and TEAEs.
Studies are beginning to expand combination treatment regimens to include locoregional liver-directed therapies for incurable, non-metastatic disease. A phase III, multicenter, randomized, double-blinded, controlled trial of lenvatinib and pembrolizumab in combination with transarterial chemoembolization (TACE) in patients with incurable, non-metastatic HCC was recently announced.49 The experimental arm will receive lenvatinib (12 mg orally daily if weight ≥60 kg or 8 mg if weight <60 kg) and pembrolizumab 400 mg IV every 6 weeks with TACE. The control arm will receive oral and IV placebo with TACE.
Alternative Combination Therapy
It is worth mentioning recently updated results from CheckMate-040, which, based on favorable data from earlier cohorts, trialed combination cabozantinib and nivolumab with or without ipilimumab for advanced HCC.50 Stratification was based on prior sorafenib exposure, and 171 patients were randomized to receive nivolumab 240 mg every 2 weeks and cabozantinib 40 mg orally daily (n=36) or nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks and cabozantinib 40 mg orally daily (n=35). After median follow-up of 19 months, ORR was 17% in the nivolumab and cabozantinib group (six PRs) and 26% (nine PRs) in the nivolumab, ipilimumab, and cabozantinib group. This is in contrast to prior studies of combination ipilimumab and nivolumab in which ORR was greater than 30%.32 Median PFS was 5.5 months in the doublet arm and 6.8 months in the triplet arm. Triplet therapy had a high rate of grade 3–4 TRAEs, occurring at a rate of 71% compared to 47% in the doublet arm. These most commonly consisted of AST increase (23%), lipase increase (17%), ALT increase (17%), hypertension (17%), and palmar–plantar erythrodysesthesia (9%). Longer duration of follow-up may be useful in revealing the true risk–benefit ratio of either combination group.
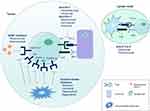
There remains great enthusiasm to explore other combination therapies, particularly in the frontline setting. Figure 1 highlights the main targets of systemic treatment for advanced HCC, which have been combined with the goal of potentiating a synergistic response. The open-label, phase Ib COSMIC-021 study (NCT03170960) seeks to assess the safety and preliminary efficacy of cabozantinib plus atezolizumab across several gastrointestinal tumor types, including untreated advanced HCC patients with well-preserved liver function (Child–Pugh class A).51 In the multicenter, open-label, phase III trial COSMIC-312 (NCT03755791), patients with untreated advanced HCC have been randomized to an experimental arm of cabozantinib (40 mg orally daily) plus atezolizumab (1200 mg IV infusion every 3 weeks) versus a control arm of sorafenib (400 mg orally twice daily).52 Co-primary endpoints include OS and PFS, while secondary endpoints include ORR, safety, pharmacokinetics, and correlation of biomarker analyses with clinical outcomes. Other ongoing phase I/II combination therapy trials include regorafenib plus pembrolizumab as first-line treatment for advanced HCC (NCT03347292),53 regorafenib plus anti-PD-L1 antibody avelumab in patients with advanced solid tumors (NCT03475953),54 and cabozantinib plus anti-PD-L1 antibody durvalumab in patients with gastrointestinal malignancies, including both treated and untreated advanced HCC (NCT03539822).55 In the phase Ib study of ramucirumab plus durvalumab in previously treated patients with advanced malignancies, an ORR was observed in three of the 28 patients (11%) in the HCC cohort, with a median OS of 10.7 months.56 The compares to a median OS of 8.5 months in REACH-2.21. Table 2 depicts the FDA approval status of current immunotherapy regimens for advanced HCC.
 |
Table 2 FDA Approval Status of Immunotherapy for Advanced HCC* |
Conclusions
Advanced HCC remains a deadly disease; however, with the emergence of multiple new treatment options, median survival is now reported at longer than a year. Further, results from combination antiangiogenic therapy and checkpoint inhibitor trials offer patients a realistic hope for some additional years of life. Combination therapy with antiangiogenic treatments and immunotherapy provides new promise for a disease with an otherwise grim prognosis. Preclinical studies suggest that the immunomodulatory effects of antiangiogenic therapy may be potentiated by the addition of immunotherapy, and this has been well illustrated in much of the available clinical trial data. As the first treatment to demonstrate superiority in the frontline setting for advanced HCC in over a decade, the combination of atezolizumab and bevacizumab is expected to change clinical practice, and with its recent FDA approval, it now represents an overwhelming improvement on the other currently FDA-approved first-line treatment options, sorafenib and lenvatinib. Similarly, phase I data from combination lenvatinib and pembrolizumab as first-line therapy appear extremely promising. While we anticipate combination therapy to alter the treatment landscape for patients with advanced HCC, it also raises important questions regarding treatment selection as well as optimal sequence strategy. Our current second-line agents have proven benefit only in sorafenib-experienced patients, and we cannot infer conclusions about their efficacy following treatment with various combination strategies. Future areas of investigation should work on isolating predictive biomarkers that can identify the subset of patients who may benefit from immunotherapy most, and on understanding the appropriate sequence of combination treatment with current standard-of-care first- and second-line agents.
Disclosure
Dr Hanna Sanoff reports grants from Bayer, outside the submitted work. The authors report no other conflict of interest in this work.
References
1. American Cancer Society. Key statistics about liver cancer. [Internet]. Available from: https://www.cancer.org/cancer/liver-cancer/about/what-is-key-statistics.html.
2. Rawla P, Sunkara T, Muralidharan P, Raj JP. Update in global trends and aetiology of hepatocellular carcinoma. Contem Oncol. 2018;22(3):141–150.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
4. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 19752014, featuring survival. J Natl Cancer Inst. 2017;109.
5. Giannini G, et al. Prognosis of untreated hepatocellular carcinoma. Hepatol. 2015;61(1):184–190. doi:10.1002/hep.27443
6. Bruix J. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona 2000 EASL conference. J Hepatol. 2001;35:421–430. doi:10.1016/S0168-8278(01)00130-1
7. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Eng Journal Med. 2008;359:378–390. doi:10.1056/NEJMoa0708857
8. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
9. Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol. 2016;65:1140–1147. doi:10.1016/j.jhep.2016.07.020
10. Cheng AL, Kang Y, Lin DY, et al. Sunitinib versus sorafenib in advanced HCC cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–4075. doi:10.1200/JCO.2012.45.8372
11. Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK_FL study. J Clin Oncol. 2013;31:3517–3524. doi:10.1200/JCO.2012.48.4410
12. Cainap C, Qin S, Huang WT, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172–179. doi:10.1200/JCO.2013.54.3298
13. Zhu AX, Rosmorduc O, Evans TR, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:559–566. doi:10.1200/JCO.2013.53.7746
14. Vilgrain V, Pereira H, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARA): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. doi:10.1016/S1470-2045(17)30683-6
15. Kudo M, Ueshima K, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroentero. 2018;3(6):424–432. doi:10.1016/S2468-1253(18)30078-5
16. Abou-Alfa GK, Galle PR, et al. PHOCUS: A phsae 3 randomized, open-label study comparing the oncolytic immunotherapy Pexa-Vec followed by sorafenib (SOR) vs SOR in patients with advanced hepatocellular carcinoma (HCC) without prior systemic therapy. Journ Clin Oncol. 2016;34.15_suppl. doi:10.1200/JCO.2016.34.15_suppl.TPS4146.
17. Chow P, Gandhi M, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. Journ Clin Oncol. 2018;36(19):1913–1921. doi:10.1200/JCO.2017.76.0892
18. Ghassan K, Abou A, et al. Assessment of treatment with sorafenib plus doxorubicin vs sorafenib alone in patients with advanced hepatocellular carcinoma: phase 3 CALGB 80802 randomized clinical trial. JAMA Oncol. 2019;5(11):1582–1588. doi:10.1001/jamaoncol.2019.2792
19. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled Phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
20. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Eng J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
21. Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, Phase 3 trial. Lancet Oncol. 2015;16(7):859–870. doi:10.1016/S1470-2045(15)00050-9
22. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi:10.1016/S1470-2045(18)30937-9
23. Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52:512–519. doi:10.1007/s00535-016-1263-4
24. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
25. US FDA. FDA approves lenvatinib for unresectable hepatocellular carcinoma [Media release]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-unresectable-hepatocellular-carcinoma.
26. US FDA. FDA approves atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma [Media release]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-plus-bevacizumab-unresectable-hepatocellular-carcinoma#:~:text=On%20May%2029%2C%202020%2C%20the,not%20received%20prior%20systemic%20therapy.
27. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
28. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with Sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi:10.1016/S1470-2045(18)30351-6
29. US FDA. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib [Media release]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib.
30. US FDA. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma [Media release]. Available from: https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma.
31. Yau T, Park JW, Finn RS, et al. LBA38_PR - CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Annals of Oncol. 2019;30(5):v874v875. doi:10.1093/annonc/mdz394.029
32. He AR, Yau T, Hsu C, et al. Nivolumab + ipilimumab combination therapy in patients with advanced hepatocellular carcinoma: subgroup analysis from CheckMate 040. J Clin Oncol. 2020;38(4_suppl):512. doi:10.1200/JCO.2020.38.4_suppl.512
33. US FDA. FDA grants accelerated approval to nivolumab and ipilimumab combination for hepatocellular carcinoma [Media Release]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-and-ipilimumab-combination-hepatocellular-carcinoma.
34. Finn S, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):L198202. doi:10.1200/JCO.19.01307
35. Merck (2020). Keytruda: keytruda (pembrolizumab) injection, for intravenous use. Initial U.S. Approval: 2014. [Media Release]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s066lbl.pdf.
36. ClinicalTrials.gov [Internet]. current. Identifier NCT03062358. A Phase III randomized double-blind study of pembrolizumab plus best supportive care vs. placebo plus best supportive care as second-line therapy in asian subjects with previously systemically treated advanced hepatocellular carcinoma (KEYNOTE-394). Bethesda (MD): National Library of Medicine (US); 2017 April 27. Available from: https://clinicaltrials.gov/ct2/show/study/NCT03062358.
37. Lin YY, Tan CT, Chen CW, et al. Immunomodulatory effects of current targeted therapies on hepatocellular carcinoma: implications for the future of immunotherapy. Semin Liver Dis. 2018;38(4):379–388. doi:10.1055/s-0038-1673621
38. Kwilas AR, Donahue RN, Tsang KY, Hodge JW. Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron. 2015;2:1.
39. Terme M, Colussi O, Marcheteau E, et al. Modulation of immunity by antiangiogenic molecules in cancer. Clin Dev Immunol. 2012;2012:492920. doi:10.1155/2012/492920
40. Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993–4002. doi:10.1111/cas.13806
41. Kudo M. Combination cancer immunotherapy with molecular targeted agents/anti-CTLA-4 antibody for hepatocellular carcinoma. Liver Cancer. 2019;8:1–11. doi:10.1159/000496277
42. US FDA. FDA approves atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma [Media Release]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-plus-bevacizumab-unresectable-hepatocellular-carcinoma#:~:text=On%20May%2029%2C%202020%2C%20the,not%20received%20prior%20systemic%20therapy.
43. Pishvaian MJ, Lee MS, Ryoo B, et al. Updated safety and clinical activity results from a phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC). Ann Oncol. 2018;29.
44. Finn RS, Qin S, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. NEJM. 2020;382:1894–1905. doi:10.1056/NEJMoa1915745
45. Zhu A, Finn RS, et al. A phase Ib study of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC). J Clin Oncol. 2020;38(15_suppl):4519. doi:10.1200/JCO.2020.38.15_suppl.4519
46. Merck. Merck and eisai receive third breakthrough therapy designation from FDA for KEYTRUDA (pembrolizumab) plus LENVIMA (lenvatinib) Combination Treatment [Press release]. eisai public relations department 2019 [July 23]. Available from: http://eisai.mediaroom.com/Merck-and-Eisai-Receive-Third-Breakthrough-Therapy-Designation-from-FDA-for-KEYTRUDA-R-pembrolizumab-plus-LENVIMA-R-lenvatinib-Combination-Treatment.
47. Llovet JM, Kudo M, Cheng AL, et al. Lenvatinib (len) plus pembrolizumab (pembro) for the first-line treatment of patients (pts) with advanced hepatocellular carcinoma (HCC): phase 3 LEAP-002 study. J Clin Oncol. 2019;37.
48. ClinicalTrials.gov [Internet]. current. Identifier NCT03895970. Lenvatinib combined pembrolizumab in advanced hepatobiliary tumors. Bethesda (MD): National Library of Medicine (US); 2019 March 29. Available from: https://clinicaltrials.gov/ct2/show/NCT03895970.
49. ClinicalTrials.gov [Internet]. Current. safety and efficacy of Lenvatinib (E7080/MK-7902) with pembrolizumab (MK-3475) in combination with Transarterial Chemoembolization (TACE) in participants with incurable/non-metastatic hepatocellular carcinoma (MK-7902-012/E7080-G000-318/LEAP-012). Bethesda (MD): National Library of Medicine (US); 2020 January. Available from: https://clinicaltrials.gov/ct2/show/NCT04246177.
50. Yau T, Zagonel V, Santoro A, et al. Nivolumab + ipilimumab + cabozantinib combination therapy in patients with advanced hepatocellular carcinoma: results from CheckMate 040. J Clin Oncol. 2020;38(4_suppl):478. doi:10.1200/JCO.2020.38.4_suppl.478
51. Spencer KR, Ramsingh G, Mohamed N, et al. Phase Ib trial of cabozantinib (C) in combination with atezolizumab (A) in patients (pts) with advanced hepatocellular carcinoma (HCC), gastric or gastroesophageal junction cancer (GC/GEJC), or colorectal cancer (CRC). J Clin Oncol. 2019;37(4_suppl.TPS478). doi:10.1200/JCO.2019.37.4_suppl.TPS478.
52. Kelley RK, Cheng AL, Braiteh FS, et al. Phase 3 (COSMIC-312) study of cabozantinib (C) in combination with Atezolizumab (A) versus sorafenib (S) in patients (pts) with advanced hepatocellular carcinoma (aHCC) who have not received previous systemic anticancer therapy. J Clin Oncol. 2019;37:
53. ClinicalTrials.gov [Internet]. Current. regorafenib plus pembrolizumab in first line systemic treatment of HCC. Bethesda, MD: National Library of Medicine (US); 2017 Nov Available from: https://clinicaltrials.gov/ct2/show/NCT03347292.
54. ClinicalTrials.gov [Internet]. Current. A Phase I/II study of regorafenib plus avelumab in solid tumors (REGOMUNE). Bethesda, MD: National Library of Medicine (US); 2018 March. Available from: https://clinicaltrials.gov/ct2/show/NCT03475953.
55. ClinicalTrials.gov [Internet]. Current. Cabozantinib in combination with durvalumab in patients with gastroesophageal cancer and other gastrointestinal malignancies (CAMILLA). Bethesda, MD: National Library of Medicine (US); 2018 May. Available from: https://clinicaltrials.gov/ct2/show/NCT03539822.
56. Yung-Jue B, Talia G, Lin C, et al. Ramucirumab (ram) and durvalumab (Durva) treatment of metastatic non-small cell lung cancer (NSCLC), gastric/gastroesophageal junction (G/GEJ) adenocarcinoma, and hepatocellular carcinoma (HCC) following progression on systemic treatment(s). J Clin Oncol. 2019;37(15_suppl.2528).
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.