Back to Journals » Cancer Management and Research » Volume 13
Columbamine-Mediated PTEN/AKT Signal Pathway Regulates the Progression of Glioma
Authors Niu HT, Liu Y, Wang YZ, Tian Y, Yang M, Jiang HS
Received 14 October 2020
Accepted for publication 3 December 2020
Published 19 January 2021 Volume 2021:13 Pages 489—497
DOI https://doi.org/10.2147/CMAR.S286866
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
This paper has been retracted.
Hai-Tao Niu, Yang Liu, Yan-Zhou Wang, Yong Tian, Ming Yang, Hong-Sheng Jiang
Department of Neurosurgery, Cangzhou Central Hospital, Cangzhou, Hebei 061000, People’s Republic of China
Correspondence: Hai-Tao Niu
Department of Neurosurgery, Cangzhou Central Hospital, No. 16, Xinhua West Road, Cangzhou, Hebei 061000, People’s Republic of China
Email [email protected]
Purpose: At present, comprehensive therapy has been widely used in the treatment of glioma, but the curative effect is not good, and the survival rate of patients is low. Therefore, it is crucial to explore further the regulatory mechanism of the occurrence and development of glioma and find potential therapeutic targets. We aimed to investigate the columbamine (a tetrahydroisoquinoline alkaloid derived from the rhizome of Chinese herbal medicine Rhizoma Coptidis) on glioma progression.
Methods: MTT, clone formation assay, wound healing assay, and transwell assay were performed to detect the cell viability, proliferation, migration, and invasion ability. Flow cytometry, TUNEL, and Western blot were used to identify the apoptosis level in glioma cells. PTEN inhibitor (SF1670) and AKT activator (SC79) were used to explore the mechanism of columbamine on glioma cell progression.
Results: Columbamine inhibits proliferation, migration, invasion, and induces apoptosis in glioma cell lines (SHG44 and U251). Columbamine prevents phosphorylation of AKT and promotes the expression of PTEN. Blocking PTEN level or inducing phosphorylation of AKT attenuates columbamine function on SHG44 cells proliferation, metastasis, and apoptosis.
Conclusion: In this research, we find that columbamine could inhibit proliferation and metastasis of glioma cell lines, and promote apoptosis of glioma cell lines via regulating PTEN/AKT signal pathway. It provides a new theoretical basis for the development of anti-glioma drugs.
Keywords: columbamine, glioma, proliferation, PTEN, AKT
Introduction
Glioma is one type of tumor originating from central nerve cells.1 It is characterized by high incidence, high mortality, substantial invasion, and poor prognosis.2,3 In recent years, the prevalence of glioma has been increasing year by year.4 At present, the treatment of gliomas is mainly surgery, adjuvant chemotherapy, and radiotherapy. However, glioma often has infiltrative growth, so it is difficult to achieve the ideal effect of surgical treatment.5 The existence of a blood-brain barrier is difficult; it is difficult for drugs to penetrate the blood-brain barrier and play a role, resulting in the poor prognosis, high recurrence rate, and mortality of glioma.6 Therefore, more potential treatments need to be explored.
Recently studies showed that kinds of alkaloids acted as an anti-tumor role in glioma. Wang et.al found that nor-monoterpenoid indole alkaloids could inhibit the development of glioma stem cells.7 Two isoquinoline alkaloids from Scolopendra subspinipes mutilans promoted apoptosis and prevented the proliferation of glioma cells.8 Alkaloids abstracting from Lycoris Caldwell could induce glioma cell death by performing specific cytotoxicity.9 Chi G found that matrine could promote apoptosis and autophagy in glioma cells via controlling circRNA-104075/BCL-9 signal pathway.10
Columbamine is an alkaloid that was extracted from calumba.11 In the previous research, it was found that Columbamine has anti-inflammatory and anti-tumor effects. Columbamine prevented the growth and deterioration of colon cancer by blocking the Wnt/β-catenin signaling pathway.12 Columbamine also inhibits hepatocellular carcinoma development by preventing AKT and ERK1/2 signaling pathways.13 Meanwhile, Columbamine has a cytotoxic effect on osteosarcoma cells to inhibit tumor development. Traditional Chinese medicine therapy also occupies a particular position in the process of tumor treatment. Columbamine’s effect on glioma has not been reported so far, so we were asked to explore its value. In this study, we investigated the function of Columbamine on glioma cell proliferation and metastasis.
Methods and Materials
Ethics Statement
The animal study was reviewed and approved by Cangzhou Central Hospital. The research was carried out based on the proposals in the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health.
Cell Culture
The human glioma cell lines SHG44 and U251 cells were purchased from the Science Cell Laboratory. Cell lines were cultured in PRIM 1640 (Thermo-life, USA) with 10% FBS (Thermo Fisher, USA) and 100 μL/mL penicillin and streptomycin (Beyotime, China) and placed at 37°C with 5% CO2. SHG44 and U251 cells were added with columbamine (0, 10, 20, 30, 40, and 50 μM) for 24 h, 48 h, and 72 h.
Western Blot
Total protein was isolated from cells with RIPA lysis Mix (Beyotime, China). Briefly, 60 μg protein extraction was loaded via SDS-PAGE and transferred onto nitrocellulose membranes (MILLIPORE, USA), then put them into a 5% blocking solution for 3 h. The membranes were incubated with primary antibodies at 4°C for one night. After incubation with secondary antibodies, the membranes were scanned using an Odyssey, and data were analyzed with Odyssey software (LI-COR, USA). Anti-PCNA (10205-1-AP, 1:500), anti-E-cadherin (20874-1-AP, 1:1000), anti-MMP2 (10373-2-AP, 1:500), anti-MMP7 (10374-2-AP) were purchased from Proteintech, anti-N-Cadherin (#14215, 1:500) was purchased from CST. MMP9 (ab73734) was purchased from Abcam, and Gapdh (60004-1-Ig, 1:2000) was used as an internal control.
Cell Viability Detection
MTT assay was used to detect cell viability. Cells were cultured in the 96-well plates for 1×103/well. Overnight, cells were starved for 12 h. After columbamine treatment, the cells were added with 5 mg/mL MTT (20 µL/well) for 4 h. Removing supernatant and adding DMSO (150 µL/well), the plate was shaking 0.5 h at room temperature. The absorbance was read at 490 nm using an Infinite 200PRO microplate spectrophotometer (BioTek, USA). The absorbance values were normalized to the control.
Clone Formation Assay
The cell pores in logarithmic growth phase were inoculated in 6-well plate, 4 mL PRIM 1640 was added to each well and cultured in an incubator for 14 days; the culture medium was discarded, washed with PBS for 3 times, fixed with 4% paraformaldehyde solution for 30 min; 30 min was stained with 0.1% crystal violet, washed, and dried at room temperature. Take pictures with the camera and observe the colony formation.
Wound-Healing Assay
The wound-healing assay was carried out on SHG44 and U251cell. A total of 5 ×105 cells were cultured in 6-well plates, and then the cells were gently scratched with a pipette tip. The fresh medium was changed. After columbamine treatment, the scratched spaces on the plate were evaluated by microscopy.
Transwell Assay
Cells in the logarithmic growth phase were adjusted to 2 × 105 cells/well of medium (without serum) and plated 1μg/μL Matrigel into the upper chamber. The lower chamber was added with 500 μL of the medium, and then incubate the plate at 37°C for 48 h. Then, the invading cells were visualized by the crystal violet and inverted microscope. In the same way, the transwell migration experiment was carried out without the addition of the Matrigel matrix.
Cell Apoptosis Assay
The 9 cells were counted, about 5×105cells/mL. Then, 1 mL cells were centrifuged, 1000 rpm, 10 min, 4°C, and the medium was thrown away. The cells were washed with PBS and dropped medium. The cells were resuspended and avoid light for 15 min, 200 μL Binding Buffer with10 μL Annexin V-FITC, and 10 μL PI. Flow cytometry was used to measured apoptosis rate within 1 h.
Cell Cycle Assay
Cells were collected with 1mL trypsin for 2 min, suspension the cell with 5 mL PBS, centrifuge at 1000 RPM for 5 min at 4°C. 10mL PBS buffer was used to re-washed and discarding medium, Then the cells were fixed with 70% ethanol overnight. The next day, the cell medium was filtered with a 300-mesh sieve, centrifuged at 1000 RPM at 4°C for 5 min, and the supernatant was discarded. The cells were avoided light and fixed with 1 mL PI solution and stated at 4°C for 30 min. Flow cytometer was used to evaluate the cell cycle.
TUNEL Staining
TUNEL staining was performed with a One Step TUNEL Apoptosis Assay Kit (Beyotime, China) according to protocol. The TUNEL-positive cells containing apoptotic bodies were stained red. The apoptotic cells were statistics, and the rate of apoptosis cells among the total cells was statistical.
Statistical Analysis
All values are expressed as the mean ± SEM. Statistical significances were measured by Student’s t-test and ANOVA. A two-tailed value of P < 0.05 was indicated as a statistically significant difference. Data statistics were used the Prism 8.4.
Results
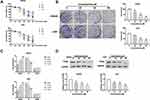
Columbamine Inhibits the Proliferation of Glioma Cell Lines
To explore the function of Columbamine on glioma, we treated SHG44 and U251 cells with Columbamine (0, 10, 20, 30, 40, and 50 μM) for 24, 48, 72 h, and determining cell viability with the MTT assay. The cell viability of glioma cells was shown to concentration and time-dependent reduction following the treatment with Columbamine (Figure 1A). In the next experiment, we treated the glioma cells with 0, 20, 30, and 50 μM columbamine for 24 h. The clone formation experiment showed the higher the concentration, the less the number of clones (Figure 1B). Next, we detected the cell cycle in SHG44 and U251 cells after columbamine treatment via flow cytometry. As Figure 1C shows, Columbamine blocks cell cycle progression from the G0/G1 to S phase. Proliferating cell nuclear antigen (PCNA) is a helper protein of DNA polymerase δ, which may play a key role in cell cycle control. Then, we found the protein level of PCNA in SHG44 and U251 cells were concentration-dependent reduction after columbamine treatment for 24 h (Figure 1D). In summary, Columbamine could inhibit the proliferation of glioma cells.
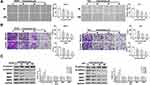
Columbamine Prevents the Migration and Invasion of Glioma Cells
Next, we discussed the effect of Columbamine on glioma cell migration and invasion. Wound healing assay showed that Columbamine significantly inhibited cell migration, and 50 μM Columbamine almost completely inhibited cell migration (Figure 2A). In our experiments with glioma via transwell and Matrigel invasion assay, we observed that Columbamine could inhibit the migration and invasion of SHG44 and U251 cells in a dosage-dependent manner (Figure 2B). The migration and invasion of glioma cells are accompanied by changes in molecular proteins. Then, we detected the expression of metastasis-related proteins, E-cadherin, N-cadherin, MMP2, MMP7, and MMP9. We observed that Columbamine induced expression of E-cadherin and inhibited the expression of N-cadherin, MMP2, MMP7, and MMP9 (Figure 2C). In summary, Columbamine could inhibit the migration and invasion of glioma cells.
Apoptosis of Glioma Cells Induced by Columbamine
Inducing tumor cell apoptosis is another way to limit tumor development. Further, we estimated the effect of Columbamine on glioma cell apoptosis. The apoptosis rates of SHG44 and U251 cells were detected by flow cytometry. The results showed that columbamine treatment significantly increased the apoptosis rate of glioma cells (Figure 3A). Additionally, TUNEL assay results showed that columbamine treatment could induce DNA damage and cell apoptosis (Figure 3B). Meanwhile, we appraised the expression of the apoptosis-associated protein. The level of Cleaved-caspase3 and Cleaved-PARP were both upregulations after 30 and 50 μM columbamine treatment, while, 30 and 50 μM Columbamine could induce upregulated of Bax and downregulated of Bcl2 (Figure 3C). The above results indicated that Columbamine could trigger apoptosis of glioma cells.
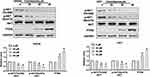
The Columbamine Regulation on Glioma Cells is Associated with PTEN/AKT Signal Pathways
To further investigate the mechanism on Columbamine inhibited the development of glioma, we examined the potential pathways of glioma. PTEN/AKT signal pathway involved in series of tumor programs. Focusing on PTEN/AKT signal pathways, we carried out Western blotting assay to explore the components of PTEN/AKT signaling pathways. The results performed that Columbamine inhibited the phosphorylation of AKT and promoted the expression of PTEN in SHG44 and U251 (Figure 4). Then, we speculated that Columbamine might regulate the development of glioma cells via controlling PTEN/AKT signaling pathways.
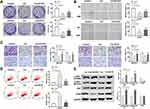
To verify this conjecture, we treated SHG44 cells with PTEN inhibitor (SF1670, 2 μM) and AKT activator (SC79, 10μM) for 24 h. After columbamine incubation, we performed colony formation assay to detect the proliferation ability, as Figure 5A shows, SF1670 and SC79 blocked the columbamine function on colony formation of SHG44 cells. Wound healing assay and Transwell migration assay revealed that SF1670 and SC79 recovered the migration ability of SHG44 cells (Figure 5B and C). Transwell invasion assay verified that SF1670 and SC79 could invert the role of Columbamine in SHG44 cells (Figure 5C). Furthermore, SF1670 and SC79 abolished the function of Columbamine in the cell apoptosis level (Figure 5D). Western blot showed that SF1670 could recover the phosphorylation level of AKT and PTEN, while SC79 could restore the phosphorylation level of AKT, but not the expression of PTEN, which revealed that PTEN was upstream of AKT (Figure 5E). Taken together, Columbamine regulated glioma cell progression via PTEN/AKT signal pathway (Figure 6).
Discussion
In the past years, the genetic basis of glioma has been elucidated in genomic research. In recent years, glioma has made significant progress in surgical and medical imaging technology, as well as radiotherapy, electric field treatment, chemotherapy, and immunotherapy.14–16 However, the inherent trend of the widespread of glioma cells in normal brain parenchyma severely limits the therapeutic effect, and the therapeutic effect of chemotherapy and biological regulators has not yet been confirmed. Therefore, we need to develop more effective and less toxic treatment methods to guide clinical application.
At present, for the treatment of tumors, not only the use of chemotherapy drugs, but traditional Chinese medicine is also constantly emerging.17–19 Because of the pharmacological effects of traditional Chinese medicine, such as inhibiting tumor cell proliferation, promoting cell differentiation and dissipation, preventing tumor metastasis to the whole body or nearby, reducing the side effects of chemotherapy, enhancing patients’ self-immunity.20–22 In clinical, traditional Chinese medicine anti-tumor drugs have been widely used.23,24 It is proved that allicin can activate the p53 gene and JNK pathway to play a role in G-M regulatory points of the cell cycle, and block tumor cells in the M phase.25 Oridonin, an effective component extracted from Rabdosia rubescens, can inhibit DNA synthesis and prolong cell cycle time by inhibiting the expression of cyclinB1, leading to G phase arrest of tumor cells.26 Wang et al confirmed that ethanol extract of hedyotis diffusa could effectively stimulate tumor cells to produce superoxide. Finally, the apoptotic signal network was activated to induce apoptosis of HL-60 cancer cells.27
The previous report showed that Columbamine suppressed colon cancer cells via blocking Wnt/β-catenin signaling pathway.12 Lin et al found that Columbamine inhibited hepatocellular carcinoma cells via abolishing of PI3K/AKT, p38 and, ERK1/2 MAPK signaling pathways.13 Columbamine prevented the development of metastatic osteosarcoma U2OS cells with low cytotoxicity.28 At present, no research has shown any effect of Columbamine on glioma. Here, we performed experiments to explore the function of Columbamine on glioma cell progression. We elucidated the inhibition effect of Columbamine on glioma cell proliferation, migration, invasion, and apoptosis level, which have specific guiding significance for the future clinical application. Meanwhile, NHA (normal human astrocytes) cells were treated with 50 μM Columbamine. The results performed that Columbamine did not affect the progression of NHA cells (Figure S1).
PTEN is a precise tumor suppressor gene. Its full name is 10q deleted tensin homologous gene, and its expression is deleted, mutated, or down-regulated in series of malignant tumors.29 PTEN could inhibit cell proliferation and hinder the development of the cell cycle, and the downstream PI3K/AKT pathway is an essential pathway for PTEN to inhibit cell proliferation. PTEN can inhibit the activation of PI3K and block the phosphorylation of AKT and its downstream protein kinases. On the one hand, it can cause cell cycle arrest in the G1 phase; on the other hand, it can also induce the expression of a variety of pro-apoptotic molecules and promote apoptosis. When the expression of PTEN decreases, the inhibition of PI3K/AKT pathway weakens, and the phosphorylation of downstream protein kinases increases, thus promoting cell proliferation.30,31 In our research, we found that Columbamine induced the expression of PTEN and prevented the phosphorylation of AKT, PTEN inhibitor, and AKT activator could abolish the function of Columbamine on glioma cell progression, which indicateds the underlying mechanism of Columbamine on glioma. In this study, we found that Columbamine can regulate AKT/PTEN pathway to control the occurrence and development of glioma, indicating that the expression of AKT and PTEN may be related to the development of glioma and may be used as an index for clinical detection and diagnosis of glioma.
Conclusion
In this research, we investigated the function of columbamine on glioma progression. It was found that columbamine could inhibit proliferation and metastasis of glioma cell lines, and promote apoptosis of glioma cell lines via regulating PTEN/AKT signal pathway.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019;15:405–417. doi:10.1038/s41582-019-0220-2
2. Jung E, Alfonso J, Osswald M, et al. Emerging intersections between neuroscience and glioma biology. Nat Neurosci. 2019;22:1951–1960. doi:10.1038/s41593-019-0540-y
3. Jones C, Baker J. Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nat Rev Cancer. 2014;14.
4. Cuddapah VA, Robel S, Watkins S, Sontheimer H. A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 2014;15(7):455–465. doi:10.1038/nrn3765
5. Glass Consortium. Glioma through the looking GLASS: molecular evolution of diffuse gliomas and the Glioma Longitudinal Analysis Consortium. Neuro Oncol. 2018;20(7):873–884. doi:10.1093/neuonc/noy020
6. Wen PY, Reardon DA. Neuro-oncology in 2015: progress in glioma diagnosis, classification and treatment. Nat Rev Neurol. 2016;12(2):69–70. doi:10.1038/nrneurol.2015.242
7. Wang B, Dai Z, Yang XW, et al. Novel nor-monoterpenoid indole alkaloids inhibiting glioma stem cells from fruits of Alstonia scholaris. Phytomedicine. 2018;48:170–178. doi:10.1016/j.phymed.2018.04.057
8. Ding D, Guo YR, Wu RL, Qi WY, Xu HM. Two new isoquinoline alkaloids from Scolopendra subspinipes mutilans induce cell cycle arrest and apoptosis in human glioma cancer U87 cells. Fitoterapia. 2016;110:103–109. doi:10.1016/j.fitote.2016.03.004
9. Cao P, Pan DS, Han S, et al. Alkaloids from Lycoris caldwellii and their particular cytotoxicities against the astrocytoma and glioma cell lines. Arch Pharm Res. 2013;36:927–932. doi:10.1007/s12272-013-0089-3
10. Chi G, Xu D, Zhang B, Yang F. Matrine induces apoptosis and autophagy of glioma cell line U251 by regulation of circRNA-104075/BCL-9. Chem Biol Interact. 2019;308:198–205. doi:10.1016/j.cbi.2019.05.030
11. Wang Y, Han Y, Chai F, et al. The antihypercholesterolemic effect of Columbamine from Rhizoma Coptidis in HFHC-diet induced hamsters through HNF-4alpha/FTF-mediated CYP7A1 activation. Fitoterapia. 2016;115:111–121. doi:10.1016/j.fitote.2016.09.019
12. Lei C, Yao Y, Shen B, et al. Columbamine suppresses the proliferation and malignization of colon cancer cells via abolishing Wnt/beta-catenin signaling pathway. Cancer Manag Res. 2019;11:8635–8645. doi:10.2147/CMAR.S209861
13. Lin Z, Li S, Guo P, et al. Columbamine suppresses hepatocellular carcinoma cells through down-regulation of PI3K/AKT, p38 and ERK1/2 MAPK signaling pathways. Life Sci. 2019;218:197–204. doi:10.1016/j.lfs.2018.12.038
14. Avila EK, Chamberlain M, Schiff D, et al. Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro Oncol. 2017;19:12–21. doi:10.1093/neuonc/now190
15. Walsh KM, Wiencke JK, Lachance DH, et al. Telomere maintenance and the etiology of adult glioma. Neuro Oncol. 2015;17:1445–1452. doi:10.1093/neuonc/nov082
16. Jones C, Karajannis MA, Jones DTW, et al. Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol. 2017;19(2):153–161. doi:10.1093/neuonc/now101
17. Xue T. Synergy in traditional Chinese medicine. Lancet Oncol. 2016;17:e39. doi:10.1016/S1470-2045(15)00557-4
18. Oncology The Lancet. Rethinking traditional Chinese medicines for cancer. Lancet Oncol. 2015;16(15):1439. doi:10.1016/S1470-2045(15)00406-4
19. Xue T, Roy R. Studying traditional Chinese medicine. Science. 2003;300:740–741. doi:10.1126/science.300.5620.740
20. Sun L, Mao JJ, Vertosick E, Seluzicki C, Yang Y. Evaluating cancer patients’ expectations and barriers toward traditional chinese medicine utilization in China: a patient-support group-based cross-sectional survey. Integr Cancer Ther. 2018;17:885–893. doi:10.1177/1534735418777117
21. Parekh HS, Liu, G, Wei MQ. A new dawn for the use of traditional Chinese medicine in cancer therapy. Mol Cancer. 2009;8(1):21. doi:10.1186/1476-4598-8-21
22. Efferth T, Li PC, Konkimalla VS, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 2007;13(8):353–361. doi:10.1016/j.molmed.2007.07.001
23. Shi YL, Li MF. Biological effects of toosendanin, a triterpenoid extracted from Chinese traditional medicine. Prog Neurobiol. 2007;82:1–10. doi:10.1016/j.pneurobio.2007.02.002
24. Chan KK, Yao TJ, Jones B, et al. The use of Chinese herbal medicine to improve quality of life in women undergoing chemotherapy for ovarian cancer: a double-blind placebo-controlled randomized trial with immunological monitoring. Ann Oncol. 2011;22(10):2241–2249. doi:10.1093/annonc/mdq749
25. Zeng T, Li Y, Zhang CL, et al. Garlic oil suppressed the hematological disorders induced by chemotherapy and radiotherapy in tumor-bearing mice. J Food Sci. 2013;78:H936–942. doi:10.1111/1750-3841.12137
26. Shen QK, Deng H, Wang SB, Tian YS, Quan ZS. Synthesis, and evaluation of in vitro and in vivo anticancer activity of 14-substituted oridonin analogs: a novel and potent cell cycle arrest and apoptosis inducer through the p53-MDM2 pathway. Eur J Med Chem. 2019;173:15–31. doi:10.1016/j.ejmech.2019.04.005
27. Wang C, Zhou X, Wang Y, et al. The antitumor constituents from hedyotis diffusa Willd. Molecules. 2017;22.
28. Bao M, Cao Z, Yu D, et al. Columbamine suppresses the proliferation and neovascularization of metastatic osteosarcoma U2OS cells with low cytotoxicity. Toxicol Lett. 2012;215:174–180. doi:10.1016/j.toxlet.2012.10.015
29. Malaney P, Uversky VN, Davé V. PTEN proteoforms in biology and disease. Cell Mol Life Sci. 2017;74(15):2783–2794. doi:10.1007/s00018-017-2500-6
30. Elhag R, Mazzio EA, Soliman KF. The effect of silibinin in enhancing toxicity of temozolomide and etoposide in p53 and PTEN-mutated resistant glioma cell lines. Anticancer Res. 2015;35(3):1263–1269.
31. Xia M, Tong JH, Ji NN, et al. Tramadol regulates proliferation, migration, and invasion via PTEN/PI3K/AKT signaling in lung adenocarcinoma cells. Eur Rev Med Pharmacol Sci. 2016;20(12):2573–2580.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.